Abstract
Purpose
The purpose of this study was to conduct a systematic review to assess the effect of exercise on the quality of life among people with breast cancer.
Methods
We conducted a systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Cochrane Library, PubMed, EMBASE, Web of Science, CINAHL, and four Chinese databases were searched for studies published until January 2018. The review included all randomized controlled trials that evaluated the effect of exercise on quality of life compared with that of usual care for people with breast cancer. Two reviewers independently assessed the quality of all the included studies using the Cochrane Handbook for Systematic Reviews of Interventions.
Results
In total, 36 studies (3914 participants) met the inclusion criteria. We divided the exercise into three modes: aerobic, resistance, and a combination of aerobic and resistance. All three modes of exercise intervention showed a significant effect on quality of life between groups.
Conclusions
Exercise is a safe and effective method of improving the quality of life in patients with breast cancer. In particular, combined training was associated with a significant improvement in quality of life. In future research, more high-quality, multicenter trials evaluating the effect of exercise in breast cancer patients are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women; more than 1.3 million women are diagnosed with breast cancer every year [1]. In the USA, the 5-year relative survival rate is 90.6% [2]. As the survival rate of breast cancer increases, many breast cancer patients are confronted with cancer-related side effects, with severe impact on physical, psychological, social, and spiritual aspects of quality of life (QOL) [3]. In addition, significant treatment-related sequelae may persist for many years and continue to influence long-term quality of survival [4]. Consequently, there is an imperative for increased attention to their quality of survivorship.
The benefits of exercise intervention on QOL for patients of breast cancer are increasingly attracting attention. Exercise interventions may be particularly appropriate for cancer patients because they have the potential to improve physical and psychological functioning, including QOL [5, 6]. There is evidence that exercise improves lean body mass, adiposity, and muscular strength in breast cancer patients, which has been associated with improved physical function and QOL [7]. Exercise prescribed two to three times a week, during or after chemotherapy, has been shown to improve QOL [8, 9]. Recent reviews on cancer and exercise reported that participation in an exercise program during and after treatment improved QOL and health status, and decreased side effects [10].
There have been several published meta-analyses of the effect of exercise interventions on the QOL of breast cancer patients. Duijts et al. [10] conducted a comprehensive meta-analysis of the effects of physical exercise on QOL for both breast cancer patients and survivors, but included randomized controlled trials (RCTs) only up to 2009. A meta-analysis published in 2013 evaluated the effect of exercise training on QOL for breast cancer survivors only [11]. This study supported the idea that exercise interventions have statistically significant effects on overall QOL. However, in recent years, there have been an increasing number of trials of motion interventions for breast cancer patients. Therefore, we undertook the present systematic review to evaluate the most recent and convincing evidence.
Methods
Protocol and registration
The review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [12].
Search strategy
A thorough search (computerized and manual) was undertaken to identify all relevant literature. We searched the Cochrane Library, PubMed, EMBASE, Web of Science, CINAHL, and four Chinese databases (CBM, CNKI, WanFang Data, and VIP). The search comprised the terms “breast cancer” and “quality of life”, with exercise intervention terms such as “exercise,” “exercise therapy,” “aerobic exercises,” “physical activity,” “resistance training,” “running,” “walking,” “sports,” “yoga,” “tai chi,” and “qigong.” The search was limited to human studies. All searches were from database inception to January 17, 2018. We also performed a manual search of references cited by the original published studies and relevant review articles. A search of Google Scholar was conducted using the same key words to identify any additional relevant articles.
Eligibility criteria
Types of studies
Only RCTs were included in the present review. No publication date restrictions were imposed on the initial search.
Participants
The participants were adults (> 18 years) diagnosed with breast cancer.
Interventions
The inclusion criteria were as follows: (1) the intervention group underwent exercise intervention; and (2) the control group did not undergo any exercise intervention. However, studies in which exercise training was part of an intervention with multiple components (e.g., combined with a diet intervention) were excluded.
