Abstract
Few randomized controlled trials have examined the effects of combined aerobic and resistance training in breast cancer survivors soon after completing adjuvant therapy. Breast cancer survivors (N = 58) within 2 years of completing adjuvant therapy were randomly assigned to an immediate exercise group (IEG; n = 29) or a delayed exercise group (DEG; n = 29). The IEG completed 12 weeks of supervised aerobic and resistance exercise, three times per week. The DEG completed the program during the next 12 weeks. Participants completed patient-rated outcomes at baseline, 6, 12, 18 and 24 weeks. The primary endpoint was overall quality of life (QoL) measured by the Functional Assessment of Cancer Therapy-Breast scale. Secondary endpoints were fatigue, social physique anxiety, and physical fitness. Follow-up data was obtained on 97% of participants and exercise adherence was 61.3%. Repeated measures analyses of variance revealed a significant group by time interaction for overall QoL (P < 0.001). Specifically, QoL increased in the IEG from baseline to 12 weeks by 20.8 points compared to a decrease in the DEG of 5.3 points (mean group difference = 26.1; 95% CI = 18.3–32.7; P < 0.001). From 12 to 24 weeks, QoL increased in the DEG by 29.5 points compared to an increase of 6.5 points in the IEG (mean group difference = 23.0; 95% CI = 16.3–29.1; P < 0.001). Similar results were obtained for the secondary endpoints. Combined aerobic and resistance exercise soon after the completion of breast cancer therapy produces large and rapid improvements in health-related outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer incidence has nearly doubled in Australia since 1983, with Australian women facing a lifetime risk of 1 in 8 [1]. Although survival rates have improved, many women still suffer from long-term psychological and physical distress from current adjuvant therapies. Breast cancer survivors can face issues such as premature menopause, body image and sexual and relationship problems [2], fatigue [3], comorbidities as a consequence of cancer treatments [4], and greater risk of psychological distress, depressive episodes and lifestyle disruption [5]. Indeed, cancer survivors can still experience distressing symptoms for a number of years after their diagnosis [3–5].
Exercise has been shown to improve some aspects of QoL among breast cancer survivors but few studies have assessed the efficacy of a combined aerobic and resistance exercise program soon after the completion of adjuvant therapies [6, 7]. Focusing on the post-adjuvant setting may be important because preliminary evidence from systematic reviews has suggested that QoL and fatigue appear to be improved more in the post-adjuvant setting than during adjuvant therapy [6, 7] and survey studies have shown that the majority of cancer survivors prefer to start an exercise program after they complete treatments [8]. Moreover, combining aerobic and resistance exercise may provide greater QoL and fatigue benefits than either modality alone. Finally, no study to date has examined social physique anxiety (SPA) [9] among breast cancer survivors. Social physique anxiety is experienced when individuals perceive others to be negatively evaluating their physique and may be particularly relevant to breast cancer survivors who have experienced potentially disfiguring treatments.
The purpose of the present study was to examine the effects of a 12-week, supervised, combined aerobic and resistance exercise program on QoL, fatigue, SPA, and physical fitness in breast cancer survivors who have recently completed adjuvant therapy except for hormone therapy. We hypothesized that exercise training would result in significant and meaningful improvements in all patient-rated outcomes as well as objectively measured fitness outcomes. We also hypothesized that changes in fitness would be associated with changes in patient-rated outcomes.
Methods
Setting and participants
The trial was conducted at the Health and Rehabilitation Program (UHRP) Clinic at The University of Western Australia (UWA). Ethics approval was granted from the UWA Human Research Ethics Committee, while written informed consent was obtained from all participants. Participants were recruited using posters that were displayed in surgical and oncology wards in metropolitan hospitals, as well as through advertisements published in community newspapers. Eligibility criteria included women with stage I–II breast cancer, ≥18 years old, English speaking, within 24 months of their cancer diagnosis, and having completed all treatments except hormone therapy. Women were excluded if they had evidence of recurrent disease, had previously engaged in any formal exercise programs for 6 months prior to participation in this study, or if they failed the revised Physical Activity Readiness Questionnaire (rPAR-Q) [10].
Design and procedures
The study was a prospective, two-armed, randomized controlled trial with a complete crossover design. The immediate exercise group (IEG) completed the exercise program from baseline to 12 weeks whereas the delayed exercise group (DEG) completed the exercise program from 13 to 24 weeks.
