Abstract
Sperm cells of Solanum verbascifolium L. were isolated by using an in vivo/in vitro method. Hand-pollinated styles were first grown in vivo for several hours, then cut off from their base and cultured in vitro until pollen tubes grew out from the cut end. When the pollen tubes were transferred to broken solution, the sperm cells were released from broken tubes. For isolation of egg cells and associated cells, ovules were digested in an enzymatic solution for 10–20 min and then transferred to an isolation solution without enzymes for the dissection. The digested ovules were cut transversely and the micropyle end was compressed to release the egg apparatus and central cell. Typically, 7–10 egg cells could be isolated in 1 h and batches of cells could be collected together and concentrated using a micromanipulator. The protocol reported here for the isolation of sperm cells and egg cells of S. verbascifolium potentially provides a basis for in vitro fertilization of this dicotyledonous plant, and for the provision of materials for molecular-biological investigations of the mechanisms of fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isolated sperm cells and egg cells provide useful materials for the study of fertilization in higher plants, under controlled conditions, in the absence of somatic tissue. In this way, it is possible to investigate the mechanisms controlling the processes of fertilization and zygote activation by male and female gametes. Kranz et al. (1991) first achieved in vitro fertilization of maize using isolated sperm and egg cells, and finally generated fertile plants (Kranz and Lörz 1993). However, a second instance of in vitro fertilization of higher plants was not reported until 14 years later, in rice (Uchiumi et al. 2007). This suggests that the establishment of in vitro fertilization methods for flowering plants has been challenging. In this respect, a major obstacle appears to be the isolation of sperm cells and egg cells (Wang et al. 2006).
Besides their potential for the study of in vitro fertilization, isolated sperm cells and egg cells could also provide a resource for experimental investigations of the molecular biology of the development of the sperm, egg and zygote. In recent years, several molecular studies of sperm and eggs have been published. Numerous sperm expressed genes have now been identified (Ning et al. 2006; von Besser et al. 2006; Berger 2008; Russell and Dresselhaus 2008; Bayer et al. 2009; Frank and Johnson 2009) and their transcribed products have been shown to be essential for fertilization and normal embryogenesis (Gou et al. 2009; Russell et al. 2010, 2012). The mechanisms regulating the transition from egg to zygote have been progressively elucidated (Okamoto et al. 2004, 2005; Sauter et al. 1998; Sprunck et al. 2005; Yang et al. 2006; Zhao et al. 2011). Recently, Leljak-Levanić et al. (2013) identified de novo transcribed genes in isolated wheat zygote and Abiko et al. (2013) have examined gene expression profiles in isolated eggs and zygotes of rice. These molecular studies of male and female germ cells require the use of isolated cells to avoid interference by genes from somatic tissues, and to permit the location of genes specifically controlling, or contributing to, the development of these reproductive cells.
Solanum verbascifolium L. is a herbaceous annual with a nearly worldwide distribution. The species contains derivations of cinnamamide and other compounds (Zhou and Ding 2002). In China, it also has medicinal applications in the treatment of scabies, eczema, dermatitis, trauma infection and other diseases (Wu 1989). In the present study, we describe a protocol for the successful isolation of sperm cells, egg cells, synergids and central cells of S. verbascifolium.
Materials and methods
Solanum verbascifolium L. (Solanaceae) is widely found throughout the Xiamen district. The flowers at anthesis were collected from the Xiamen University campus.
Disruption of pollen grains
Fresh pollen grains did not burst spontaneously when they were incubated in solutions containing 5–15 % mannitol (0.273–1.179 mOsmol/kg) for 5–20 min. However, in these solutions, it was possible to disrupt the grains by compressing them between two dissecting needles, which released the pollen cytoplasm.
In vitro pollen tube induction
Pollen grains were placed in media containing 0.1 % (w/v) KH2PO4, 0.01 % (w/v) KH2PO4, 0.01 % (w/v) H3BO3, or 0.05 % (w/v) CaCl2, and 10–15 % sucrose at pH 6.5 at room temperature (25 °C). Initial observations indicated that some pollen grains germinated and produced short pollen tubes. When the in vitro pollen tubes were transferred to 5–15 % mannitol solutions, they burst and released the tube contents, including the generative cell.
