Abstract
Key message
A detailed, step-by-step protocol for isolation of rice gametes for transcriptional profiling, with a general workflow that includes controls for RNA contamination from surrounding cells and tissues is presented.
Abstract
Characterization of the transcriptome and other -omics studies of flowering plant gametes are challenging as a consequence of the small sizes and relative inaccessibility of these cells. Collecting such poorly represented cells is also complicated by potential contamination from surrounding sporophytic, adjacent gametophytic tissues and difficulties in extracting high-quality intact cells. Here we present detailed, step-by-step procedures for collecting intact, unfixed rice (Oryza sativa) egg cells and sperm cells without enzymatic treatments. In addition, we also present a general workflow for assessing sample purity by RT-PCR, using primers specific for marker genes preferentially expressed in surrounding cells and tissues. These protocols should facilitate future studies of genome-scale characterization of gametes in this important model crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular studies of flowering plant gametes have been impeded by the relatively small size and inaccessibility of these cells. The female gamete or egg cell is embedded within layers of maternal tissue, whereas the two male gametes or sperm cells are engulfed by the pollen vegetative cell. Despite recent advancements in sequencing technology, the quality of transcriptome data of plant gametes, embryos and endosperm has been debated due to potential contamination from surrounding sporophytic tissues (Schon and Nodine 2017). Most of these studies have used Arabidopsis for its many advantages as a plant model, but it presents additional challenges due to the small size of the floral organs and reproductive cells. Rice (Oryza sativa) is a particularly appropriate model as it is one of the most important food crops in the world, in addition to being the subject of extensive genomic characterization (Kawahara et al. 2013), and has served as a model cereal for genetic and molecular studies of gene regulation and epigenetics.
Isolation of rice egg cells for global expression studies has been previously published (Abiko et al. 2013; Anderson et al. 2013, 2017; Ohnishi et al. 2011; Zhang et al. 1999). Gene expression has also been profiled in rice sperm cells (Anderson et al. 2013; Okamoto 2017; Russell et al. 2012, 2017), and a detailed but involved sperm cell isolation method has been published recently (Russell et al. 2017). Here we present a detailed, step-by-step protocol for the isolation of rice gametes, that can be easily adapted by researchers new to the field, in which we also present a general workflow to sufficiently reduce RNA contamination from surrounding cells to be considered negligible. Egg cells are isolated by manual dissection, each captured individually, without fixation or enzymatic treatments. Additionally, we present an improved sperm cell isolation method, also in the same protocol format as for the egg cell. The previous sperm cell isolation protocol (Gou et al. 2011) begins with isolated late pre-anthesis anthers, which are limited by the number of anthers separated. However, based on mass isolations of maize and tobacco sperm cells (Xu and Tsao 1997; Xu et al. 2002), we found that harvesting spikelets was less time-consuming than hand selecting anthers. The current protocol eliminates the tedious manual collection of large amounts of anthers, significantly shortens pollen collection time, and thus facilitates collecting greater volumes and improves repeatability. Sperm cells are then purified by a series of Percoll density gradient centrifuge steps. During isolation of each of the respective gametes, we incorporated extra rinse steps, which in theory should reduce RNA contamination from surrounding cells. The degree of contamination can be subsequently assessed by RT-PCR amplification of marker genes preferentially expressed in nearby cells.
Materials
List I: materials for egg cell isolation
-
1.
Flowering rice plants;
-
2.
0.3 M mannitol solution: add 27.3 g of mannitol into ~ 200 mL distilled deionized water (ddH2O). Fill up final volume to 500 mL. Autoclave;
-
3.
Scissors (to remove florets from panicle);
-
4.
50-mL test tubes;
-
5.
Weighing boats or petri dishes;
-
6.
Dissecting microscope and light source for initial dissection and cleaning;
-
7.
Inverted phase contrast microscope for final collection;
-
8.
Fine tweezers, needles (Covidien 8881250305 Monoject Hypo Needle, 25G × 1″);
-
9.
Thin razor blades (Merkur double edge razor blades);
-
10.
Acupuncture needles or insect pins;
-
11.
Transfer pipettes (Thermo Scientific Samco Standard Disposable Transfer Pipettes, 13-711-9D);
-
12.
Microcapillaries to collect egg cells (microcapillaries are purchased from World Precision Instruments, Inc., Sarasota, Florida USA TW120F-4, pulled to a fine tip using a PUL-1 micropipette (WPI, Inc.) Micropipettes are screened using a dissecting microscope and cleaved to an appropriate hole diameter to allow egg cells and zygotes to be collected with a minimum of damage and contamination).
