Abstract
Key message
Sperm cells can be isolated from the mature pollen grains of medicinal wild rice (Oryza officinalis) using an osmotic shock method, and the viable egg cells can be isolated by enzymatic digestion and mechanical dissection steps.
Abstract
Favorable alleles for rice breeding have been identified in natural cultivars and wild rice by association analysis of known functional genes with target trait performance. Transferring these genes from wild rice into cultivated rice varieties is one of the important objectives for rice breeders. The isolation of the sperm and egg cells of wild and cultivated rice is a prerequisite for the in vitro hybridization of distantly related cultivated rice and wild rice lines. Here, we provide a technical approach for isolating the sperm and egg cells of wild rice (Oryza officinalis). In this method, sperm cells were isolated from the mature pollen grains of medicinal wild rice (O. officinalis) according to an osmotic shock method. Additionally, viable O. officinalis egg cells were isolated following enzymatic digestion and mechanical dissection steps. Specifically, ovules were digested in an enzymatic solution containing pectinase and cellulase for 30 min, after which the ovule was cut into two halves. Three egg apparatus cells were released by gently applying pressure to the micropylar end. Generally, six or seven egg cells could be isolated from 20 ovules in 60 min. The same method was used to isolate zygotes from flowers at 24 h after pollination. This technology solved the difficulty of isolating sperm and egg cells in O. officinalis and allowed the isolated sperm and egg cells to be combined by in vitro hybridization of distantly related wild and cultivated rice lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild rice is an ancestor of cultivated rice. After long evolution and myriad selection processes, wild rice developed into modern cultivated rice. However, during this selection many wild rice alleles and genotypes were lost, presumably including myriad genes encoding desirable traits, including disease resistance, insect resistance, weed resistance, and stress tolerance, as well as genes related to ideal nutrient quality, high grain yield, and quality (Zhong et al. 2000; Uchiumi et al. 2006; Guo et al. 2016). Selectively transferring such genes from wild rice into cultivated rice varieties is an important objective for rice breeders. Studies involving hybridization between cultivated rice and wild rice have been conducted for many years within China as well as internationally. However, crosses between cultivated rice and distantly related wild rice can be difficult, with problems including inadequate hybrid seed set, especially for some wild rice lines that are highly resistant to biotic factors. Thus, there is a need for new and effective methods for the hybridization between distantly related rice lines.
In vitro fertilization techniques involving male and female gametes completely isolated from their paternal and maternal structures have been successfully applied to maize (Kranz and Lörz 1993) and rice (Uchiumi et al. 2007; Maryenti et al. 2019). Isolating the sperm and egg cells avoids any interference from the somatic cells of the stigma and style tissues. Thus, the sperm and egg cells can then be directly fused to produce a hybridized zygote that may not be obtainable using typical hybridization techniques. Kranz et al. (1991) fused maize egg cells with the sperm cells of genetically remote species from the genera Coix, Sorghum, Hordeum, and Triticum, and, respectively, 78% (21/27), 50% (17/34), 43% (13/30), and 24% (9/38) of the resulting hybrid zygotes divided to form microcalli. The relatively high frequency of hybrid zygotes dividing to form microcalli suggests the fusion of egg cells with the sperm cells of diverse species or genera represents a potentially viable method for hybridization between angiosperm species from different genera (Wang et al. 2006).
The sperm and egg cells of cultivated rice have been isolated (Zhang et al. 2010; Li et al. 2019), which eliminate sporophytic barriers to the in vitro hybridization of cultivated and wild rice. Because the genome of medicinal wild rice (Oryza officinalis) includes many important genes conferring resistance to various biotic factors, including Sogatella furcifera, the brown planthopper, and the pathogen responsible for bacterial blight, wild rice progenitors may represent a valuable genetic resource for rice breeding (Zhang et al. 2009). Some rice breeders have tried to cross-cultivated rice with medicinal wild rice for many years, but have so far been unsuccessful in producing hybrid seed (Professor Xiang Dong Liu, South China Agricultural University, private conversation). In the present study, we developed a protocol for isolating medicinal Oryza officinalis sperm cells using an osmotic shock treatment and combining them with egg cells obtained using a method involving enzymatic maceration and mechanical dissection.