Randomization
Participants were randomly assigned to either the IEG or the DEG in a 1:1 ratio using a computer-generated program. Group assignments were concealed from the project director who recruited participants to the trial.
Exercise training intervention
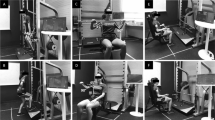
Participants were asked to attend the rehabilitation clinic three times a week for 12 weeks any time during clinic hours. The sessions were supervised by two exercise physiologists who ensured every participant received one-on-one contact during each session. The program included an aerobic component that utilized the cycle and rowing ergometers, the mini-trampoline, and the step-up blocks. The cardiovascular component of the program was conducted for 20 min and ended with a 5 min cool down. The resistance training component consisted of 12 different exercises. The specific exercises were: chest press, chest extension, biceps curls, triceps extension, leg extension, leg curls, hip abduction and adduction, back extension, abdominal crunches, standing fly’s and leg press. For each exercise, participants performed two sets of 10–15 repetitions of lightweights and progressed to a heavier weight once the current weight and repetitions could be achieved easily and with good form. Finally, 5 min of stretching was performed at the beginning and end of each session in order to increase flexibility. During weeks 1–12, the IEG were provided with the supervised exercise program and the DEG was asked not to participate in exercise during this time period. To maintain study interest, the DEG was telephoned at weeks 3, 6, 9, and 12. During weeks 13–24, the DEG was provided with the same supervised exercise program while the IEG received telephone calls at weeks 15, 18, 21 and 24. The IEG were not given any specific exercise instructions to follow during weeks 13–24.
Assessments
The rPAR-Q [10] was administered, and self-reported medical and demographic information was collected at baseline. Additionally, both groups completed a questionnaire package at baseline and weeks 6, 12, 18 and 24 that assessed QoL using the Functional Assessment for Cancer Therapy-Breast (FACT-B) scale [11]; fatigue using the Schwartz Cancer Fatigue Scale (SCFS) [12]; and SPA using the Social Physique Anxiety Scale-7 items (SPAS-7) [9].
Submaximal fitness tests were performed before and after the exercise training (i.e. baseline and week 12 for the IEG; and week 13 and 24 for the DEG). Aerobic fitness was measured using the Aerobic Power Index (API) cycle test [13]. Participants commenced cycling on a cycle ergometer at 25 W and this workload was increased by 25 W every minute. The test was terminated at the end of the minute that participants reached 75% of their estimated maximal heart rate (220 − age × 0.75) [14]. The fitness test score was calculated as the power output that coincided with the 75% maximal heart rate as determined through interpolation techniques [13]. This calculated result was then divided by body mass (kg) and expressed as Wkg−1. Ratings of perceived exertion were measured using the 15-grade Borg scale [15]. Strength was measured by recording the weight used during the performance of specific exercises (i.e. bicep curls, leg presses and chest extensions) at baseline and postintervention.
Statistical analyses
Twenty-five participants were needed per group to detect a large standardized effect size (d = 0.80) with a power of 0.80 and a two-tailed alpha of 0.05. A large standardized effect size on the FACT-B is approximately 10 points based on a standard deviation of 13 and exceeds the 7–8 point minimally important difference (MID) identified for this scale [16]. Our goal was to randomize 60 participants to allow for a 10% loss-to-follow-up.
Statistical analysis was performed using SPSS version 12.0 software (SPSS Inc, Chicago, IL). Baseline descriptive statistics were compared using independent t-tests for continuous data and Pearsons χ2 analysis for categorical data. Missing data were remedied using last-observation-carried-forward and all analyses were intention-to-treat. The primary analysis employed a 2 (group) by 5 (time) Repeated Measures Analysis of Variance (RM-ANOVA) to test for interactions between Time and Group on QoL, fatigue, and SPA. Post-hoc analyses were performed on significant interactions using paired and independent-sample t-tests. These analyses were repeated using analysis of covariance with the respective baseline score as a covariate.
Paired sample t-tests were used in order to assess changes in fitness over the 12-week intervention period in both the IEG and the DEG, while an independent sample t-test was used to compare fitness values between the groups at 12 weeks (i.e., the IEG’s post-intervention scores and the DEG’s baseline score). Pearson product-moment correlations were used to examine the associations between changes in physical fitness and changes in patient-rated outcomes.