Outcomes
The studies were required to report QOL as outcome measure.
Study selection
The eligibility assessment was performed by two independent reviewers. All papers identified using the search strategy were assessed for eligibility by evaluating their titles and/or abstracts. If insufficient information was available to evaluate an article, then a full-text version was obtained and reviewed by two independent reviewers. Disagreements were resolved by discussion with a third reviewer. When insufficient information or data were available in the included articles, the authors were contacted to obtain additional information if possible.
Quality assessment
The quality of included studies was assessed independently by two authors. The quality items assessed were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each trial was classified into low risk, unclear, or high risk following the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions [13] with the aim of estimating the selection, performance, attrition, and detection biases.
Data extraction and analysis
The data were extracted from the included articles using a data extraction form. Sample sizes were collected. Details on exercise interventions were recorded including the exercise type, exercise session, frequency, and program duration. The effects of the exercise training on QOL were collected, including QOL measures and the study result. One investigator performed the data extraction, which was verified by a second investigator. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions, a meta-analysis was not performed because of the heterogeneity due to a variety of different measurement tools.
Results
Study searching and selection
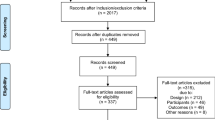
We identified 4133 studies based on the database searches, and two new studies were retrieved after reviewing the bibliographies of the full-text papers collected during the initial search. After removing duplicates and studies that did not fulfill the criteria, 36 studies [8, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] with 3914 participants were included in the final analysis (Fig. 1).
Description of studies
The characteristics of the included articles are presented in Table 1. Sample size ranged from 20 [25] to 460 [34] participants. The duration of the training sessions varied from at least 15 min [18, 33] to 90 min [8, 26, 40, 46, 49], excluding three studies [16, 24, 39] that did not report the duration of the training sessions. The frequency of the training sessions varied from at least once a week [8, 33, 36] to seven times a week [32], excluding one study [21] that did not report the frequency of the training sessions. The duration of the total training period varied from 4 weeks [17, 47] to 8 months [44], with the exception of one study [16] that did not report the duration of the total training period. We divided the exercise into three modes: aerobic, resistance, and a combination of aerobic and resistance (Table 1). Among the included studies, four studies [16, 24, 37, 42] compared two intervention types with a control group, and three studies [14, 43, 44] reported two forms of the same exercise intervention trial.
Quality assessment
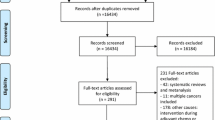
All 36 studies were included in the quality assessment (Fig. 2). Most had low risk of bias. Major sources of risk of bias were from lack of blinding study subjects or research personnel, incomplete outcome data, and blinding of outcome assessment. Of the 36 trials, most studies described the method of randomization and their methods of sequence generation or allocation concealment. Two studies did not provide the reason for loss of follow-up; this may induce attrition bias. Due to the nature of exercise interventions, it may be difficult to blind participants to intervention delivery.
Outcomes
Aerobic exercise
Twenty-five studies comprising 2327 participants examined the effect of aerobic training on QOL in patients with breast cancer [8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29, 31,32,33,34,35,36,37, 42]. Walking was the most common aerobic training [8, 14, 17, 20, 21, 25, 27, 28, 34, 37]. Other forms of aerobic training were yoga [22, 26, 31, 32, 35, 36, 42], treadmills or bicycle ergometers [8, 16, 18, 24, 33], qigong [23, 29], tai chi [19], and dancing [36].
Among the 25 studies, three studies (sample sizes between 20 [18] and 175 [14]) reported no significant effects of aerobic training on QOL in patients with breast cancer compared with that of usual care [14, 18, 24]. Cadmus et al. [14] reported the effect of a home-based walking intervention on QOL among recently diagnosed breast cancer survivors undergoing adjuvant therapy and post-treatment survivors. Hornsby et al. [18] evaluated the effect of moderate-to-high-intensity aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Adams et al. [24] evaluated the effects of the treadmill, cycle ergometer, or elliptical-based aerobic exercise on sarcopenia and dynapenia in breast cancer patients.
