Abstract
Exercise for Health was a randomized, controlled trial designed to evaluate two modes of delivering (face-to-face [FtF] and over-the-telephone [Tel]) an 8-month translational exercise intervention, commencing 6-weeks post-breast cancer surgery (PS). Outcomes included quality of life (QoL), function (fitness and upper body) and treatment-related side effects (fatigue, lymphoedema, body mass index, menopausal symptoms, anxiety, depression and pain). Generalised estimating equation modelling determined time (baseline [5 weeks PS], mid-intervention [6 months PS], post-intervention [12 months PS]), group (FtF, Tel, Usual Care [UC]) and time-by-group effects. 194 women representative of the breast cancer population were randomised to the FtF (n = 67), Tel (n = 67) and UC (n = 60) groups. There were significant (p < 0.05) interaction effects on QoL, fitness and fatigue with differences being observed between the treatment groups and the UC group. Trends observed for the treatment groups were similar. The treatment groups reported improved QoL, fitness and fatigue over time and changes observed between baseline and post-intervention were clinically relevant. In contrast, the UC group experienced no change, or worsening QoL, fitness and fatigue, mid-intervention. Although improvements in the UC group occurred by 12-months post-surgery, the change did not meet the clinically relevant threshold. There were no differences in other treatment-related side effects between groups. This translational intervention trial, delivered either FtF or Tel, supports exercise as a form of adjuvant breast cancer therapy that can prevent declines in fitness and function during treatment and optimise recovery post-treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With over one million women worldwide diagnosed with breast cancer each year, and improving survival rates [1], there is an imperative for increased attention to breast cancer survivorship. Receiving treatment for breast cancer has long been associated with an array of physical and psychosocial consequences with the type, prevalence and severity of concerns evolving with changes in the way breast cancer is treated [2]. Current estimates indicate that at 6-months post-diagnosis, 90 % of women report at least one adverse treatment effect and 60 % report multiple sequelae, which influence function, the ability to adhere to adjuvant breast cancer treatment, quality of life (QoL) and potentially survival [3]. Notably, even 6 years out from breast cancer, 30 % of women report multiple, significant treatment-related sequelae that continue to influence longer-term morbidity and mortality [3]. Thus, there is a clear need for identifying strategies which can be integrated among current breast cancer care to optimize quality and quantity of survival.
There exists a growing and compelling body of evidence supporting the benefits of exercise following the diagnosis of cancer, in particular breast cancer, with results summarized in meta-analyses and systematic reviews [4–6]. Exercise interventions implemented during and/or following treatment lead to improvements in cardiorespiratory fitness, body composition (i.e. muscle mass and bone health), immune function, strength and flexibility, body image, self-esteem and mood, and allow for better adjustment to illness. Exercise interventions have also been shown to reduce stress, depression, anxiety and the number and severity of side effects, including nausea, fatigue and pain. These benefits have been observed in exercise interventions involving aerobic- and/or resistance-based exercise undertaken for 90+ min/week [6]. Further, evidence from large cohort studies indicate that exercise may also improve survival [7] with results supporting a dose–response relationship, whereby more exercise is better than less, but possibly only up to levels which meet national physical activity recommendations (150 min of moderate-intensity activity per week).
To date, the majority of exercise intervention trials among women with breast cancer has evaluated supervised and clinic-based interventions with eligibility criteria which restricts participation to those with early stage disease and no additional co-morbidities or complications that may interfere with participating in an exercise intervention [4]. While a limited number of trials have evaluated non-FtF intervention delivery methods [8–16], effect of the exercise intervention on QoL, function and treatment-related side effects varies and none of these trials have compared the effect of different modes of delivery of the same exercise intervention. Thus, the current evidence base in support of exercise post-breast cancer largely pertains to the ‘healthier’ woman with breast cancer, who is able and willing to attend clinic-based exercise treatment.
The call to include exercise as a part of the standard of care provided to women following breast cancer is getting louder [17]. However, to change clinical practice, there is a clear need for comparative effectiveness trials demonstrating feasibility and effect of exercise associated with alternative delivery modalities. It is also necessary for benefits to be observed on a heterogeneous population of women with breast cancer, such that the intervention is suitable for all women, irrespective of health and disease status and participation needs to be feasible irrespective of place of residence. Exercise for Health (EfH) was a pragmatic trial designed to evaluate the feasibility and effect of an exercise intervention delivered either FtF or Tel, that if successful, could be integrated within clinical practice. The 8-month intervention delivered via both modalities was designed to assist women during active treatment periods (up to 6 months post-diagnosis), to more quickly and fully recover following treatment, and to develop the skills and confidence to become and stay physically active for the longer term. The purpose of this paper is to compare the effectiveness of the FtF and telephone-delivered exercise intervention on QoL, and patient-reported and clinically measured function and treatment-related side effects.