List II: materials for sperm isolation using blender method
Items 1 and 8 from List I.
-
1.
Scissors (to cut rice stem at the lower part);
-
2.
Bucket (2~3 gallons);
-
3.
300 mL 45% sucrose: dissolve 135 g sucrose into ~ 250 mL ddH2O, fill the final volume with ddH2O up to 300 mL and store at 4 °C or on ice;
-
4.
100 mL 15% sucrose: dissolve 15 g sucrose into ~ 80 mL ddH2O, fill the final volume with ddH2O up to 100 mL and then filter (0.2 µm) and store at 4 °C or on ice;
-
5.
25 mL 40% Percoll solution in 15% sucrose: dissolve 3.75 g sucrose in 10 mL ddH2O, add 10 mL Percoll, fill the final volume up to 2 5 mL with ddH2O and store at 4 °C or on ice;
-
6.
25 mL 15% Percoll solution in 15% sucrose: dissolve 3.75 g sucrose in 10 mL ddH2O, add 3.75 mL Percoll, fill the final volume up to 25 mL with ddH2O and store at 4 °C or on ice;
-
7.
Centrifuge tubes, sterile plastic, 50 mL (Corning 430290) and 15 mL (Corning 430052);
-
8.
Three-milliliters syringe with needle (B–D, 309579) bent in right angle at ~8 mm from tip (for preparing the discontinuous Percoll solution and collecting sperm-rich portion);
-
9.
Four or more isolation tubes: in 15 mL centrifuges, layer 2.5 mL 15% Percoll in 15% sucrose above 2.5 mL 40% Percoll in 15% sucrose;
-
10.
200-mL flasks;
-
11.
100-mL beakers;
-
12.
Funnels (2 fl. oz);
-
13.
Electric blender (Hamilton Beach);
-
14.
Three pieces of nylon mesh, 80–100 cm2 each, with mesh sizes of 100, 30 and 10 µm, respectively;
-
15.
Centrifuge with swinging bucket capable of temperature control and speeding up to 4000 × g;
-
16.
Light microscope.
List III: materials for RNA extraction and quality control
-
1.
Nuclease free water;
-
2.
100% ethanol;
-
3.
Ambion RNAqueous-Micro Total RNA Isolation Kit (AM1931);
-
4.
Qiagen RNase free DNase (79254);
-
5.
Bench top centrifuge capable of spinning 12,000×g or higher;
-
6.
PCR thermal cycler;
-
7.
Agilent Bioanalyzer 2100 and RNA Pico kit (5067-1513);
-
8.
NuGEN Ovation RNA-seq System V2 (7102);
-
9.
Qiagen MinElute Reaction Cleanup Kit (28204);
-
10.
ND 1000 Nanodrop Spectrophotometer (Thermo Fisher Scientific);
-
11.
PCR and agarose gel electrophoresis supplies.
Rice plant growth condition
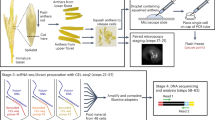
Rice (Kitaake variety) seeds are surface-sterilized (70% bleach for 10 min followed by washes with autoclaved water) and germinated in ddH2O in petri dishes wrapped with parafilm for 2 weeks in growth chamber (28 °C, 500 μmol m−2 s−1 light for 14.5 h and 25 °C, dark for 9.5 h, with constant 80% relative humidity). Two weeks after sowing, seedlings are then transplanted to greenhouse. The temperature of greenhouse is 28 °C for daytime (7:00 am to 9:30 pm) and 25 °C for nighttime, with 14.5 h light per day. Plants are irrigated with deionized water twice a week and supplied with nutrient water every other week (Fig. 1).
Methods
Method I: isolation of rice egg cells
-
1.
Collect rice florets into 0.3 M mannitol in a 50-mL test tube in the morning before anthesis. Rice florets (Kitaake variety) usually enter anthesis around 10:00 am under our growth conditions.Footnote 1
-
2.