In vivo/in vitro induction of pollen tubes
The style of S. verbascifolium is approximately 7 mm long and pollen tubes require 10 h to travel to the ovary. Therefore, our protocol was as follows: each flower was emasculated at anthesis and the stigma was hand-pollinated with fresh pollen. Pollen tubes were subsequently grown in vivo for 4–10 h. After growth, the styles were cut near the ovary and immersed in a medium containing 0.01 % (w/v) H3BO3, 0.01 % (w/v) KH2PO4, 0.05 % (w/v) CaCl2 and 15 % (w/v) sucrose at pH 6.0 for 3–5 h, until pollen tubes emerged from the cut end of the style. The styles with pollen tubes were transferred into a 5–15 % solution of mannitol. The tubes burst in the mannitol solution and released their contents, including pairs of sperm cells.
Isolation of egg cells, synergids and central cells
The ovules of S. verbascifolium were excised from flowers at anthesis and incubated in an enzyme solution containing 0–1 % (w/v) cellulase RS (Yakult Pharmaceutical Industry Co., Ltd., Higashi Shinbashi Minato-Ku, Tokyo, Japan), 0–1 % (w/v) hemicellulase (Sigma-Aldrich Chemical Company, St. Louis, MO), 0–0.3 % (w/v) pectolyase Y-23 (Kikkoman Corporation, Koamicho Nihobashi Chuo-ku Tokyo, Japan), 0–1 % (w/v) pectinase (Serva Feinbiochemica GmbH. & Co., Munich, Germany), 0.05 % (w/v) CaCl2, 1 % (w/v) bovine serum albumin (BSA), and 6–12 % (w/v) mannitol for 10–20 min, with gentle shaking at 25–27 °C. The ovules were then washed twice with the above-mentioned solution but lacking the enzymes and dissected under an inverted microscope. An ovule was cut transversely into two parts at its mid-point. After gently depressing the micropyle end of the ovule, the cells of the egg apparatus and the central cell were released from the cut end. Isolated egg cells, synergid cells, and central cells were collected using a micromanipulator and transferred to an Eppendorf® microcentrifuge tube in preparation for molecular-biological assays of the cells. Cell viability was evaluated by fluorescein diacetate (FDA) staining.
The osmolalities of all solutions were measured using an osmometer (Osmomat 030, Gonotech GmbH, Berlin, Germany).
Results
Isolation of generative cells
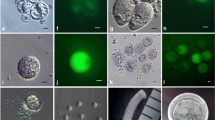
Pollen grains of S. verbascifolium are released at anthesis from a pore at the top of each anther (Fig. 1a). The pollen grains did not burst when they were incubated in 5, 10, or 15 % mannitol solutions for 5–10 min (Fig. 1b), or after the addition of equal volume of water to generate an osmotic shock. However, when the pollen grains in these mannitol solutions were compressed between two dissecting needles (Fig. 1c), they broke and released their contents, including a generative cell (Fig. 1d). Viability of the released generative cells was related to the concentration of the osmotic regulator. In 5 % mannitol solution, the generative cells became round soon after release from the pollen grain and the FDA fluorescence lasted only 1–2 min. In a 10 % mannitol solution, the generative cell remained elongated and were viable for about 10 min (Fig. 1e). In 15 % mannitol solution, however, the released generative cells retained their fusiform shapes for much longer times but they exhibited no FDA fluorescence. Therefore, the 10 % mannitol solution was considered optimal for isolating generative cells. The released generative cells were easily collected and purified in large numbers (Fig. 1f).