Methods
The EfH trial (ACT RN: 012606000233527) was reviewed and approved by the Institutional Review Board (i.e., Ethics Committee) at the Queensland University of Technology and at each of the four participating hospitals. The analyses presented in this manuscript adhere to those outlined in the original grant application and presented in the baseline manuscript [18].
Patients
A total of 194 women with a first diagnosis of invasive breast cancer were recruited into the ‘Exercise for Health’ trial through four participating Brisbane (Australia) hospitals between October 2006 and June 2008. Breast cancer nurses introduced patients to the trial after their initial surgery. Interested patients were then contacted by research staff 3–4 weeks post-surgery to discuss the trial in detail and confirm informed consent. Women aged 20–69 years, and residing within a 30-km radius of the Brisbane central business district (to enable participation in FtF sessions) were eligible to participate. Exclusions were made for women who were pregnant or lactating, had plans for breast reconstructive surgery during the study period or with poor English.
Study design
While a detailed explanation of trial methods and baseline participant characteristics have been published elsewhere [18], key features of study design are summarised below.
Timing of assessments and randomisation
Disease and treatment characteristics were extracted from the Queensland Cancer Registry, including type of cancer, type of surgery, tumour size, cancer stage and lymph node status, while a self-reported questionnaire and battery of physical tests were implemented by Exercise Physiologists blinded to group allocation at pre-intervention/baseline (5.7 weeks post-surgery, 95 % CI: 5.2, 6.1 weeks); mid-intervention/6 months (25.6 weeks; 95 % CI: 25.1, 26.2 weeks) post-surgery and 8 weeks post-intervention/12 months (51.4 weeks; 95 % CI: 51.0, 51.9 weeks) post-surgery (Fig. 1). The mid-intervention assessment coincides with mid to end of adjuvant treatment period and enables measurement of intervention effect on treatment-related symptoms at a time when they are expected to be at their highest. The final data collection time point (approximately 2 months post-intervention) allows measurement of longer-term effect on outcomes and coincides with 12 months post-surgery, which is the time point when many of the outcomes have been previously shown to stabilise [19–21].
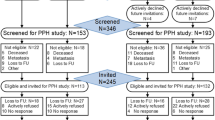
After baseline assessment, women were individually randomised into one of the three groups via a computer-generated, unblocked sequence of random numbers. Sixty-seven women were each randomised into the FtF-delivered exercise intervention group (FtF) and the telephone-delivered exercise intervention group (Tel), while sixty women were randomised into the usual-care group (UC). Figure 2 details the recruitment, randomisation and followup process.
Intervention
For those in the FtF and Tel groups, the 8 month exercise intervention began in the week following baseline assessment (6 weeks post-surgery) (Fig. 1). Key features of the intervention are presented in Table 1. The intervention involved 16 scheduled sessions (in person or via telephone) with a designated Exercise Physiologist, starting weekly and tapering to monthly contacts after 4 months. Exercise prescription was Exercise Physiologist-driven during the first third to half of the programme and became more patient-driven over time. This approach allowed patients to have their exercise prescription clinically led during their active treatment period, when treatment-related symptoms were likely to be presenting and/or fluctuating and exercise confidence, skills and knowledge was at their lowest. The approach also acknowledges the need for longer-term behaviour change (beyond the treatment period), which would only occur if patients developed skills, knowledge and confidence to become and stay independent exercisers. At all stages of the intervention, women were progressing towards (or maintaining) the overall goal of exercising at least 4 days per week for 45 min (accumulating 180+ min of exercise per week) and incorporating both aerobic and strength-based exercises (on at least 2 days per week). Exercise starting parameters and rate of progression was individually tailored, taking into account existing level of fitness, the presence of treatment-related side effects and exercise preferences.