To open the floret, a floret is held by two pairs of tweezers with the opening of the floret facing down. Use one pair of tweezers to hold the palea, while the other pair holds the lemma (Fig. 2a). Gently separate these until the lemma falls off. Gently remove the stamens at the base using a pair of tweezers (Fig. 2b). Tearing apart the palea along its symmetric axis should give access to the base of stamen. After all six stamens are removed, use the tip of the tweezers to very gently remove the ovary from the base of the floret (Fig. 2c). Care should be taken not to damage the ovary. After the ovaries are removed, they are allowed to float on 0.3 M mannitol in a weighing boat or petri dish until further dissection. Note: take care to control evaporation or molarity of mannitol may excessively increase. Dissect ovaries out from all the florets before proceeding to the next steps.Footnote 2
Fig. 2 Key steps of rice egg cell isolation a: a rice floret after the bracts are pulled open; b: floret after lemma and anthers are removed; c: dissected rice ovary; d: scheme of inner structure of rice ovary used to indicate the incision line and the positions of embryo sac cells; e: cut ovary; f: egg cell (indicated by the black arrowhead) has been released from ovary; g: differential interference contrast image of isolated egg cell; h: FDA staining of isolated egg cell; i: fine capillary used to collect egg cells connected to the tip of a transfer pipette. Bar = 5 mm for a–d; 1 mm for e, f, h; 50 µm for i
-
3.
Rinse a microscope slide with deionized water while rubbing it with an ungloved thumb. Pipette 6 µL of 0.3 M mannitol onto the slide, and the liquid should assume a small convex droplet and not spread out.
-
4.
Under a dissecting stereomicroscope, examine the stigma of the ovary to ensure no pollen has landed. Mount the ovary into the mannitol droplet. Remove any remaining stamens using a needle. Use a thin razor blade to make a transverse cut through the middle region of the ovary (Fig. 2d). A clean cut should be made taking care not to squeeze or crush the ovary. Sharp blades have a better chance in producing clean cuts and therefore each side of the razor blade should be used only once in a given position to avoid dulling of the blade (Fig. 2e).
-
5.
To reduce the risk of RNA contamination from floral organs, once the ovary is cut, pipette a new 6 µL droplet of 0.3 M mannitol onto the slide and move the basal part of ovary so as to rinse the ovary in the new droplet.
-
6.
Using an inverted phase contrast microscope (10× objective lens), use an acupuncture needle or insect pin to very gently exert pressure on the ovary with the goal of relaxing tension in the cell wall. In around 3 min, the egg cell may be released from the ovary (Fig. 2f). The rice egg cell is about 50 µm wide, having many large vacuoles. There is only one egg cell per floret. Pay attention in picking the egg cell based on the cell morphology. Egg cells may not always be captured because: (1) the ovary has been damaged and the egg cell has been ruptured or (2) staging of the flower is not correct and the egg cell has not yet developed.
-
7.
Once the egg cell floats out of the incision in the ovary, it is captured by a fine capillary connected to a transfer pipette (Fig. 2i), under the 40× object lens (Fig. 2g). To reduce the risk of RNA contamination, pipette the egg-containing liquid into a new 0.3 M mannitol droplet to rinse the egg cell.
-
8.
The isolated egg cell can be stained with fluorescent diacetate (FDA) to examine its viability (Fig. 2h). Alternatively, for transcriptome or other omics studies, the egg cell is then immediately transferred into a 1.5-mL centrifuge tube and frozen in liquid nitrogen. The captured cells should always be kept frozen in liquid nitrogen or dry ice and maintained at − 80 °C freezer temperatures until RNA extraction. Around 35 egg cells are adequate as a biological replicate that will provide sufficient RNA for reverse transcription.
Synergid cells are sometimes also released at step 6. However, the two cell types can easily be distinguished (Supplementary Fig. 1). The egg cell appears clearer and more transparent, with larger vacuoles. In contrast, the synergid, which is slightly smaller in size, appears more opaque with many small vesicles. This same protocol can also be adapted for rice zygote isolation if the starting material is pollinated, time-staged florets (Anderson et al. 2017).
Depending on the skill of the experimenter, our protocol processes 10 ovaries (post-step 2) in about 40 min per person, with a 30~50% frequency in capturing intact egg cells. In a 2-h period, 10–15 egg cells can be collected by one person. The success rate increases as the experimenter becomes more experienced with the protocol. The non-enzymatic isolation method from Uchiumi et al. (2007) can collect 30–40 egg cells per day. This protocol has the potential to achieve similar yields if sufficient rice plants at the appropriate reproductive stage are available. The previous method from Zhang et al. (2010) could collect 3–5 egg cells from 30 ovaries in an hour. Although fewer ovaries are processed in an hour, this protocol is more efficient than that described in Zhang et al. (2010).
Method II: sperm isolation using blender method
-
1.