The other 22 studies reported a significant effect of aerobic exercise on the QOL in patients with breast cancer compared with that of patients in the control group.
In the study by Basen-Engquist et al. [15], a 6-month moderate walking intervention was incorporated into daily routines and intervention was conducted in group meetings ranging from 7 to 15 participants. A positive effect was detected on the bodily pain and general health subscales of breast cancer survivors. Rogers et al. [21] evaluated the effects of a walking intervention for sedentary breast cancer survivors; participants were encouraged to convert the minutes spent in physical activity recorded on their weekly exercise logs into miles, which were graphed on a map indicating travel across the USA to the west coast. The results demonstrated significantly greater improvements in social well-being and waist-to-hip ratio in the intervention group. Shobeiri et al. [28] reported that moderate walking significantly improved global QOL in the exercise group in women suffering from breast cancer, among whom exercise intensity increased from 50 to 75%. Heart rate reserve, function (body image, sexual function), and symptoms (side effects of treatment, breast symptoms, and arm symptoms) showed a significant improvement on EORTC QLQ-BR23. Baruth et al. [25] evaluated a 12-week home-based walking intervention with telephone counseling sessions in breast cancer survivors. The walking prescription was a gradual increase in the frequency, duration, and intensity at moderate to vigorous intensity. Pedometers and physical activity logs were given to encourage self-monitoring. They found improvements in general aspects of QOL and in breast cancer-specific QOL. Ergun et al. [37] and Lahart et al. [27] reported that a moderate-intensity home-based walking intervention resulted in small significant improvements in body mass index and breast cancer-specific QOL. Participants were encouraged to achieve 150 min of moderate-vigorous physical activity over each week, followed by an in-person counseling session, telephone call, and mailed information encouraging participation in activity. Patients were taught how to measure their heart rates and maximal heart rate for their ages were calculated. Jacobsen et al. [34] and Fillion et al. [17] evaluated the effects of home-based walking on QOL in breast cancer patients receiving chemotherapy; participants were provided with a video, booklet, audio, and use of coping self-statements to manage stress. They found that the combined stress management and exercise intervention yielded effects on QOL.
Five studies evaluated yoga in people with breast cancer and found that yoga had a significant effect on QOL. Littman et al. [31] evaluated the effects of 6-month facility- and home-based yoga in overweight and obese breast cancer survivors on QOL. Participants received a yoga mat and strap, blankets, and chairs to aid with poses. Practices were designed specifically for the study. They found that yoga practice improved fatigue and decreased waist circumference; the greatest improvements were observed in women who practiced a mean of three times per week. Loudon et al. [32] reported the effect of yoga on women with breast cancer-related lymphedema, who underwent a weekly 90-min teacher-led class and a 40-min daily session delivered by DVD. The yoga intervention reduced tissue induration of the affected upper arm and decreased the QOL sub-scale of symptoms. Danhauer et al. [35] reported that restorative yoga had significant effects for health-related QOL and fatigue for women with breast cancer who attended ≥ 7 classes/week; better intervention adherence was associated with higher self-reported physical health and health-related QOL. Kiecolt-Glaser et al. [26] and Chandwani et al. [42] reported that yoga practice substantially reduced fatigue, and resulted in increased physical functioning and physical component scale scores in women with breast cancer undergoing radiotherapy compared with the control group.
Courneya et al. [8] and Voogd et al. [33] reported the effects of exercise training on recumbent or upright cycle ergometers on cardiopulmonary function and QOL in post-menopausal breast cancer survivors. The training intensity corresponded to approximately 70–75% of maximal oxygen consumption in untrained subjects. Beneficial effects on QOL were found, with adverse events in the exercise group reported in three participants (lymphedema, gynecologic complication, and influenza). Courneya et al. [16] reported that a cycle ergometer exercise program resulted in improvements in body composition and QOL in breast cancer patients.Mutrie et al. [20] evaluated the effects of a supervised group exercise program on bicycle ergometers on QOL for women being treated for early stage breast cancer. Women were monitored to ensure that they were exercising at a moderate level (50–75% of age-adjusted maximum heart rate) and showed benefits in physical and psychological functioning at the 6-month follow-up.