Results
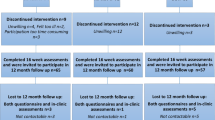
Participant recruitment took place between January and March 2005 and the trial commenced in April 2005. Figure 1 presents the flow of participants through the study. We recruited 58 of 131 (44.3%) eligible participants. Common reasons for refusal included not willing to travel (n = 13) and having family or holiday commitments over the study period (n = 16). Table 1 presents the baseline characteristics. The groups were balanced on all medical and demographic variables. The average IEG attendance was 60.4% (21.7 of 36 sessions) with a median of 23 (63.9%) and a range of 11–36. The average DEG attendance was 62.2% (22.4 of 36 sessions) with a median of 24 (66.7%) and a range of 12–35. Groups were balanced on baseline values for the primary and secondary endpoints.
Changes in the primary endpoint
Repeated measures ANOVA revealed a significant Time by Group interaction for the FACT-B [F = 21.8, P < 0.001; Fig. 2]. Follow-up paired t-tests revealed that the IEG demonstrated a significant increase in QoL from baseline to week 6 [mean change = 12.6; 95% CI = 7.8 to 17.4, P < 0.001] and from week 6 to week 12 [mean change = 8.2; 95% CI = 4.8 to 11.5, P < 0.001] with a borderline significant increase from week 12 to week 18 [mean change = 3.1; 95% CI = −0.6 to 6.9, P = 0.096] and a further significant increase from week 18 to week 24 [mean change = 4.9; 95% CI = 2.0 to 7.8, P = 0.002]. Conversely, the DEG demonstrated a borderline significant decrease in the FACT-B from baseline to week 6 [mean change = −3.0; 95% CI = −6.4 to 0.2, P = 0.063], a significant decrease from week 6 to week 12 [mean change = −2.3; 95% CI = −4.5 to −0.2, P = 0.034], and significant increases from week 12 to week 18 [mean change = 27.7; 95% CI = 22.6 to 32.8, P < 0.001] and from week 18 to week 24 [mean change = 4.0; 95% CI = 1.4 to 6.6, P = 0.003]. Follow-up independent t-tests revealed that there were no differences between the groups at baseline [mean group difference = 1.8; 95% CI = −4.9 to 8.5, P = 0.593], however, scores for the IEG were significantly higher at week 6 [mean group difference = 17.9; 95% CI = 11.8 to 24.0 P < 0.001] and week 12 [mean group difference = 28.6; 95% CI = 22.0 to 35.1, P < 0.001]. FACT-B scores were not significantly different at week 18 [mean group difference = 4.1; 95% CI = −2.6 to 10.9, P = 0.224] but were borderline higher in the IEG at week 24 [mean group difference = 5.1; 95% CI = −1.6 to 11.7, P = 0.059]. The FACT-B subscales of FACT-G, TOI, physical well-being, emotional well-being, functional well-being and breast cancer subscale (BCS) all demonstrated the same pattern of results (Table 2). Results were not altered after adjusting for baseline values.
Changes in the secondary endpoints
Repeated measures ANOVA revealed a significant Time by Group interaction for fatigue [F = 8.8, P < 0.001; Fig. 3]. Follow-up paired t-tests revealed that the IEG demonstrated a significant decrease in fatigue from baseline to week 6 [mean change = −2.7, 95% CI = −4.1 to −1.4, P < 0.001], a borderline significant decrease from week 6 to week 12 [mean change = −1.0; 95% CI = −2.2 to 0.2, P = 0.096], a significant decrease from week 12 to week 18 [mean change = −1.8; 95% CI = −3.0 to −0.6, P = 0.005], and no significant change between weeks 18 and 24 [mean change = −0.1; 95% CI = −1.0 to 0.7, P = 0.749]. Conversely, scores for the DEG did not significantly change from baseline to week 6 [mean change = −0.5; 95% CI = −2.2 to 1.3, P = 0.587] or from week 6 to week 12 [mean change = −0.5; 95% CI = −1.9 to 0.9, P = 0.466], but did significantly decrease from week 12 to week 18 [mean change = −6.3; 95% CI = −8.2 to −4.3, P < 0.001] with no further change occurring between week 18 to week 24 [mean change = −0.9; 95% CI = −2.0 to 0.2, P = 0.108]. Follow-up independent t-tests revealed that there were no significant differences between the groups at baseline [mean group difference = −0.8; 95% CI = 1.3 to −2.9, P = 0.458], week 18 [mean group difference = −1.1; 95% CI = 0.7 to −3.0, P = 0.234] or week 24 [mean group difference = −0.4; 95% CI = 1.5 to −2.3, P = 0.448], however, scores for the IEG were significantly lower at week 6 [mean group difference = −3.9; 95% CI = −1.8 to −6.0, P < 0.001] and week 12 [mean group difference = −5.4; 95% CI = −3.3 to −7.6, P < 0.001].