Select ~ 50 rice panicles with mature florets around 10 am (for variety Kitaake) before anthesis. Cut panicles at their base and place them into the bucket with ~ 200 mL water to keep hydrated.
-
2.
Collect all mature and almost mature florets (1 day before flowering) into the blender (Hamilton Beach, with single speed setting), containing ~150 mL 45% sucrose (Fig. 3a).
Fig. 3 Key steps for rice sperm cell isolation using blender method. a: rice florets collected into the blender containing 45% sucrose solution; b: pollen-containing solution in flask separated from the blended mixture through 100-µm mesh; c: pass the filtrate of step B through 30-µm mesh; d: pollen on the mesh from step C transferred to a clean beaker for washing in 45% sucrose; e: pollen washed twice; f: intact and almost clean pollen grains viewed under microscope note that minor debris can be observed, likely arising from individual burst pollen grains; g: DAPI staining to show the two sperm cells within the pollen grain; h: pollen incubated with 15% sucrose to release sperm cells; i: sperm cell-containing filtrate in a 50-mL tube; j: Sperm cell-rich layer indicated by arrow at the interface of 40/15% Percoll (Russell et al. 2017, with permission of the publisher); k: rice sperm cells under microscope from different isolations to show repeatability of this method
-
3.
Blend tissues for 3 times to release pollen grains from anthers: 30 s each time.
-
4.
Separate pollen-containing liquid into a flask from the blended mixture using 100 µm nylon mesh on a funnel (Fig. 3b).
-
5.
To remove floral organ cytoplasm from pollen, pass the 100 µm filtrate (solution containing pollen) through a 30-µm nylon mesh (Fig. 3c). Pollen will be trapped on the mesh.
-
6.
Carefully transfer pollen from 30-µm mesh into a beaker (Fig. 3d) by rinsing the mesh with 45% sucrose using a transfer pipette. Then add an additional ~ 50 mL 45% sucrose into the beaker and swirl for ~ 1 min.
-
7.
Repeat Steps 5 and 6 once or more until the filtered sucrose solution is clear and colorless (Fig. 3e). Intact and pure pollen grains are on the 30-µm mesh (Fig. 3f, g).
For sperm cell isolation, following procedures are based on the protocol developed by Russell et al. (2017) with modifications.
-
8.
To burst pollen for sperm isolation, quickly rinse 1–2 mL of the pollen into a 50-mL tube in ~20 mL 15% sucrose (Fig. 3h), seal the cap and rotate horizontally and slowly at room temperature for ~ 25 min.
-
9.
Filter the mixture through a 30-µm mesh to separate sperm cells from sperm-depleted pollen (Fig. 3i).
-
10.
Equally split the filtrate into two aliquots. Carefully layer each aliquot using a syringe with a 90° tip-bent needle on top of a 15% Percoll solution in an isolation tube.
-
11.
Centrifuge at 4 °C, 4000×g for 45 min with slower acceleration and deceleration. For example, for a Thermo Scientific Heraeus Multifuge X3R Centrifuge, set acceleration to 8 (with 9 being fastest) and deceleration at 9 (with 10 being fastest); the purpose is to prevent the interface of discontinuous Percoll solutions from being disturbed by rapid acceleration or deceleration. The sperm-rich portion at the interface of 40/15% Percoll will be visible after centrifuging (Fig. 3j, Russell et al. 2017 with permission of the publisher).
-
12.
Remove supernatant by pipetting until 0.5–1 cm above the interface and collect up to ~ 0.5 mL sperm-rich layer using the syringe with the tip-bent needle from each aliquot.
-
13.
Dilute sperm-rich fraction with 4 volumes of 15% sucrose in a new tube and filter with 10-µm mesh to prevent the potential aggregation of sperm cells (aggregated sperms, along with impurity, may form a new layer on the surface of 15% Percoll and thus reduce the yield of sperm cells).
-
14.
Layer the filtrate on the top of 15% Percoll in a new isolation tube, and centrifuge at 4 °C, 3000×g for 25 min.
-
15.
For higher purity (but lower sperm cell yield), repeat Steps 13 and 14 once or more.
-
16.
Collect 0.2–0.5 mL from the interface into a new tube, add 3 mL of 15% sucrose, and centrifuge at 4 °C, 1000×g for 10 min; sperm cells are collected at the tube bottom.
-
17.
Remove most supernatant by pipetting but leave 0.1–0.2 mL from the bottom. Add 1 mL 15% sucrose and centrifuge again (4 °C, 1000×g, 10 min).