Oh et al. [23] reported that a 10-week medical qigong exercise program improved QOL in women with metastatic breast cancer; the intervention included gentle stretching and body movements in standing postures, meditation, and breathing exercises. Participants were encouraged to undertake home practice every day for at least 30 min. Chen et al. [29] reported lower levels of depressive symptoms and fatigue and better overall QOL in their qigong cohort.
Mustian et al. [19] compared the efficacy of Yang-style tai chi chuan with psychosocial support therapy for health-related QOL. Participants were instructed to keep a daily log; patients undertaking tai chi chuan demonstrated significant improvements in QOL and self-esteem.
Sandel et al. [36] evaluated a 12-week dance program incorporating a variety of traditional music and movements, and reported significant improvements in QOL, shoulder range of motion, and body image scale in breast cancer survivors. The control group also showed significant improvements after crossover to active treatment in weeks 13 to 25.
Overall, these results indicate that the effect of aerobic training on QOL in patients with breast cancer is beneficial.
Resistance exercise
Eight studies comprising 1150 participants examined the effect of resistance training on QOL in patients with breast cancer [16, 24, 30, 38,39,40,41,42]. The studies examined resistance training with free weights, resistance training machines [38,39,40,41], and progressive stretching [16, 30, 42]. One study did not specify resistance training type [24]. Most of the training sessions were 30 min [30, 41, 42]. The most common training session frequency was two or three times a week [16, 24, 30, 39,40,41,42].
Among the eight studies, one (Kilbreath et al. [38]) reported no significant effects of resistance training on QOL in patients with breast cancer compared with that of usual care; they evaluated the shoulder muscles with progressive resistance and stretching training following surgery for early breast cancer and precipitate lymphedema.
Seven studies reported a significant effect of resistance exercise on the QOL in patients with breast cancer compared with that of patients in the control group.
Hagstrom et al. [39] reported that a 16-week supervised resistance training session had significant difference in global QOL and the specific physical wellbeing subscale in previously sedentary breast cancer survivors. Training included barbell squats, deadlifts, free weight barbell bench presses, leg presses, bent over barbell row, and assisted chin ups in one-on-one or group training sessions. Three sets of eight to 10 repetitions were performed for each exercise. They found a significant correlation between improvements in strength of the treated limb and improvements in global QOL. Speck et al. [40] showed twice-weekly strength training (included seated row, supine dumbbell presses, lateral or front raises, bicep curls and triceps pushdowns, leg presses, back extension, leg extension, and leg curl) positively impacted self-perceptions of appearance, health, mental summary scores, and social functioning for breast cancer survivors with or at risk for lymphedema, and found the intervention was beneficial regardless of prior diagnosis of lymphedema. Ohira et al. [39] and Chandwani et al. [42] evaluated the effects of weight-training exercises using variable resistance machines and free weights. They reported improved physical and psychosocial global QOL score in recent breast cancer survivors. Participants were encouraged to train with other survivors to foster friendships and found weight training-associated changes in body composition and upper body strength to improve QOL.
Steindorf et al. [30] reported that a 12-week progressive resistance training program significantly larger improved the QOL sub-scales of role function and pain. The control group carried out progressive muscle relaxation without any aerobic or muscle strengthening components. Adherence to the intervention program as well as completion rate were 97%. Adams et al. [24] reported that resistance exercise significantly reversed sarcopenia and dynapenia in breast cancer patients and greatly improved QOL. Training was performed at 60–70% of predicted one repetition maximum. Courneya et al. [16] reported that resistance exercise improved self-esteem, QOL, and chemotherapy completion rate in breast cancer patients receiving adjuvant chemotherapy.