Materials and method
Plant growth and egg cell isolation
The medicinal wild rice (O. officinalis) used in this study, which was provided by Professor XD Liu of South China Agricultural University, China, normally blooms over 20 days in September in the Xiamen region of China. Medicinal wild rice egg cells were isolated using enzymatic maceration and micromanipulation. The glumous flowers at anthesis were selected, and the pistil was dissected in a mannitol solution. The ovule was peeled from the ovary and incubated at 25 °C for 30 min with gentle shaking in an enzyme solution comprising 0–0.2% (w/v) Cellulase RS (Yakult Pharmaceutical Industries, Tokyo, Japan), 0–0.2% (w/v) Pectolyase Y-23 (Kikkoman Corporation, Tokyo, Japan), 0.05% (w/v) CaCl2·2H2O, 1% (w/v) bovine serum albumin, and 8–15% (w/v) mannitol. The macerated ovules were then transferred to the same solution without the above enzymes for removal of cells using a dissection with a dissecting needle and an inverted microscope. Each ovule was transversely cut into two halves, after which gentle pressure was applied to the micropylar end of the ovule to release the egg apparatus cells from the cut end. The released egg cells were collected with a Leica DC-180 micromanipulator. Using the same method, zygotic cells were isolated from flowers at 24 h after anthesis.
Sperm cell isolation
Mature wild rice pollen grains are tricellular and consist of two sperm cells and a vegetative cell at anthesis. When pollen grains collected from early post-anthesis flowers were incubated in a 4–12% mannitol solution, many pollen grains ruptured and released their contents, including the two sperm cells, which were collected with a micromanipulator.
The viability of the isolated egg cells and zygotes was evaluated using fluorescein diacetate (FDA) fluorescence (Heslop-Harrison and Heslop-Harrison 1970). The osmolality of all solutions was measured using an Osmomat 030 osmometer (Gonotech GmbH, Berlin, Germany). Using a dissecting microscope, the ovary wall was cut lengthwise and removed to release the ovule. The collected ovules were digested in an enzyme solution for 30 min. The outline of the ovule and its micropylar position were observable and provided a transverse line for cutting the ovule into two parts with a dissecting needle. The micropylar end of each ovule was held in position with a dissecting needle, and another needle was used to apply gentle pressure to release the egg apparatus cells from the cut end of the ovule.
Results
Egg cell isolation
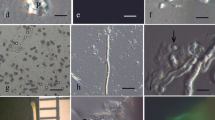
Compared with cultivated rice, medicinal wild rice plants are taller (almost 2 m tall, twice as tall as some cultivars) and grow more loosely (Fig. 1A). During anthesis, the spikelets are also loosely arranged in the inflorescence. Each spikelet has a long awn (Fig. 1B). After removing upper and lower glumes from a flower, the pistil attached to the enlarged ovary and two purple pinnatiform stigmata are revealed (Fig. 1C). Using a dissecting microscope, the ovary wall was cut lengthwise and removed to release the ovule (Fig. 1D). The released embryo sac cells were easily distinguishable in the solution because they are larger than the nucellar cells. The egg cell and synergid cells were also distinguished based on their structural and morphological features. Of the three cells in the egg apparatus, the two synergid cells contained more cytoplasm than the egg cell, which was highly vacuolated (Fig. 1E). The three isolated cells were readily labeled with FDA—the fluorescence implying that these cells remained viable during and after their isolation (Fig. 1F). The egg cell was separated from the other surrounding cells with a dissecting needle (Fig. 1G) and remained intensely viable fluorescent and thus viable for 1 h (Fig. 1H). The egg cell was collected using a micromanipulator (Fig. 1I). Generally, 5–7 egg cells were isolated within 1 h.