Usual care
Women in the UC group were given no advice outside of that provided through usual care. This varied depending on treating clinician and/or hospital and may have included receipt of verbal or written encouragement for participating in physical activity during and beyond breast cancer, but with no formal or regular advice about what to do and how to do it.
Outcomes of interest
Primary outcome: quality of life
The primary outcome measure was QoL, as measured by the Functional Assessment of Cancer Therapy-Breast (FACT-B +4) questionnaire. This scale has been used widely in cancer research with high reliability and validity [22, 23]. The FACT-B +4 includes 40 items rated on a five-point Likert scale ranging from 0 ‘not at all’ to 4 ‘very much’. This questionnaire includes the FACT-General (FACT-G) [24], consisting of four domains (physical, social, emotional and functional well-being) and a breast cancer-specific subscale (FACT-B) [22] with an additional four questions specific to arm morbidity [23]. Scores were calculated according to the FACT manual [25], resulting in total scores of 0–160, with higher scores representing better well-being. A priori sample size calculations indicated a minimum of 40 women per group was required to detect a clinically important difference of 8 units in overall QoL between groups or change over time (standard deviation of change in FACT-B +4 over 12 months = 10 units) [22], with 90 % power and 5 % type I error (two tailed).
Secondary outcomes: patient-reported function and treatment-related symptoms
Upper-body function and treatment-related symptoms including fatigue, menopausal symptoms (including psychological, anxiety, depression, somatic and vasomotor scales) and neuropathic pain were measured by the Disabilities of the Arm, Shoulder and Hand Questionnaire (scale: 0–100, higher score denotes worse function) [26], the Functional Assessment of Chronic Illness Therapy–Fatigue Subscale (scale: 0–52, higher score denotes lower fatigue) [27], the Greene Climacteric Scale (scale 0–63, higher score denotes higher symptom presence) [28] and the Neuropathic Pain Scale (scale 0–100, higher score denotes higher pain [29], respectively. The Greene Climacteric Scale was also used to evaluate anxiety and depression with women reporting scores of >10 for scale items 1–6 and 7–11 (assessed separately) being classified as clinically anxious and clinically depressed, respectively. Participants were also asked whether they had received a clinical diagnosis of lymphoedema, and if so, by whom and when.
Secondary outcomes: clinically measured function and treatment-related symptoms
The 3-min step test was used as a measure of aerobic fitness [30]. The step height was modified from 12 inches to 6 inches to accommodate knee and hip limitations of some of the participants (within-patient step height was standardised across all assessments). The metronome was set at 96 beats/min and heart rate on test completion was used as the outcome measure. Lower heart rate indicates higher fitness. Upper-body strength and endurance was measured by an incremental exercise protocol combining a traditional upright row and shoulder press exercise using hand weights. Each stage lasted 20 s in duration and progression was made through number of repetitions and weight held. The prerequisite for advancement to the next stage was defined by maintaining correct form, range of motion and speed (as determined by the Exercise Physiologist). Stages ranged from 1 (no weight) through to 24 (3.5 kg). The amount of weight held incremented by 0.5 kg after three completed stages with 10 repetitions performed for each stage. The last successfully completed stage for each arm was recorded. This protocol has been successfully used in our prior work [31]. Lymphedema status was assessed by bioimpedance spectrophy (BIS), which is a previously well-described objective method of subclinical and/or pitting lymphoedema [32, 33]. In brief, BIS measurements on each arm were carried out using an Imp SFB7 monitor (Impedimed, Brisbane, Australia). The impedance of the extracellular fluid for each limb was calculated by means of the manufacturer’s software. The ratio of impedance values, comparing the treated and untreated sides, was then calculated and converted into a lymphedema index (L-Dex) score. A participant was classified as having lymphedema when the L-Dex score was 10 or greater. Height was assessed at pre-intervention with the participant barefoot and measured to the nearest 0.5 cm at baseline. Body weight (kg) was measured at all three assessments using analogue Seca™ scales. Weight was recorded to the nearest 0.5 kg. Weight and height were used to calculate body mass index (BMI) using the metric formula weight (kg)/height2 (m2) to produce a unit of measurement of kg/m2. The Active Australia Survey was used to collect information on total minutes of walking and moderate and vigorous physical activity mid- and post-intervention [34].