Overall, these results indicate that resistance training in patients with breast cancer is safe and has beneficial effect on QOL.
Combined exercise
Seven studies comprising 703 participants examined the effect of combined exercise on QOL in patients and breast cancer survivors [37, 43,44,45,46,47,48]. Combined exercise consisted of a combination of aerobic (e.g., walking, running, cycling, swimming) and resistance training (e.g., upper and lower limb resistive exercise, stretching exercise).
Milne et al. [46] evaluated a combined exercise program supervised by two exercise physiologists. The program included an aerobic component (cycle and rowing ergometers, a mini-trampoline) and resistance training consisted of different exercises (e.g., chest presses, etc.). Improvements in QOL and fatigue were detected in breast cancer survivors, and improvements in aerobic fitness were associated with improvements in QOL. Ariza-Garcia et al. [43] evaluated the effects of an 8-week combined aerobic and strength exercise program in water versus on land on QOL in breast cancer survivors. The water exercise group trained in a swimming pool with a water temperature of 30–32 °C, using pool noodles, pull buoys, and swimming board. They reported that exercise on land produced a greater increase in lean body mass, whereas water exercise was better for improving breast symptoms; both interventions improved QOL. Do et al. [47] evaluated the effects of aerobic exercises as well as stretching and strengthening exercises on QOL, cardiopulmonary function, and fatigue in breast cancer patients. Aerobic exercise was performed for 40 min at 40–75% of maximum oxygen consumption (VO2 max), and strengthening exercises comprised two sets of 8–12 repetitions using the T-bar and gym ball at 60–80% of one repetition maximum. The intensity of exercises was set according to the guidelines for older adults, provided by the American College of Sports Medicine. They found significant differences in global health scores, physical functioning, emotional functioning, and cancer-related symptoms on EORTC QLQ-C30 and cancer-related symptoms such as arm and breast symptoms on EORTC QLQ-BR23. Hayes et al. [44] evaluated an 8-month intervention incorporating both aerobic and strength-based exercises. The multimodal exercise intervention was effective in preventing fatigue and optimizing QOL. The goals of the intervention were accumulation of 180+ min of exercise per week, remain independent exercisers, and to demonstrate that delivery of the intervention face-to-face and over-the-telephone had similar effect. Galiano-Castillo et al. [48] evaluated an Internet-based, personalized resistance and aerobic exercise program. The system could send instant messages and set up video conference sessions. QOL, pain, and muscle strength were significantly improved in breast cancer survivors. Herrero et al. [45] evaluated a combined cardiorespiratory and resistance exercise training program in breast cancer survivors and all of the subjects performed a cardiorespiratory test to measure peak VO2 (VO2peak). They found improved VO2peak, QOL, and overall physical fitness. Ergun et al. [37] evaluated the effects of combined exercise on angiogenesis and apoptosis-related molecules and QOL in patients with breast cancer. Functional scores and global health scores increased significantly after the exercise program.
Overall, these results indicate that combined training in patients with breast cancer was safe and beneficial for QOL.
Discussion
The present review systematically assessed the effectiveness of aerobic exercise, resistance exercise, and combined exercise on QOL in patients with breast cancer. In this systematic review, the 36 included studies were rather heterogeneous in terms of type of intervention exercise, frequency and duration of exercise sessions, program duration, and QOL measurements. It is important to determine what types of exercise program are optimal for the improvement of QOL of breast cancer patients. Twenty-two of the 25 studies reported a significant effect of aerobic exercise on QOL in patients with breast cancer compared with that of the control group. Seven of the eight studies reported a significant effect of resistance exercise on QOL in patients with breast cancer compared with that of the control group. All seven studies reported a significant effect of combined training on the QOL in patients with breast cancer compared with that of the control group. Future research should be directed at the effects of combined exercise on QOL in patients with breast cancer.