Isolation of the sperm, egg cell, and zygote of medicinal wild rice. A Medicinal wild rice plant. B Glumous flower at anthesis. C Pistil of medicinal wild rice. D Ovule after the ovary wall has been stripped. E Three released egg apparatus cells (two synergids and an egg cell). F Egg apparatus cells stained with fluorescein diacetate. G Isolated egg cell. H Egg cell stained with fluorescein diacetate. I Isolated egg cells. J Isolated zygote from a flower at 24 h after pollination. K Isolated zygote. L Zygote stained with fluorescein diacetate. M Ruptured pollen grains releasing their contents. N Single ruptured pollen grain releasing its contents. O Two released sperm cells (arrows). P Sperm cells population
Zygote isolation
In our experience, the fertilization of cultivated rice egg cells occurs 3 h after pollination, and the zygote divides 13 h after pollination. However, it is unclear when the fertilization and zygote division occur for medicinal wild rice. In our assay, the medicinal wild rice zygote did not divide at 24 h after pollination. We selected flowers at 24 h after pollination for isolating the zygote with the same method as that used to isolate the egg cells. The open flowers were marked at about 9:00 am and then selected at the same time on the following day. The ovules generally released only one cell, not all three egg apparatus cells (Fig. 1J). The zygote was similar in size to the egg cells (Fig. 1K), but was differentially fluorescently labeled, with fluorescence concentrated surrounding the nucleus (Fig. 1L). In the 10% mannitol solution (0.638 mOsmol/kg−1H2O), the zygote appeared to be structurally and morphologically stable and remained viable fluorescence for up to 6 h.
Sperm cell isolation
The medicinal wild rice pollen grains contain a vegetative nucleus and two sperm cells. When pollen grains were treated with osmotically active solutions, the pollen grains took up water. The increase in turgor pressure caused the pollen grains to rupture and release the contents, including two sperm cells per pollen grain. Because rice pollen grains are rich in starch, a large number of starch grains were released (Fig. 1M) along with the two sperm cells. When the pollen grains started to rupture, the two sperm cells were packaged in the released pollen cytoplasm and were not distinguishable (Fig. 1N). However, as the cytoplasmic material dispersed, the two sperm cells were identifiable (Fig. 1O) and were collected using a micromanipulator (Fig. 1P).
Discussion
Determining the optimal conditions for isolating medicinal wild rice egg cells
In rice breeding, analyses of the cultivated rice genome have resulted in the identification of most genes, many of which have been used in screening for stress resistance; however, some alleles for resistance to certain diseases, insects, weeds, and other stresses are not present in cultivated rice, but do exist in wild rice (Zhong et al. 2000; Guo et al. 2016). Therefore, introducing these alleles into cultivated rice is a strategy that could be useful in generating novel cultivars resistant to various stresses. However, the wild rice egg and sperm cells must first be isolated for the in vitro hybridization of wild and cultivated rice lines.