Clinically relevant changes in outcomes
Clinically relevant changes in outcome were determined a priori, with cut-offs identified previously by us or others. Specifically, a change of >8 quality of life units [22], >5 fatigue units [27], >1.5 stages for clinically measured upper-body function[31] and >1 BMI unit (approximately 2.5 kg change in body weight)[35] is clinically relevant with respect to perceived quality of life, fatigue, upper-body function and longer term health outcomes, respectively. Of note, these magnitudes of changes are equivalent to ≥1/2 SD of baseline scores. As such, in the absence of previous work to guide clinically relevant cut-offs for our other outcomes of interest, a change of ≥1/2 SD of baseline scores was a priori deemed clinically relevant and was equivalent to a change of >8 beats/min for fitness (heart rate) and >7.5, >7.5 and >9 units for upper-body function (self-report), menopausal symptoms and pain scores, respectively.
Statistical analysis
Summary descriptive statistics for baseline characteristics included counts and percentages for categorical variables or means (SD), alternatively medians (ranges), for continuously-scaled variables. Continuous outcomes were modelled using generalised estimating equations (GEE) to determine time (baseline, mid- and post-intervention) and intervention group (FtF, Tel, UC) effects and the interaction between time and group. Means and 95 % confidence intervals (CI) are reported for each estimate. GEEs were considered the most appropriate multivariate modelling technique, as unlike conventional repeated measures approaches, it is able to incorporate baseline data as well as all available data including those from participants with missing data over time. Intention-to-treat principles were applied to the analysis of data. No imputation was generated. All analysis was undertaken by SPSS version 18 software (SPSS inc, Chicago, IL).
Results
Flow of participants through the trial has been reported in detail elsewhere [18] and is summarised in Fig. 2. Briefly, of the 402 women who were approached about the trial, 318 were deemed eligible and 194 women (61 %) gave informed consent, completed the baseline assessment and were randomly allocated into one of the three trial groups (FtF n = 67, Tel n = 67, UC n = 60). Reasons given for non-participation included had too many other commitments/were too busy, felt the programme was not needed or were not coping. Trial participants were on average younger, but had similar disease characteristics to the Queensland breast cancer population (data not shown). The trial retention rate was 94 % at 6 months and 93 % at 12 months. Women who withdrew (n = 14) were similar in age, socioeconomic status and had similar disease characteristics compared to women who completed the trial and withdrawal rate did not differ by group allocation (6, 4 and 4 in the FtF, Tel and UC groups, respectively).
Participant characteristics
Median age of trial participants was 52 years (age range: 29–70 years). Personal and diagnostic characteristics, including body mass index, lymph node status, stage of disease and receipt of adjuvant therapy were similar for participants in the FtF, Tel and UC groups (Table 2). Women in the Tel group were more likely to be treated at a private hospital when compared with those in the UC group (61 and 50 %, respectively) and less likely to have a mastectomy than the FtF and UC groups (22, 39 and 43 %, respectively). During the trial, 69 % of women underwent chemotherapy, 71 % underwent radiotherapy and 64 % began hormone therapy. Type of adjuvant therapy was balanced between all three groups.
Exercise trial adherence
Information about adherence in the Exercise for Health trial has been previously reported [18]. On average, the FtF group participated in 88 % (14 of 16) of their scheduled sessions with their Exercise Physiologist. Those in the Tel group participated in 81 % (13 of 16) of scheduled telephone calls.
Quality of life
The interaction effect between time and group was statistically significant (p = 0.03) for QoL. The FtF and Tel exercise groups both reported increased QoL scores over time; by 12 months post-treatment, showed clinically higher QoL (>8 FACT-B +4 units) compared with baseline scores (Table 2). In contrast, the UC group showed a delayed QoL recovery (no change between baseline and mid-intervention) and level of improvements observed by post-intervention failed to meet the clinically relevant threshold. While the differences in change in QoL between baseline and mid-intervention, and baseline and post-intervention were similar for the FtF and Tel groups, only change in QoL for the Tel group differed significantly (p < 0.05) compared with the UC groups (Table 3).
Function, treatment-related side effects and physical activity levels
Change in function (specifically, aerobic fitness) and fatigue between groups and over time differed (p < 0.05). The FtF and Tel groups reported improvements in fitness over time, which were clinically relevant by post-intervention (>8 beats/min heart rate declines) (Table 3). In contrast, the UC group reported worsening fitness at mid- and post-intervention and the differences between treatment groups and the UC in change in resting heart rate at mid- and post-intervention were significant (p < 0.05). Fatigue improved over time for the treatment groups with change between baseline and post-intervention fatigue scores being clinically relevant for the Tel group (Table 3). In contrast, the UC group showed worsening fatigue between baseline and mid-intervention and improved fatigue between baseline and post-intervention although magnitude of change was neither statistically nor clinically relevant.