Isolation of generative cells and sperm cells of S. verbascifolium. a: Anther of S. verbascifolium at anthesis; b: pollen grains in medium (×200, bar = 40 μm); c: A pollen grain is compressed between two dissection needles (×400, bar = 20 μm); d: A released generative cell (arrow) from a pollen grain (×800, bar = 10 μm); e: The generative cell displaying viable fluorescence (×800, bar = 10 μm); f: A collected population of generative cells (×800, bar = 10 μm); g: Pollen grains with germinating pollen tubes (×40, bar = 200 μm); h: An in vitro pollen tube (×100, bar = 80 μm); i: A generative cell after pollen tube rupture (arrow) (×400, bar = 20 μm); j: Style of S. verbascifolium (mm scale shown); k: Pollen tubes releasing a pair of sperm cells (arrows) from ruptured pollen tubes, which grew out from the cut end of the style (×100, bar = 80 μm); l: Sperm cells displaying fluorescence (arrows) (×100, bar = 80 μm); m: A pair of sperm cells (×1000, bar = 10 μm); n: Two sperm cells displaying fluorescence (×1000, bar = 10 μm); o: A collected population of sperm cells (×400, bar = 20 μm)
In vitro pollen germination
When pollen grains were incubated for 3 h in the saline containing 10–15 % sucrose, 19.9 % of the pollen grains germinated and produced pollen tubes (Fig. 1g), which reached a mean length of 46.09 μm (Fig. 1h). When the sucrose-saline was then replaced with one containing 10 % mannitol solution, the tubes burst at the end and released their contents, including the generative cell (Fig. 1i).
Sperm cell isolation
In the above-mentioned medium, the generative cells within the pollen tubes did not divide in vitro, even following 3 h of culture. However, the in vivo/in vitro method induced generative cell division. Pollen tubes were permitted to grow within the style in vivo and then the styles were cut and immersed in a medium containing 0.01 % (w/v) H3BO3, 0.01 % (w/v) KH2PO4, 0.05 % (w/v) CaCl2 and 15 % (w/v) sucrose for 3–5 h. The style of S. verbascifolium is about 7 mm long (Fig. 1j). The pollinated styles grew in vivo over 3–10 h. They were then cut at the base and transferred to medium to grow in vitro. Following approximately 2–8 h of incubation, a number of pollen tubes emerged from the cut end of the style (Fig. 1k) and exhibited conspicuous cytoplasmic streaming. To release the sperm cells, the cut end of the style was transferred and immersed into a bursting solution of 10 % mannitol. A number of the pollen tubes ruptured and pairs of elongate sperm cells were released (Fig. 1k and l). In 10 % mannitol solution, these sperm cells were initially elongated but rapidly became ellipsoidal and finally round in appearance (Fig. 1m). The two sperm cells remained connected and FDA staining indicated they were viable (Fig. 1n). In some cases, the two sperm cells appeared to be associated with the vegetative nucleus as a male germ unit (MGU). The vegetative nucleus swelled and broke quickly but the sperm cells remained associated with each other for at least 30 min (Fig. 1m) and retained viable fluorescence. During this 30 min, the two sperm cells were easily collected using a micromanipulator (Fig. 1o).
The quality of pollen tubes is vital for successful release of the tube contents, including the two joined sperm cells. Generally, the pollen tubes growing out from the cut end of style achieved suitable quality within 2–3 h after placement of the style in the in vitro culture solution. However, if the styles did not grow in vivo to an adequate length, or if the in vitro culture period was too short, pollen tubes did not emerge from the cut end of the style. Conversely, if styles grew too long in vivo, or were held in the culture solution for more than 5 h, the pollen tubes grew too long and were irregularly curved. These pollen tubes rarely burst during transfer to the breaking solution. After a number of trials, it was considered that an in vivo growth period of 8 h followed by in vitro culturing for 3 h was optimal for best growth of the pollen tubes and release of the pair of sperm cells (Fig. 1k and l).
Isolation of the embryo sac cells
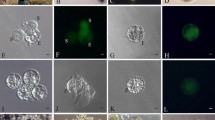
The flowers of S. verbascifolium generally bloom at noon (Fig. 2a). The perianth and stamens were peeled off the flowers at anthesis (Fig. 2b), and then the ovary wall was removed, revealing the ovules (Fig. 2c). Ovules were collected and subjected to enzyme treatment. Following 10–30 min of incubation in the enzymatic solution, the outline of the embryo sac was visible (Fig. 2d). This indicated the precise position for the ovule dissection. The embryo sac of S. verbascifolium was deeply embedded in the ovule. The ovule dissection could not be managed without the enzyme treatment.