The egg cells of higher plants are deeply embedded in the ovule, which is located within the ovary. An often employed method for isolating egg cells involves the use of dilute enzyme treatments to loosen the surrounding tissues, followed or accompanied by micromanipulation to separate the remaining ovule cells from the embryo sacs. Although egg cells can be isolated without the enzymatic maceration of the ovules (Cao and Russell 1997; Uchiumi et al. 2006), the yield is very low. Previous studies on rice (Zhang et al. 2010), Datura stramonium (He et al. 2012), and Solanum verbascifolium (Yang et al. 2015) revealed the difficulties associated with dissecting ovules, fixing ovules in an isolation solution, and removing the integument without an enzymatic digestion step. To efficiently dissect ovules, the optimal concentration and proportion of enzymes should be determined (Hoshino et al. 2006). Excessive enzyme concentrations adversely affect the separation of the three egg apparatus cells after release because they remain tightly connected, making it difficult to isolate the egg cell (Zhang et al. 2010; He et al. 2012; Yang et al. 2015; Li et al. 2019). Thus, relatively low enzyme concentrations are used for isolating the egg cells. The ideal enzyme concentration varies among species depending on the ovule cell wall contents and may also be influenced by the diversity in the research methods used. For example, Zhao et al. (2000) isolated rice egg and zygote cells with 0.8% pectinase, 0.2% Pectolyase Y23, 0.5% cellulase Onozuka R-10, and 0.5% hemicellulase. In contrast, Khalequzzaman and Haq (2005) isolated cultivated rice egg cells by treating ovules with 2.0% cellulase and 0.55% pectinase for 5 h. Additionally, Uchiumi et al. (2006) isolated cultivated rice egg cells using a 10–15-min incubation in an enzyme solution comprising 0.5% Pectolyase Y-23, 1.5% pectinase, 1% Cellulase RS, and 1% hemicellulase, after which the isolated egg and sperm cells were fused with an in vitro fertilization system (Uchiumi et al. 2007). In the present study, a comparison of various enzymatic solutions revealed that an enzyme solution containing 0.1% (w/v) Pectolyase Y-23 and 0.1% (w/v) Cellulase RS was suitable, with an average of six egg cells isolated from 20 ovules in 1 h (Table 1). This low enzyme concentration enabled the isolated egg cells to remain more intensely fluorescent for a longer period compared with egg cells isolated in a solution using a higher enzyme concentration. We observed that Pectolyase Y-23 softens the ovules of medicinal wild rice, making them easier to dissect. Cellulase RS added to the enzymatic solution helps to remove the cell wall of the egg apparatus cells, enabling them to be released from the embryo sac by gently pushing the micropylar end with a needle.
Determining the optimal conditions for isolating the medicinal wild rice sperm cells
There are two types of pollen grains in higher plants at anthesis, namely bicellular and tricellular pollen. Bicellular pollen consists of a vegetative cell and a generative cell, which is precursor to the sperm cells. Isolating sperm cells is generally more difficult in species with bicellular pollen because the generative cell of bicellular pollen needs to undergo mitosis to form two sperm cells within a pollen tube. Thus, to isolate the sperm cells of bicellular pollen, it is first necessary to induce pollen tube growth. In contrast, it is relatively easy to isolate sperm cells from species with tricellular pollen. When tricellular pollen grains, especially those with a large abundance of starch, are incubated in an osmotic solution, the grains hydrate and rupture, thereby releasing their contents, including the two sperm cells. The mature wild rice pollen grain is tricellular, and the two sperm cells can be isolated by directly rupturing the pollen grains. However, the osmotic solutions must be optimized for isolating sperm cells. If the osmotic pressure of the solution is low, more pollen grains will rupture, but the released sperm cells will also rapidly take up water and rupture. When distilled water without mannitol was used, over 80% of the pollen grains ruptured, but viable sperm cells were undetectable. In contrast, a treatment with a 6% mannitol solution resulted in 63.21% (134/212) of the pollen grains rupturing, after which 14.92% (20/134) of the sperm cells were detected within 5 min. If the osmotic pressure of the solution is high, fewer pollen grains will rupture, but the released sperm cells will remain intact for a relatively longer period. An incubation in a 12% mannitol solution (0.768 mOsmol kg−1) caused 27.86% (56/201) of the pollen grains to rupture, but only seven sperm cells (12.5%) were detected in the solution. In a 10% mannitol solution (0.638 mOsmol kg−1), 42.12% (88/214) of the pollen grains ruptured and 29.54% (26/88) of the released sperm cells were detected (Table 2). Therefore, a 10% mannitol solution appears to be optimal for isolating medicinal wild rice sperm cells. This mannitol concentration was also optimal for isolating egg cells. The harvested egg and sperm cells may be used for in vitro fertilizations.
Authors’ Contributions
WD performed the experiments and isolated egg cells. CC isolated sperm cells. SY designed the experiments and wrote the manuscript with the help of all other authors. CL conceived and supervised the project.