Upper-body function and treatment-related side effects such as menopausal symptoms (including anxiety and depression) and pain improved over time (p < 0.05) for all groups, and participating within the intervention did not influence the magnitude or rate of this change (Table 4). There was no difference between the mean body mass index and L-Dex measured at baseline, mid- and post-intervention for all groups (baseline BMI and L-Dex was 26.6 ± 5.2 and 1.1 ± 6.8, respectively). There were no statistical or clinically relevant differences between groups over time for the proportion of women with lymphoedema, clinical anxiety, clinical depression or having experienced BMI gains of ≥1 unit (Table 5).
Of those in the FtF and Tel groups, 25 % did not meet the intervention goal at mid- or post-intervention and did not increase their total physical activity by 30+ min (a priori deemed clinically relevant) between baseline and mid- or post-intervention. There were also 66 % of women in the UC group who participated in 180+ min of total weekly physical activity at 6 or 12 months post-surgery and/or increased their level of activity by 30+ min following baseline assessment. At 12 months post-surgery, median minutes of total physical activity and walking for exercise per week was 180 (0, 840), 120 (0, 1,110) and 120 (0, 1,120) and 90 (0, 480), 60 (0, 360) and 10 (0, 420) for those in the FtF, Tel and UC group, respectively.
Discussion
Exercise, when delivered by a pragmatic, translational approach and included as part of standard care provided to women with breast cancer, leads to significant benefits with respect to QoL, function and treatment-related side effects. Specifically, gains in QoL and fitness and declines in fatigue were observed for those in the exercise groups during and following breast cancer treatment and the benefits are similar irrespective of whether the exercise is delivered FtF or Tel. In contrast, during the treatment period (between 6 weeks and 6 months post-surgery) those in the UC group experienced declines in fitness and no change in QoL and fatigue. Beyond the treatment period, although improvements in QoL, fatigue and fitness were observed, unlike the intervention groups, the change for the UC group was of insufficient magnitude to be clinically relevant.
An important and novel aspect of the EfH trial was the evaluation of two intervention delivery modes—FtF or telephone delivered. The FtF delivery mode reflects the traditional approach used by Exercise Physiologists in the prescription of exercise, and at least in Australia there already exists public and private health reimbursement for use of such allied health services via this mode. While the telephone delivery of exercise prescription does not reflect standard practice, Cancer Councils throughout Australia commonly deliver support on a range of healthy behaviours via their telephone helpline, highlighting that the necessary infrastructure to deliver exercise prescription via this mode already exists. Also, delivering an exercise intervention using the telephone has the advantage of reaching all women, irrespective of place of residence, and thus can accommodate the 30 % of Australian women with breast cancer who live in rural, regional areas [36] and/or areas with limited access to specialist services. Further, the telephone delivery of specialist services, such as exercise, has possible cost advantages compared to FtF delivery. Therefore, the evaluation of these two modes was purposely chosen as they reflect feasible delivery modes which could be integrated among standard practice quickly should the intervention prove effective. While future analysis will specifically evaluate the cost effectiveness of EfH delivered via the telephone or FtF, findings presented here highlight the similarities in effect size of the exercise intervention between the two modes of delivery.