Repeated measures ANOVA revealed a significant Time by Group interaction for SPA [F = 3.4, P = 0.030; Fig. 4]. Follow-up paired t-tests on the interaction effects revealed that the IEG demonstrated a significant decrease in SPA from baseline to week 6 [mean change = −1.5; 95% CI = −3.2 to −0.1, P = 0.049] and from week 6 to week 12 [mean change = −2.6; 95% CI = −4.8 to −0.3, P = 0.026]. There were no significant changes from week 12 to week 18 [mean change = 1.1; 95% CI = −0.4 to 2.5, P = 0.136] or from week 18 to week 24 [mean change = 0.8; 95% CI = −0.5 to 2.1, P = 0.208]. Conversely, scores for the DEG did not significantly change from baseline to week 6 [mean change = 0.1; 95% CI = −1.6 to 1.6, P = 0.964] but demonstrated a borderline significant increase from week 6 to week 12 [mean change = 0.9; 95% CI = −0.1 to 2.0, P = 0.070] and a significant decrease from week 12 to week 18 [mean change = −4.0; 95% CI = −6.1 to −2.0, P < 0.001] and from week 18 to week 24 [mean change = −1.7; 95% CI = −3.1 to −0.2, P = 0.023]. Follow-up independent t-tests revealed that there were no significant differences between the groups at baseline [mean group difference = −0.7; 95% CI = 3.0 to −4.3, P = 0.723], at week 6 [mean group difference = −2.0; 95% CI = 1.5 to −5.5, P = 0.220], or at week 24 [mean group difference = −1.6; 95% CI = 1.2 to −4.3, P = 0.265], but scores for the IEG were significantly lower at week 12 [mean group difference = −5.5; 95% CI = −2.4 to −8.7, P = 0.001] and week 18 [mean group difference = −2.4; 95% CI = −0.5 to −5.3, P = 0.039].
Changes in physical fitness endpoints
Descriptive statistics for aerobic fitness and muscular strength are presented in Table 3. Paired t-tests showed that both the IEG [t(28) = 3.5, P = 0.002] and the DEG [t(28) = 2.2, P = 0.034] significantly improved their aerobic fitness over the course of the intervention. Independent t-tests revealed that the IEG postintervention fitness scores were significantly higher than baseline scores in the DEG [t(53) = 2.1, P = 0.049]. Results for strength revealed that weight load values recorded postintervention were significantly higher than those recorded at baseline for both groups for bicep curls: IEG [t(28) = 12.9, P < 0.001]; DEG [t(28) = 10.1, P < 0.001]; leg press: IEG [t(28) = 12.2, P < 0.001]; DEG [t(28) = 13.9, P < 0.001] and chest extension: IEG [t(28) = 9.0, P < 0.001]; DEG [t(28) = 18.3 P < 0.001]. Independent t-tests revealed that that the IEG postintervention muscular strength scores were significantly higher than the DEG baseline scores for bicep curls [t(56) = 9.3, P < 0.001], leg press [t(56) = 10.6, P < 0.001], and chest extension [t(56) = 4.1, P < 0.001].
Associations between exercise adherence and changes in endpoints
Pearson correlations among variables are presented in Table 4. Exercise adherence was significantly associated with changes in aerobic fitness (r = 0.29; P = 0.034), bicep curls (r = 0.43, P = 0.001), chest extensions (r = 0.30, P = 0.024), FACT-B (r = 0.26, P = 0.047), FACT-G (r = 0.30, P = 0.025), and functional well-being (r = 0.26, P = 0.048). Changes in aerobic fitness were significantly associated with changes in FACT-B (r = 0.34, P = 0.012), fatigue (r = −0.28, P = 0.038), FACT-G (r = 0.34, P = 0.012), social well-being (r = 0.30, P = 0.029), and emotional well-being (r = 0.27, P = 0.045). Change in bicep curl load was significantly associated with change in SPA (r = −0.27, P = 0.040).
Discussion
As hypothesized, a combined aerobic and resistance exercise program initiated soon after the completion of adjuvant breast cancer therapy resulted in reliable and meaningful improvements in QoL, fatigue, SPA, aerobic fitness, and muscular strength. These improvements were evident after 6 weeks of exercise training, with further improvements occurring at 12 weeks. We also found that improvements in aerobic fitness were associated with improvements in QoL and fatigue, whereas improvements in muscular strength were associated with improvement in SPA. These data provide compelling evidence that a combined aerobic and resistance exercise program is an effective intervention in breast cancer survivors after the completion of adjuvant therapy.