Availability of data and materials
All relevant data are provided in the file.
Change history
13 February 2020
The funding section of the original publication gave a wrong funding number.
Abbreviations
- FDA:
-
Fluorescein diacetate
- E:
-
Egg cell
- S:
-
Synergid
- Z:
-
Zygote
References
Cao YJ, Russell SD (1997) Mechanical isolation and ultrastructural characterization of viable egg cells in Plumbago zeylanica. Sex Plant Reprod 10:368–373
Guo S, Wei Y, Li X, Gao G, Deng G (2016) Advances in excavation and utilization of elite genes from Oryza minuta. J Plant Genet Resources 17:371–376 (in Chinese with English abstract)
He EM, Wang YY, Liu HH, Zhu XY, Tian HQ (2012) Egg cell isolation in Datura stramonium (Solanaceae). Annales Botanici Fennici. 49:7–12
Heslop-Harrison J, Heslop-Harrison Y(1970) Evaluation of pollen viability by enzymatically-induced fluorescence:intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:115–120
Hoshino Y, Murata N, Shinoda K (2006) Isolation of individual egg cells and zygotes in Alstroemeria followed by manual selection with a microcapillary-connected micropump. Ann Bot 97:1139–1144
Khalequzzaman M, Haq N (2005) Isolation and in vitro fusion of egg and sperm cells in Oryza sativa. Plant Physiol Biochem 43:9–75
Kranz E, Lörz H (1993) In vitro fertilization with isolated, single gametes results in zygotic embryogenesis and fertile maize plants. Plant Cell 5:739–746
Kranz E, Bautor J, Lörz H (1991) In vitro fertilization of single, isolated gametes of maize mediated by electrofusion. Sex Plant Reprod 4:12–16
Li C, Xu H, Russell SD, Sundaresan V (2019) Step-by-step protocols for rice gamete isolation. Plant Reprod 32:5–14
Maryenti T, Kato N, Ichikawa M, Okamoto T (2019) Establishment of an in vitro fertilization system in wheat (Triticum aestivum L.). Plant Cell Physiol 60(4):835–843
Uchiumi T, Komatsu S, Koshiba T, Okamoto T (2006) Isolation of gametes and central cells from Oryza sativa L. Sex Plant Reprod 19:37–45
Uchiumi T, Uemura I, Okamoto T (2007) Establishment of an in vitro fertilization system in rice (Oryza sativa L.). Planta 226:581–589
Wang YY, Kuang A, Russell SD, Tian HQ (2006) In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sex Plant Reprod 19:103–115
Yang SJ, Wei DM, Tian HQ (2015) Isolation of sperm cells, egg cells, synergids and central cells from Solanum verbascifolium L. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-014-0290-6
Zhang H, Liu R, Guo H, Li Y (2009) Advancement on Mining and Utilization of Elite Genes in Oryza officinalis Wall. Chin Agric Sci Bull 25(19):42–45 (in Chinese with English abstract)
Zhang YN, Wei DM, He EM, Miao S, Tian HQ, Russell SD (2010) Isolation of male and female gametes of rice. Crop Sci 50:2457–2463
Zhao J, Zhou C, Yang HY (2000) Isolation and in vitro culture of zygotes and central cells of Oryza sativa L. Plant Cell Rep 19:321–326
Zhong D, Luo L, Ying C (2000) Advances on transferring elite gene from wild rice species into cultivated rice. Chinese Journal Rice Science 14(2):103–106 (in Chinese with English abstract)
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 31570297; No. 31100225), the Natural Science Foundation of Ningxia (No. NZ17033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by S. D. Russell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, W., Cheng, C., Luo, C. et al. Isolation of the sperm and egg cells in wild rice (Oryza officinalis) as a mechanism for crop improvement. Plant Reprod 33, 35–40 (2020). https://doi.org/10.1007/s00497-020-00383-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-020-00383-z