One of the many benefits associated with exercise following breast cancer is its potential to prevent treatment-related side effects particularly those associated with adjuvant therapy [4]. Participation in the EfH intervention prevented fatigue and declines in fitness, but did not seem to affect rates of lymphoedema, anxiety, depression or adverse changes in body mass index. There were also no differences observed in upper-body function, menopausal symptoms and pain between the groups. An active UC group (two-thirds were participating in 180+ min of activity per week and/or increased activity levels by 30+ min between baseline and mid- or post-intervention) may have precluded differences being observed between groups for these specific outcomes. Alternatively, a higher exercise dose (25 % of the exercise groups did not meet the intervention goal and did not increase their total physical activity levels by 30+ min between baseline and mid- or post-intervention) or different delivery setting (e.g. in a supervised, clinic-based setting) may be required to illicit prevention of these specific side effects. For the prevention of gains in body mass index, exercise alone may not be sufficient and may require dietary changes as well. Alternatively, it is plausible that the outcome measures lack sensitivity to detect exercise-induced changes. Nonetheless, EfH has clearly demonstrated that women can feasibly participate in an exercise intervention delivered FtF or Tel, commencing 6-weeks post-surgery, and can do so safely without exacerbating or initiating common treatment-related side effects. The intervention evaluated here included more frequent contact with women during the early phases of the programme (weekly for 2 months and then fortnightly for 2 months) and tapering to monthly contact during the second half of the programme. In an attempt to ensure optimal prevention of treatment-related side effects, future research may consider not only the evaluation of an intervention which tapers frequency of contact but also transitions from face-to-face contact through to telephone-contact over the intervention period. This may also involve having a more flexible protocol with regard to contact between the Exercise Physiologist and patient, whereby when certain situations arise (e.g. no previous exercise history before participating in the intervention; change in treatment-related symptoms) contact increases to two–three times per week and tapers back when the participant’s exercise self-efficacy has improved.
The strengths of this work relate to the recruitment of a sample generally representative of the wider breast cancer population, the evaluation of an exercise intervention which was delivered by a pragmatic approach to delivery suitable for translation into practice, assessors blinded to group allocation and application of intention to treat principles to analysis. However, there was a slight imbalance in numbers, place of treatment (public vs private hospital) and rates of mastectomy between groups following randomisation. While block randomisation may have led to better balance between groups, findings from adjusted analyses, which take into account rates of mastectomy and place of treatment, were no different to those presented within the manuscript. It is plausible that the EfH sample was more active at baseline compared with the wider breast cancer population, as is the case for the majority of exercise intervention trials following cancer [17]. While we are unable to confirm this using data, it is important to note that the impact of such a bias on trial findings would be in the conservative direction, making it more difficult to establish a time and/or group effect. Consequently, demonstrating an intervention effect on QoL, function and fatigue by means of a translational intervention approach is particularly compelling.
In summary, findings from this study highlight that a translational exercise intervention implemented within 6-weeks post-breast cancer surgery is safe and effective at preventing fatigue and declines in fitness and optimising QoL, while our previous published findings highlight that the participation in the intervention is feasible [18]. Women were interested and able to integrate an additional form of adjuvant treatment into their standard breast cancer care with exercise being particularly appealing since it is a form of treatment that they can control and that is associated with recovery benefits, including the potential to minimise adverse effects from their other forms of breast cancer treatment. Further, demonstrating that delivery of the intervention FtF or Tel has similar effect is a particularly novel and exciting finding with significant implications for the integration of exercise into the standard of breast cancer care provided to all women, irrespective of place of residence and access to specialist care.
References
Jemal A (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Stout N, Binkley J, Schmitz K, Andrews K, Hayes S, Campbell K, McNeeley M, Soballe P, Berger A, Cheville A, Fabian C, Gerber L, Harris S, Johansson K, Pusic A, Prosnitz R, Smith R (2012) A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 188(8):S2191–S2200
Schmitz K, Speck R, Rye S, DiSipio T, Hayes SC (2012) Prevalence of breast cancer treatment sequelae over six years of follow-up: the Pulling Through Study. Cancer 118(8):S2217–S2225
Speck R, Courneya KS, Masse L, Duval S, Schmitz K (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4(2):87–100
Schmitz K, Holtzman J, Courneya K, Masse L, Duval S, Kane R (2005) Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 14(7):1588–1595