Better intervention adherence was associated with significantly improved fatigue, physical well-being, and QOL [31, 35]. Various incentive policies were taken to reduce the dropout rate, such as recording minutes of activity, recording steps using a pedometer, telephone meetings, group meetings, face-to-face counseling sessions, video conference sessions, and one-on-one training. Among the included studies, 24 provided supervised exercise [8, 14,15,16,17,18,19,20,21, 25,26,27,28, 31, 34, 35, 37, 39, 41,42,43, 45, 46, 48]. In these 24 studies, the attendance ranged from 61 to 98.4%. Rogers et al. [21] reported that participants were encouraged to convert the minutes spent in physical activity recorded on their weekly exercise logs into miles to provide participants with a feeling of accomplishment and a possible competitive opportunity, which improved the QOL. Courneya et al. [8] reported the significant self-esteem and QOL changes in the breast cancer patients resulted from increased social interaction or a sense of accomplishment in completing the exercise program. Six studies [15,16,17, 25, 28, 46] reported group meetings resulted in significant improvement in vigor and a trend toward a beneficial effect on total mood disturbance, and that patients thought that the sessions with the exercise specialists were the most helpful intervention component. Two studies [44, 48] reported video conference sessions improved high acceptance and adherence to the intervention, improved QOL, and had cost advantages. Attendance exceeded 90% in three supervised exercise programs [18, 34, 39].
Among the included studies,12 discussed the intensity of exercise interventions [8, 14, 16, 18, 19, 21, 25, 27, 28, 41, 43, 44]; of these, eight studies [8, 14, 16, 18, 25, 41, 43, 44] reported that exercise prescriptions incorporating more than 150 min of high-intensity training per week was associated with a low incidence of adverse events in breast cancer patients and significantly improved upper and lower body strength and improved the QOL. Three studies [21, 27, 28] suggested that interventions should focus on achieving a weekly minimum of 150 min of moderate intensity activity, which would improve the QOL, and possibly derive associated benefits of reduced risk of mortality and recurrence [49]. One study [19] reported that less than 150 min/week of low- and moderate-intensity exercise improved QOL and self-esteem.
In the included studies, 11 reported lymphedema [15, 16, 25, 32, 36,37,38, 40, 41, 43, 44]. One study reported adverse events. Two of three participants who developed lymphedema had locoregional radiotherapy that included axillary irradiation, which is a strong risk factor for lymphedema. Eight studies [16, 25, 36,37,38, 40, 41, 43] reported neither aerobic training nor resistance training caused lymphedema or other adverse events. Ariza-Garcia et al. [43] found breast cancer survivors performing progressive exercise in water or land showed less increase in arm swelling than the control group and had improved QOL. Results supported the use of upper quadrant exercise programs. Loudon et al. [32] reported yoga reduced tissue induration of the affected upper arm and decreased symptoms having a negative effect on QOL. However, the benefits did not last on cessation of the intervention when arm volume related to lymphedema increased.
Ergun et al. [37] reported some physicians and researchers had reservations with regard to aerobic and resistive exercise, mainly due to the concerns of lymphedema. Patients restrict the use of their arms and upper body activities because of fear of developing lymphedema. However, studies showed that aerobic or resistive exercises did not induce or aggravate lymphedema in breast cancer patients [50]. Greater shoulder muscle strength is significantly associated with increased functional well-being in breast cancer patients [51]. The improvements in QOL scores were significantly correlated with changes in bench press strengthabilities but not in leg press [39]. A recent randomized study showed that self-reported physical functioning, general health, and vitality in breast cancer patients with lymphedema increased after participating in an 8-week upper extremity exercise program [52].
Conclusion
The limitations of this systematic review should be noted. Some studies do not report adverse events and there is a need to explore the adverse effects of exercise among breast cancer patients in future research. Second, some studies did not provide supervised exercise. We could not determine whether the participants completed the training program or whether they reached a moving target, which might have affected the QOL outcome. In addition, five studies did not report the specifics of the loss to follow-up and the measurement tools of studies were diverse; both can result in overestimation of the positive effects of exercise interventions in study results.