Isolation of egg cells, synergids and central cells of S. verbascifolium. a: A flower of S. verbascifolium at anthesis; b: A gynoecium of S. verbascifolium; c: Ovules after exciting off ovary wall; d: The structure of an ovule (×40, bar = 200 μm); e: Three cells of the egg apparatus released from an ovule, S: synergid, E: egg cell (×400, bar = 20 μm); f: Three cells of the egg apparatus displaying fluorescence (×400, bar = 20 μm); g: An isolated egg (×400, bar = 20 μm); h: An egg cell displaying fluorescence (×400, bar = 20 μm); i: Four collected egg cells (×400, bar = 20 μm); j: Egg cells displaying fluorescence (×400, bar = 20 μm); k: A pair of released synergids (×400, bar = 20 μm); l: Two synergids displaying brighter fluorescence (×400, bar = 20 μm); m: Six isolated synergids (×400, bar = 20 μm); n: Synergids displaying fluorescence (×400, bar = 20 μm); o: A released central cell (×200, bar = 40 μm); p: A central cell displaying weak fluorescence (×200, bar = 40 μm)
The treated ovules were transversely cut with a dissecting needle. The egg cell and two synergid cells were then released from the cut end by pressing the micropylar end of the ovule (Fig. 2e). The released embryo sac cells were easily distinguishable in solution because of their larger size than the nucellar cells. The egg cell, synergid cells and central cell were also distinguished by their structure and morphology. Among the four cells of the embryo sac, the central cell is twice the size of the egg apparatus cells. The two synergid cells contained more cytoplasm and their nuclei were located laterally, displaying the same polarity, which is different from the arrangement in the egg cell. The three isolated cells displayed fluorescence, which suggested the cells remained viable following the isolation procedures (Fig. 2f). The three egg apparatus cells were separated in the same way as the egg cell (Fig. 2g and h) and collected individually using a micromanipulator to specifically isolate the colony of egg cells (Fig. 2i). Strong FDA fluorescence was maintained for 1 h (Fig. 2j). Thereafter, the fluorescence decreased and was lost 3 h after isolation. Therefore, isolated egg cells should be used within 1 h in in vitro fertilization assays.
The two isolated synergid cells at anthesis contained more cytoplasm (Fig. 2k) with stronger fluorescence (Fig. 2l) than the egg cell. The two synergid cells were collected together into a group of synergid cells using a micromanipulator (Fig. 2m and n). This provides an opportunity for future study of synergid development and function.
If an appropriate incision was made in the embryo sac, which did not break the central cell, the central cell could be released through the cut by pressing the micropylar end of the ovule (Fig. 2o). Freshly isolated central cell also displayed strong FDA fluorescence but this soon decreased (Fig. 2p). The central cells were also collected into a group using a micromanipulator.
Optimal conditions for the isolation of egg cells
Typically, about 30 ovules could be dissected in 1 h. Isolation of the egg cell was difficult without enzymatic digestion but after addition of suitable enzymes to the digestion solution, dissection of the ovules was relatively easy. To avoid continuing enzyme digestion, which could reduce or eliminate egg viability, the ovules were incubated in the enzymatic solution for only 10–20 min. The enzymatic solution was then removed and the ovules were dissected in the same solution without enzymes. The composition and concentration of enzymes in the digesting solution were effective for isolation of the cells of the embryo sac. Among the enzymes, pectolyase Y-23 was necessary to digest integument cells. Ovules could not be effectively dissected out using other enzymes without pectolyase Y-23. The most effective concentration of pectolyase Y-23 was 0.07 %. At lower concentrations of pectolyase Y-23, peeling of the integument and excision of nucellar cells was difficult and fewer ovules were dissected. Increasing the pectolyase Y-23 concentration allowed isolation of egg apparatus cells but the embryo sac cells adhered and it was difficult to separate the egg cell from the two synergid cells. Pectinase also helped the digestion of embryo sac cells. Without this enzyme, the egg apparatus cells were tightly conglutinated and cytoplasmic materials from the central cell were also adhering to their surface. This increased the difficulty of separating the egg cells and a fewer egg cells could be isolated. Cellulase and hemicellulase in the digesting solution mainly assisted the digestion of the cell wall of the egg apparatus cells and released the cells as protoplasts with a clear surface. In addition, the egg cells could be separated from the two synergids with little difficulty. The optimal concentrations of both enzymes were 0.1 % (Table 1).