The improvements in QoL are larger than those reported in a recent meta-analysis of exercise RCTs in breast cancer survivors [7] of 4.8 (95% CI = 0.35 to 8.8) for the FACT-G and 6.6 (95% CI 1.21 to 12.03) for the FACT–B. Our trial reported improvements in QoL of over 26 points on the FACT-B. This magnitude of improvement is the largest reported to date in the exercise literature and is over three times the minimally important difference of 7–8 points for the FACT-B scale [17]. It is unclear why our exercise intervention provided such a large and rapid improvement in QoL compared to previous exercise trials. Possible explanations include: (a) the initial lower QoL scores of our participants, (b) the initiation of the intervention soon after adjuvant therapy when QoL may be at its lowest and motivation at its highest, (c) the fact that 75% of our participants were on hormone therapy at the time of the trial, (d) the combined aerobic and resistance exercise intervention, and (e) the group format with individual supervision. Ancillary analyses showed that higher adherence and improvements in aerobic fitness, but not muscular strength, were associated with improvements in QoL. These data are consistent with the trial by Courneya et al. [18] that also reported a positive association between improvements in aerobic fitness and improvements in QoL. Future trials should be designed to explain the anticipated improvements in QoL with exercise after adjuvant breast cancer therapy.
The second main finding of our study was that exercise training had a significant impact on reported fatigue. This result is consistent with the recent meta-analysis by McNeely et al. [7] that demonstrated a pooled moderate-to-large effect size between exercise and fatigue but only post-treatment studies showing statistically significant differences [18, 19]. Our results demonstrated significant decreases in fatigue by 5 to 6 points for both groups, which exceed the minimal important difference of 5.0 points on the SCFS [20]. This result supports (previous post-treatment research that has demonstrated a reduction in fatigue with exercise [18, 21–23]. The discernible decline in fatigue over the course of the exercise program may in part contribute to the large reported increases in QoL. This result is important given that fatigue can persist among long-term survivors [24]. Further, these findings provide additional evidence that exercise is a powerful mechanism for decreasing fatigue symptoms [18, 19].
We also found that the IEG and DEG reported decreases in SPA of 6.0 and 4.4 points, respectively. This result is important since the groups were on average 13 months post-treatment and any short-term visible side effects of adjuvant therapy such as hair loss, surgical scarring, or treatment burns that could impact self-esteem should have diminished. This result also suggests that women who experience any longer term body image or self-esteem issues could benefit from an exercise program. Further, SPA was shown to be positively associated with change in bicep curl scores, which may be indicative of a growing confidence in participants to use their arms. This result is important given the assumed increased susceptibility to lymphedema when engaging in exercise despite reports that it is unfounded [25, 26]. Our trial is the first to report the effects of exercise on this important psychosocial outcome.
Our exercise adherence rate was satisfactory but not optimal. On average, the participants in the IEG and DEG completed 61% of their training sessions (approximately two sessions per week). This finding is slightly lower than that reported in other RCTs. For example, Segal et al. [27] reported that their self-directed exercise group and the supervised exercise group completed 71.5% of prescribed exercise sessions and Courneya et al. [18] reported a 98.4% adherence rate. Despite the modest adherence rate, participants still demonstrated meaningful improvements in aerobic fitness (6–8%) and strength (50–100%). These results support previous research that demonstrated that resistance training could improve QoL [28], and that only two sessions per week was necessary to elicit strength benefits [29]. Future RCTs should attempt to determine the optimal number of training sessions needed per week to accrue health benefits.
There are a number of strengths and limitations of this trial that merit comment. The main strengths include the randomized controlled trial design, the complete cross-over design with replication of the results, the supervised exercise program, the combined aerobic and resistance exercise program, minimal loss-to-follow-up, and intention-to-treat analysis. One limitation of the study was the 61.3% adherence rate, while a second limitation was the short length of the intervention. Finally, fitness testing all participants at baseline, 12 weeks, and 24 weeks would have provided a more accurate assessment of fitness changes across the study.