We conclude that exercise is safe and effectively improves QOL in patients with breast cancer. In particular, combined training produced a positive effect on QOL and no adverse events were reported with this multimodal intervention.
Abbreviations
- BCS:
-
Breast cancer subscale
- BIRS:
-
Body Image and Relationships Scale
- EORTC QLQ-BR23:
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Breast
- EORTC QLQ-C30:
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30
- ES:
-
Effect Size
- FACT-A:
-
Functional Assessment of Cancer Therapy-Anemia scale
- FACT-B:
-
Functional Assessment of Cancer Therapy-Breast questionnaire
- FACT-G:
-
Functional Assessment of Cancer Therapy-General
- FWB:
-
Functional well-being
- HRQOL:
-
Health-related quality of life
- MCS:
-
Mental health
- PCS:
-
Physical Component Summary
- QOL:
-
Quality of life
- RCT:
-
Randomized controlled trials
- SF-36:
-
36-item Short-Form Health Survey
- TOI-An:
-
Trial Outcome Index-Anemia
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics. CA Cancer J Clin 65(2):87–108
US National Cancer Institute. The SEER cancer statistics review,1975–2010,Bethesda,MD.http://seer.cancer.gov/csr/1975_2010/results_merged/topic_survival_by_yeardx.pdf. Accessed 15 July2013
Noal S, Levy C, Hardouin A, Rieux C, Heutte N, Ségura C, Collet F, Allouache D, Switsers O, Delcambre C, Delozier T, Henry-Amar M, Joly F (2011) One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 81(3):795–803
Schmitz KH, Speck RM, Rye SA, DiSipio T, Hayes SC (2012) Prevalence of breast cancer treatment sequelae over 6 years of follow-up: the pulling through study. Cancer 118(S8):2217–2225
Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A (2007) Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol 25(13):1713–1721
Courneya KS, Friedenreich CM (2007) Physical activity and cancer control. Semin Oncol Nurs 23(4):242–252
Courney KS, Crawford JJ, Adams SC (2015) Physical activity and exercise interventions in cancer survivors. Psychooncology:515–520
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS (2003) Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol 21(9):1660–1668
Campbell A, Mutrie N, White F, McGuire F, Kearney N (2005) A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs 9(1):56–63
Duijts SFA, Faber MM, Oldenburg HSA, van Beurden M, Aaronson NK (2011) Effectiveness of behavioral functioning and health-related quality of life in breast cancer patients and survivors-a meta-analysis. Psychooncology 20:115–126
Zeng Y, Huang M, Cheng AS, Zhou Y, So WK (2014) Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer 21(3):262–274
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Higgins JPT, Green S (editors) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration
Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML (2009) Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology 18(4):343–352
Basen-Engquist K, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, Gregg X, Massey P, Valero V, Rivera E (2006) Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns 64(1):225–234
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25(28):4396–4404
Fillion L, Gagnon P, Leblond F, Gélinas C, Savard J, Dupuis R, Duval K, Larochelle M (2008) A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs 31(2):145–159
Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW (2014) Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol 53(1):65–74
Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K (2004) Tai Chi Chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer 12(12):871–876
Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, Kearney N, Walker A, Ritchie D (2007) Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 334(7592):517
Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington G, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M (2009) A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc 41(4):935–946
Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, Gopinath KS, Srinath BS, Vishweshwara MS, Madhavi YS, Ajaikumar BS, Bilimagga SR, Rao N (2009) Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med 17(5–6):274–280
Oh BBP (2014) Effects of qigong on quality of life, fatigue, stress, neuropathy, and sexual function in women with metastatic breast cancer: a feasibility study. J Clin Oncol 02(4)
Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR, Gelmon K, Friedenreich CM, Reid RD, Courneya KS (2016) Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat 158(3):497–507
Baruth M, Wilcox S, Der Ananian C, Heiney S (2015) Effects of home-based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: a 12-week pilot study. J Phys Activ Health 16(12):S110–S108
Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, Emery CF, Layman R, Mrozek EE, Glaser R (2014) Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol 32(10):1040–1049
Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR (2016) Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer 16(1):234
Shobeiri F, Masoumi SZ, Nikravesh A, Heidari Moghadam R, Karami M (2016) The impact of aerobic exercise on quality of life in women with breast cancer: a randomized controlled trial. J Res Health Sci 16(3):127–132
Chen Z, Meng Z, Milbury K, Bei W, Zhang Y, Thornton B, Liao Z, Wei Q, Chen J, Guo X, Liu L, McQuade J, Kirschbaum C, Cohen L (2013) Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer 119(9):1690–1698
Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, Beckhove P, Owen R, Debus J, Wiskemann J, Potthoff K (2014) Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 25(11):2237–2243
Littman AJ, Bertram LC, Ceballos R, Ulrich CM, Ramaprasad J, McGregor B, McTiernan A (2012) Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer 20(2):267–277
Loudon A, Barnett T, Piller N, Immink MA, Williams AD (2014) Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med 14:214
Voogd AC (2003) Exercise training improves cardiopulmonary function and quality of life in postmenopausal breast cancer survivors. Aust J Physiother 49(3):220
Jacobsen PB, Phillips KM, Jim HS, Small BJ, Faul LA, Meade CD, Thompson L, Williams CC Jr, Loftus LS, Fishman M, Wilson RW (2013) Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psychooncology 22(6):1229–1235
Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, Levine EA (2005) Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology 18(4):360–368
Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M (2005) Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs 28(4):301–309
Ergun M, Eyigor S, Karaca B, Kisim A, Uslu R (2013) Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur J Cancer 22(5):626–637
Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, Hansen R (2012) Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat 133(2):667–676
Ohira T, Schmitz KH, Ahmed RL, Yee D (2006) Effects of weight training on quality of life in recent breast cancer survivors. Cancer 106(9):2076–2083
Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, Schmitz KH (2010) Changes in the body image and relationship scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat 121(2):421–430
Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S (2016) Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer 25(5):784–794
Chandwani KD, Perkins G, Nagendra HR, Raghuram NV, Spelman A, Nagarathna R, Johnson K, Fortier A, Arun B, Wei Q, Kirschbaum C, Haddad R, Morris GS, Scheetz J, Chaoul A, Cohen L (2014) Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol 32(10):1058–1065
Fernández-Lao C, Cantarero-Villanueva I, Ariza-Garcia A, Courtney C, Fernández-de-las-Peñas C, Arroyo-Morales M (2013) Water versus land-based multimodal exercise program effects on body composition in breast cancer survivors: a controlled clinical trial. Support Care Cancer 21(2):521–530
Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, Saunders C, Battistutta D, Eakin E (2013) Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat 137(1):175–186
Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, Earnest CP, Foster C, Lucía A (2006) Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. Int J Sports Med 27(7):573–580
Milne HM, Wallman KE, Gordon S, Courneya KS (2008) Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 108(2):279–288
Do J, Cho Y, Jeon J (2015) Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer 18(1):87–96
Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, Ariza-García A, Díaz-Rodríguez L, Del-Moral-Ávila R, Arroyo-Morales M (2016) Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 122(20):3166–3174
Ibrahim EM, Al-Homaidh A (2011) Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28(3):753–765
Hayes S, Reul-Hirche HJ (2009) Exercise and secondary lymphedema: safety, potential benefits, and research issues. Med Sci Sport Exer 41(3):483–489
Fong SS, Ng SS, Luk WS, Chung JW, Chung LM, Tsang WW, Chow LP (2013) Shoulder mobility, muscular strength, and quality of life in breast cancer survivors with and without Tai Chi Qigong training. Evid Based Complement Alternat Med 2013(1):787169
Mckenzie DC, Kalda AL (2003) Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. J Clin Oncol 21(3):463–466
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, X., Li, Y. & Liu, D. Effects of exercise on the quality of life in breast cancer patients: a systematic review of randomized controlled trials. Support Care Cancer 27, 9–21 (2019). https://doi.org/10.1007/s00520-018-4363-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4363-2