The osmolalities of the enzymatic digestion and dissection solutions also affected the isolation of the embryo sac cells and the viability of the egg cells. At 6 % mannitol, no embryo sac cells were released. In 8 % mannitol (0.523 mOsmol/kg), 3 egg cells could be dissected within 1 h, but the cells became increasingly inflated and the cytoplasm density was low. The FDA stain indicated that these egg cells were viable for only 10 min post-isolation. A higher osmolality (12 % mannitol, 0.829 mOsmol/kg) in the digestion and dissection solutions had no obvious effect on the isolation frequency of egg cells; 4 egg cells and 6 synergid cells could be isolated but they remained viable for only 20 min. Therefore, based on the isolation success of embryo sac cells and their viability, an osmolality of 0.633 mOsmol/kg (10 % mannitol) in the digesting and dissecting solutions was considered most suitable (Table 2). In this solution, 8 egg cells could be isolated from 30 ovules in 1 h and they retained viability, indicated by FDA fluorescence, for 60 min.
Discussion
To date, successful in vitro fertilization of higher plants has been achieved in only two monocotyledons, maize (Kranz and Lörz 1993) and rice (Uchiumi et al. 2007). It is unknown whether the fertilization features of maize and rice are representative. Therefore, there is a need to examine in vitro fertilization in more species, particularly dicotyledons. The mature pollen grains of S. verbascifolium are bicellular, and its ovules are crassinucellate so that sperm and egg isolation display some specific characteristics.
Sperm cell isolation of S. verbascifolium
Sperm cell isolation is central to performing in vitro fertilization and investigating spermatogenesis (Wang et al. 2006). Two types of pollen are characteristic of angiosperms at anthesis: bicellular and tricellular pollen. Sperm isolation is relatively easy in tricellular pollen species. Generally, when tricellular pollen grains are incubated in a medium of moderately high osmolality, the grains readily break releasing the contents, including two sperm cells. The isolation of sperm cells in bicellular pollen species is more difficult because the generative cell undergoes mitosis to form two sperm cells within the pollen tube. Therefore, to isolate the sperm cells, it is first necessary to induce pollen tube growth. However, in some species, such as tobacco, the generative cell does not divide completely in the in vitro pollen tubes unless specific amino acids are added (Read et al. 1993). In addition, the style of tobacco is 4 cm long and pollen tubes must travel for two days before arriving at the ovary (Tian and Russell 1998). By contrast, the in vitro pollen tubes of tobacco were only a few mm long. Mature sperm cells could not be isolated from the in vitro pollen tubes. These observations indicate that in vitro pollen tubes may be deficient in some respects, especially in some long-style species.
The mature pollen grains of S. verbascifolium are bicellular, comprising only a vegetative and a generative cell. Generative and sperm cell isolation in S. verbascifolium had not been achieved prior to the present study. The pollen grains of S. verbascifolium could not be disrupted in mannitol media to release their contents. Here, we used two dissecting needles to compress the pollen grains, rupturing some of them to release the generative cells. To isolate sperm cells, we applied an in vivo/in vitro technique (Shivanna et al. 1988) in which entire pollinated styles were first grown in vivo for 8 h; the whole style then was cut off and transferred to a medium for in vitro culture. When the pollen tubes emerged from the cut end of the style following 3 h of in vitro growth, the styles with pollen tubes were transferred to a solution to burst the tubes. Two sperm cells were released from the disrupted tubes. At this point, a ‘population’ of sperm cells can be collected with a micromanipulator. By these means, our sperm isolation method could provide the basis for in vitro fertilization of S. verbascifolium and the identification of unique genes and proteins that may be specifically involved in spermatogenesis in this and other species.