-
18.
Slowly remove the supernatant. Leave 30–50 µL, the sperm-rich portion, at the bottom.
-
19.
Use 1 µl for microscopy (Fig. 3k); save the rest in a new Eppendorf tube at − 80 °C; for RNA isolation, use DEPC treated Eppendorf tube and freeze it in liquid nitrogen, then store at − 80 °C until use.
Method III: RNA extraction and quality control
RNA extraction
Efficient total RNA isolation from low input materials, such as egg cell and sperm cell, can be achieved using the Ambion RNaqueous Micro Total RNA kit. We also perform an on-column DNase treatment using Qiagen DNase. Our protocol, which also recovers RNA species < 200 nt that would be useful for miRNA and siRNA profiling, is included here:
-
Make 20 µL DNase solution per sample.
5 µL DNase I
15 µL RDD buffer.
20 µL Total.
-
Pre-warm DNase solution at 37 °C.
-
Bring wash buffers 1 and 2/3 to room temperature.
-
Add 200 µL lysis solution to cells and immediately vortex until sample is completely thawed. Do not thaw samples prior to adding lysis solution.
-
Add 250 µL of 100% ethanol, vortex briefly and centrifuge briefly.
-
Load lysate/ethanol mixture (up to 150 µL) on to column, spin at maximum speed for 10 s.
-
Load remaining lysate, spin at maximum speed for 10 s.
-
Add 180 µL Wash Solution I and spin at maximum speed for 10 s.
-
Add 20 µL DNase solution to the center of the filter.
-
Incubate at 37 °C for 30 min.
-
Pre-warm elution buffer at 75 °C, 25 µL per sample.
-
Wash with 180 µL Wash Solution I, spin at maximum speed for 10 s.
-
Wash with 180 µL Wash Solution 2/3, spin at maximum speed for 10 s.
-
Repeat with a second 180 µL aliquot of Wash Solution 2/3, spin at maximum speed for 10 s.
-
Empty flow through, spin at maximum speed for 1 min to dry.
-
Elute 2× with 7 µL heated elution buffer into a new tube, incubate 1 min and then spin at maximum speed for 30 s.
-
Pipette 1.5 µL into 0.2-mL tubes to be quality checked using RNA 6000 Pico Kit on the Bioanalyzer, using the Eukaryotic Total RNA Pico program. Typically, a high-quality RNA sample should return a Bioanalyzer trace with two rRNA peaks in addition to the marker peak, whereas the higher molecular weight peak should be twice as intense as the lower molecular weight peak (Supplementary Fig. 2).
-
Store RNA at − 80 °C.
cDNA synthesis
Due the extreme difficulties of collecting rice gametes, the number of cells in each biological replicate is low and thus the RNA concentration of each sample is also low. We adapted NuGEN Ovation RNA-seq System V2 for reverse transcription to produce ~1 µg range cDNA from total RNA input as low as 1 ng. After cDNA synthesis, the cDNA products are purified using Qiagen MinElute Reaction Cleanup Kit and quantified on a Nanodrop spectrophotometer. The RNA input can be variable across samples; however, equal amount of cDNA should be used for RT-PCR for each sample. One microgram of cDNA sample can be sheared or fragmented for subsequent RNA-seq library construction.
Alternatively, RNA samples can be quantified using a Qubit fluorometric instrument (Thermo Fisher Scientific) by RNA HS assays (Q32852). Equal amounts of RNA across samples can then be used in reverse transcription reactions using iSript Select cDNA Synthesis Kit (BIO-RAD 1708896) or similar. After cDNA synthesis, 2 µL of cDNA product can be used in a 20 µL PCR.
PCR and gel electrophoresis
To address the issue of possible contamination from sporophytic tissue, we surveyed the literature for marker genes expressed preferentially in floral organs but very low in gametes (Anderson et al. 2013). A list of primer sequences can be found in Supplementary Table 1. For each gene, 25 PCR cycles were performed, and equal volumes of PCR products were run on a 2% agarose gel, at 120 V for 30 min.