Overall, this trial provides compelling evidence that a combined aerobic and resistance exercise program is an effective strategy for improving overall QoL, reducing fatigue and SPA, and improving physical fitness soon after the completion of adjuvant therapy for breast cancer. Explanatory trials examining how and why exercise improves patient-rated outcomes may provide information on how to further optimize the exercise prescription. Cancer care professionals should feel comfortable recommending a combined exercise program to breast cancer survivors under their care.
References
Australian Institute of Health and Welfare & National Breast Cancer Centre (2006) Breast cancer in Australia: an overview, 2006. Cancer series no. 34. cat. no. CAN 29. AIHW, Canberra
Avis NE, Crawford S, Manuel J (2005) Quality of life among younger women with breast cancer. J Clin Oncol 23:322–3330
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meverowitz BE, Belin TR (2000) Fatigue in breast cancer survivors: occurrence, correlates and impact on quality of life. J Clin Oncol 18:743–753
Ganz P (2006) Monitoring the physical health of cancer survivors: a survivorship-focused medical history. J Clin Oncol 24:5105–5111
Stanton AL (2006) Psychosocial concerns and interventions for cancer survivors. J Clin Oncol 24:5132–5137
Conn V, Hafdahl AR, Porock DC, McDaniel R, Nielsen PS (2006) A meta-analysis of exercise intervention among people treated for cancer. Support Care Cancer 14:699–712
McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS (2006) Effects of exercise on breast cancer patients and survivors; a systematic review and meta-analysis. Can Med Assoc J 175:34–41
Jones L, Courneya KS (2002) Exercise counseling and programming preferences of cancer survivors. Cancer Prac 10:208–215
Hart EA, Leary MR, Rejeski WJ (1989) The measurement of social physique anxiety. J Sport Exerc Psychol 11:94–104
Canadian Society for Exercise Physiology. PAR-Q and you (1994) Canadian Society for Exercise Physiology, Gloucester, Ontario
Cella DF, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale: development and validation of the central measure. J Clin Oncol 11:570–579
Schwartz AL (1998) Reliability and validity of the Schwartz cancer fatigue scale. Oncol Nurs Forum 25:711–719
Telford RD, Minikin BR. Hahn AG (1989) A simple method for the assessment of general fitness: the tri-level profile. Aust J Sci Med Sport 21:6–9
Telford RD, Minikin BR, Hooper LA, Hahn AG, Tumilty MP (1987) The tri-level fitness profile. Excel 4:11–13
Borg GA (1982) Psychophysical basis of perceived exertion. Med Sci Sports Exerc 14:377–381
Eton DT, Cella D, Yost KJ et al (2004) A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 57:898–910
Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE (2002) Combining anchor and distribution based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage 24:547–561
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS (2003) Randomized controlled trial of exercise training in postmenopausal breast cancer patients: cardiopulmonary and quality of life outcomes. J Clin Oncol 21:1660–1668
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH (2005) Home-based physical activity intervention for breast cancer patients. J Clin Oncol 23:3577–3587
Schwartz AL, Meek PM, Nail LM et al (2002) Measurement of fatigue determining minimally important clinical differences. J Clin Epidemiol 55:239–244
Berglund G, Bolund C, Gustafsson U-L, Sjödén P-O (1994) One-year follow-up of the ‘Starting Again’ group rehabilitation program for cancer patients. Eur J Cancer 30A:1751–1756
Segal RJ, Reid RD, Courneya KS et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21:1653–1659
Segar ML, Katch VL, Roth RS et al (1998) The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum 25:107–113
Jacobsen PB, Stein K (1999) Is fatigue a long-term side effect of breast cancer treatment? Cancer Control 6:256–263
Harris SR, Niesen-Vertommen SL (2002) Challenging the myth of exercise-induced lymphoedema following breast cancer; a series of case reports. J Surg Oncol 74:95–99
Hock K (1998) Lymphedema and exercise. Oncol Nurs Forum 25:1310
Segal RJ, Evans W, Johnson D et al (2001) Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized clinical trial. J Clin Oncol 19:657–665
Schmitz KH, Ahmed RL, Hannan PJ, Yee D (2005) Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 14:1672–1680
Ohira T, Schmitz KH, Ahmed RL, Yee D (2006) Effects of weight training on quality of life in recent breast cancer survivors. Cancer 106:2076–2083
Acknowledgements
Kerry S. Courneya is supported by the Canada Research Chairs Program and a Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society and the NCIC/CCS Sociobehavioral Cancer Research Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milne, H.M., Wallman, K.E., Gordon, S. et al. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 108, 279–288 (2008). https://doi.org/10.1007/s10549-007-9602-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9602-z