Egg cell isolation in S. verbascifolium
The isolation of egg cells of higher plants is a precondition for carrying out in vitro fertilization and is also a basic requirement for experimental studies of the developmental mechanisms in eggs and zygotes, using molecular methods. However, the egg cell is deeply embedded in the ovule located within the ovary. Thus, egg isolation is more difficult than sperm isolation. During egg cell isolation, two primary factors affect the results. The first is the skill of researcher in dissection of ovules under the inverted microscope. Another important factor is optimization of the conditions for egg isolation, including the concentration and proportion of enzymes and the osmolalities of the enzyme solution and the isolation solution. It is difficult to dissect out ovules without enzymatic digestion, or even peel away the integument. In the digestion solution containing suitable enzymes, the ovules became soft and were relative easily dissected. In the present study, we found that including pectinase in the enzymatic digestion helped to soften the ovules, which were then easy to dissect. Cellulase in the enzymatic digestion facilitated removal of the cell wall of the egg apparatus cells, which made ovules easy to release the egg apparatus cells when the ovule micropyle was compressed. However, higher concentrations of enzymes did not improve the isolation of egg cells because the three cells of the egg apparatus were tightly adherent. At lower concentrations of enzymes it was also difficult to isolate egg cells. Using an enzymatic solution containing 0.13 % pectinase, 0.07 % pectolyase Y-23, 0.1 % cellulase RS and 0.1 % hemicellulase, we were able to isolate 9 egg cells from 32 ovules of S. verbascifolium in 1 h (Table 1). Under these conditions of relatively low concentrations of enzymes, the isolated egg cells displayed stronger and longer lasting viability fluorescence.
The osmolality of embryo sac cells is higher than that of nucellar somatic cells, although the values vary among species (van Went and Kwee 1990; Imre and Kristof 1999). If the osmolalities of the enzymatic and isolating solutions were low, the egg apparatus cells were difficult to release, and the cells tended to break during dissection. When the osmolality was higher, the released egg apparatus cells shrank and their fluorescence was short-lived, indicating reduced cell viability (Table 2). The osmotic components of the enzymatic and isolating solutions need to be carefully selected during egg isolation. The selection should take into account both the ease of release of the egg cells and the duration of viability. In the present study, the solution containing 10 % mannitol was optimal for isolating egg apparatus cells and for maintaining egg cell viability.
References
Abiko M, Maeda H, Tamura K, Hara-Nishimura I, Okamoto T (2013) Gene expression profiles in rice gametes and zygotes: identification of gamete-enriched genes and up-or down-regulated genes in zygotes after fertilization. J Exp Bot 64:1927–1940
Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W (2009) Paternal control of embryonic patterning in Arabidopsis thaliana. Science 323:1485–1488
Berger F (2008) Double-fertilization, from myths to reality. Sex Plant Reprod 21:3–5
Frank AC, Johnson MA (2009) Expressing the diphtheria toxin a subunit from the HAP2(GCS1) promoter blocks sperm maturation and produces single sperm-like cells capable of fertilization. Plant Physiol 151:1390–1400
Gou XP, Yuan T, Wei XP, Russell SD (2009) Gene expression in the dimorphic sperm cells of Plumbago zeylanica: transcript profiling, diversity, and relationship to cell type. Plant J 60:33–47
Imre K, Kristof Z (1999) Isolation and osmotic relations of developing megagametophytes of Torenia fournieri. Sex Plant Reprod 12:152–157
Kranz E, Bautor J, Lörz H (1991) In vitro fertilization of single, isolated gametes of maize mediated by electrofusion. Sex Plant Reprod 4:12–16