Hayes SC, Spence RR, Galvão DA, Newton RU (2009) Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport 12(4):428–434
Ibrahim E, Al-Homaidh A (2011) Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28(3):753–765
Schwartz A, Winters-Stone K, Gallucci B (2007) Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum 34(3):627–633
Cadmus L, Salovey P, Yu H, Chung G, Kasi S, Irwin M (2009) Exercise and quality of life during and after treatment for breast cancer: results of two randomised controlled trials. Psychooncology 18:343–352
Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R (2001) Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomised controlled trial. J Clin Oncol 19(3):657–665
Demark-Wahnefried W, Clipp E, Lipkus I, Lobach D, Clutter Snyder D, Sloane R, Peterson B, Macri J, Rock C, McBride C, Kraus W (2007) Main outcomes of the FRESH START trial: a sequentially tailored diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol 25(19):2709–2718
Morey M, Snyder D, Sloane R, Cohen H, Peterson B, Hartman T, Miller P, Mitchell D, Demark-Wahnefried W (2009) Effects of home-based diet and exercise on functional outcomes among, older, overweight long-term cancer survivors. JAMA 18:1883–1891
Pinto B, Frierson G, Rabin C, Trunzo J, Marcus B (2005) Home-based physical activity intervention for breast cancer patients. J Clin Oncol 23(15):3577–3587
Demark-Wahnefried W, Clipp E, Morey M, Pieper C, Sloane R, Snyder D, Cohen H (2006) Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol 24(21):3465–3473
Vallance J, Courneya K, Plotnikoff R, Yasui Y, Mackey J (2007) Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol 25(17):2352–2359
Eakin E, Lawler S, Winkler E, Hayes SC (2012) A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: exercise for Health-rural. Ann Behav Med 43:229–238
Hayes S, Johanssen K, Alfano C, Schmitz K (2011) Exercise for breast cancer survivors: bridging the gap. Transl Behav Med 1:539–544
Hayes S, Rye S, Battistutta D, Yates P, Pyke C, Bashford J, Eakin E (2011) Design and implementation of the exercise for health trial—a prgamatic exercise intervention for women with breast cancer. Contemp Clin Trials 32:577–585
DiSipio T, Hayes S, Battistutta D, Newman B, Janda M (2010) Patterns, correlates, and prognostic significance of quality of life following breast cancer. Psychooncology 20(10):1084–1091
Hayes S, Rye S, Battistutta D, DiSipio T, Newman B (2010) Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Hlth Qual Life Outcomes 8:92
Harrison S, Hayes S, Newman B (2009) Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology 18(4):387–394
Brady M, Cella D, Mo F, Bonomi A, Tulsky D, Lloyd S, Deasy S, Cobleight M, Shiomoto G (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast (FACT-B) quality of life instrument. J Clin Oncol 15(3):974–986
Coster S, Poole K, Fallowfield L (2001) The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat 68(3):273–282
Cella D, Tulsky D, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen S, Winicour P, Brannon J (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Cella D (1997) Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Scales. Evanston, Ill, Evanston Northwestern Healthcare
Beaton D, Katz J, Fossel A, Wright J, Tarasuk V, Bombardier C (2001) Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther 14(2):128–146
Yellen S, Cella D, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13(2):63–74
Greene J (1998) Constructing a standard climacteric scale. Maturitas 29:25–31
Galer B, Jensen M (1997) Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 48:332–338
McArdle WD, Katch FI, Katch VL (1994) Essentials of exercise physiology. Lea & Febiger, Philadelphia
Hayes S, Battistutta D, Newman B (2005) Objective and subjective upper-body function six months following diagnosis of breast cancer. Breast Cancer Res Treat 94(1):1–10
Cornish B, Chapman M, Hirst C, Mirolo B, Bunce I, Ward L, Thomas B (2001) Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 34(1):2–11
Cornish B, Ward L, Thomas B, Bunce I (1998) Quantification of lymphoedema using multi-frequency bioimpedance. Appl Radiat Isot 49(5/6):651–652
Australian Institute of Health and Welfare (AIHW) (2003) The Active Australia Survey: A guide and manual for implementation, analysis and reporting. Canberra, AIHW
Murray C, Ezzati M, Lopez A, Rodgers A, VanderHoorn S (2003) Comparative quantification of health risks: conceptual framework and methodological issues. Popul Health Metr 1:1. doi:10.1186/1478-7954-1181-1181
Australian Institute of Health and Welfare (2007) Cancer in Australia: An overview, 2006. Australian Institute of Health and Welfare, Canberra
Acknowledgments
Study investigators would like to sincerely thank those who agreed to participate in this study and who were willing to do so within weeks of receiving their breast cancer diagnosis. Thank you also to the Breast Care Nurses and the Exercise Physiologists who worked on this project for their invaluable contributions in making the study a success. This research project was supported by the National Breast Cancer Foundation. The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; nor in the writing and submission of this publication. The research positions of SH and EE are supported via an NBCF Early Career Research Fellowship and an NHMRC Senior Research Fellowship, respectively.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayes, S.C., Rye, S., DiSipio, T. et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat 137, 175–186 (2013). https://doi.org/10.1007/s10549-012-2331-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2331-y