Kranz E, Lörz H (1993) In vitro fertilization with isolated, single gametes results in zygotic embryogenesis and fertile maize plants. Plant Cell 5:739–746
Leljak-Levanić D, Juranić M, Sprunck S (2013) De novo zygotic transcription in wheat (Triticum aestivum L.) includes genes encoding small putative secreted peptides and a protein involved in proteasomal degradation. Plant Reprod 26:267–285
Ning J, Peng XB, Qu LH, Xin HP, Yan TT, Sun M (2006) Differential gene expression in egg cells and zygotes suggests that the transcriptome is restricted before the first zygotic division in tobacco. FEBS Lett 580:1747–1752
Okamoto T, Higuchi K, Shinkawa T, Isobe T, Lörz H, Koshiba T, Kranz E (2004) Identification of major proteins in maize egg cells. Plant Cell Physiol 45:1406–1412
Okamoto T, Scholten S, Lörz H, Kranz E (2005) Identification of genes that are up- or down-regulated in the apical or basal cell of maize two-celled embryos and monitoring their expression during zygote development by a cell manipulation- and PCR-based approach. Plant Cell Physiol 46:332–338
Read SM, Clarke AE, Bacic A (1993) Requirement for division of generative nucleus in cultured pollen tubes of Nicotiana. Protoplasma 174:101–105
Russell SD, Dresselhaus T (2008) Deciphering molecular mechanisms of fertilization in seed plants. Sex Plant Reprod 21:1
Russell SD, Gou X, Wei X, Yuan T (2010) Male gamete biology in flowering plants. Biochem Soc Trans 38:598–603
Russell SD, Gou X, Wong CE, Wang X, Yuan T, Wei X, Bhalla PL, Singh MB (2012) Genomic profiling of rice sperm cell transcripts reveals conserved and distinct elements in the flowering plant male germ lineage. New Phytol 195:560–573
Sauter M, von Wiegen P, Lörz H, Kranz E (1998) Cell cycle regulatory genes from maize are differentially controlled during fertilization and first embryonic cell division. Sex Plant Reprod 11:41–48
Shivanna KR, Xu H, Taylor P, Knox RB (1988) Isolation of sperms from the pollen tubes of flowering plants during fertilization. Plant Physiol 87:647–650
Sprunck S, Baumann U, Edwards K, Langridge P, Dresselhaus T (2005) The transcript composition of egg cells changes significantly following fertilization in wheat (Triticum aestivum L.). Plant J 41:660–672
Tian HQ, Russell SD (1998) The fusion of sperm cells and the function of male germ unit (MGU) of tobacco (Nicotiana tabacum L.). Sex Plant Reprod 11:171–176
Uchiumi T, Uemura I, Okamoto T (2007) Establishment of an in vitro fertilization system in rice (Oryza sativa L.). Planta 226:581–589
Van Went JL, Kwee HS (1990) Enzymatic isolation of living embryo sacs of Petunia. Sex Plant Reprod 3:257–262
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133:4761–4769
Wang YY, Kuang A, Russell SD, Tian HQ (2006) In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sex Plant Reprod 19:103–115
Wu XR (1989) Concise Edition of Guangdong Medicinal Plants. Guangdong Higher Education Press (In Chinese), Guangzhou, p 426
Yang H, Kaur N, Kiriakopolos S, McCormick S (2006) EST generation and analyses towards identifying female gametophyte-specific genes in Zea mays L. Planta 224:1004–1014
Zhao J, Xin H, Qu L, Ning J, Peng X, Yan T, Ma L, Li S, Sun MX (2011) Dynamic changes of transcript profiles after fertilization are associated with de novo transcription and maternal elimination in tobacco zygote, and mark the onset of the maternal-to zygotic transition. Plant J 65:131–145
Zhou LX, Ding Y (2002) A cinnamide derivative from Solanum verbascifolium L. J Asian Nat Prod Res 4:185–187
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31100225,31170289) and the Zhejiang Provincial Natural Science Foundation of China (Grant No. Y3110395).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S.J., Wei, D.M. & Tian, H.Q. Isolation of sperm cells, egg cells, synergids and central cells from Solanum verbascifolium L.. J. Plant Biochem. Biotechnol. 24, 400–407 (2015). https://doi.org/10.1007/s13562-014-0290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-014-0290-6