To validate the identity of the egg cells collected by the protocol, we used ECA-like 1/2 as egg cell marker genes (Ohnishi et al. 2011). Strong signals were obtained with PCR products for ECA-like 1/2 transcripts, confirming their egg cell identity (Fig. 4a). To assess contamination from ovary, we used as a marker the MADS box gene OsMADS16, which is preferentially expressed in the ovary, and has low expression in the egg cell (Anderson et al. 2013; Xiao et al. 2003). We observed in the PCR products, a strong signal for ovary tissue and a weak signal for the egg cells, indicating that there is minimum contamination from the sporophytic ovary (Fig. 4a). For RNA-seq and other -omics experiments, we suggest performing control collections and control libraries. For example, for each egg cell collected, ~1 µL of cell-free, tissue-free mannitol should be collected into a separate tube as a control sample. Control samples should be included in RNA extraction and subsequent library construction. Control samples with no library products will suggest the cognate cell collections are free of RNA contamination from surrounding tissues.
Confirmatory semiquantitative RT-PCR experiments for a: egg cell and ovary marker genes; b: sperm cell marker genes. M DNA size marker; OV ovary, EC egg cell, PV pollen vegetative cells, BS sperm cells collected by blender method (this study), SP sperm cells collected using Russell et al. 2017 method; gDNA genomic DNA, MSU7 LOC ID locus ID number from MSU Rice Genome Annotation Project, Release 7 (http://rice.plantbiology.msu.edu)
To verify that this protocol does collect sperm cells, we used MGH3 as the sperm marker gene (Anderson et al. 2013; Okada et al. 2005). Strong signals for PCR products for MGH3 transcript in sperm samples indicated that we were successful in collecting sperm cells (Fig. 4b). Since mature rice pollen grains are trinuclear (Fig. 3g), mature anthers yield sperm cells rather than generative cells.
To assess RNA contamination from the pollen vegetative cell, we used LAT52 as the marker gene (Cook and Thilmony 2012). A sperm cell preparation collected using our previously published method (Russell et al. 2017) was also included as a control sample. Sperm samples collected with this protocol appear to perform better than our previously published protocol (Russell et al. 2017) in terms of pollen vegetative cell contamination, since the bands for LAT52 are much fainter in the current protocol (Lanes BS vs. SP in Fig. 4b).
To assess RNA contamination from floral organs, we used a set of marker genes for floral organs. Since whole florets were collected into the blender, an ovary sample was used as a positive control. MADS3 is preferentially expressed in bracts and stamen (Kyozuka et al. 2002). MADS7 is preferentially expressed in stamen and tapetum (Lu et al. 2006). These MADS genes all have detectable expression in ovary (Fig. 4b). In addition, we also included Rubisco small unit (RBCS) as a marker gene for green tissues (Kawahara et al. 2013). RT-PCR showed that while each gene amplified a strong band for ovary samples, transcripts of these marker genes are undetectable in sperm samples collected by blender method, suggesting that sperm samples collected by this method are free of floral organ and sporophytic tissue contamination.
Author contributions statement
All authors were involved in the concepts and design of the experiments. CL and HX conducted experiments and analyzed data. CL and HX wrote the manuscript, with input from SR and VS. All authors read and approved the manuscript.
Notes
Care should be taken to identify mature florets that are staged correctly. In mature florets, stamens should occupy most of the floret prior to anthesis.
For RT-PCR, we used MyTaq Red Mix (Bioline BIO-25043). All primers listed in Supplementary Table 1 performed using an annealing temperature of 59 °C.
References
Abiko M, Furuta K, Yamauchi Y, Fujita C, Taoka M, Isobe T, Okamoto T (2013) Identification of proteins enriched in rice egg or sperm cells by single-cell proteomics. PLoS ONE. https://doi.org/10.1371/journal.pone.0069578
Anderson SN, Johnson CS, Jones DS, Conrad LJ, Gou X, Russell SD, Sundaresan V (2013) Transcriptomes of isolated Oryza sativa gametes characterized by deep sequencing: evidence for distinct sex-dependent chromatin and epigenetic states before fertilization. Plant J 76(5):729–741. https://doi.org/10.1111/tpj.12336
Anderson SN, Johnson CS, Chesnut J, Jones DS, Khanday I, Woodhouse M, Li C, Conrad LJ, Russell SD, Sundaresan V (2017) The zygotic transition is initiated in unicellular plant zygotes with asymmetric activation of parental genomes. Dev Cell 43(3):349.e4–358.e4. https://doi.org/10.1016/j.devcel.2017.10.005
Cook M, Thilmony R (2012) The OsGEX2 gene promoter confers sperm cell expression in transgenic rice. Plant Mol Biol Rep 30(5):1138–1148. https://doi.org/10.1007/s11105-012-0429-3
Gou X, Wang S, Chen F (2011) Isolation and cytological observation of viable sperm cells of rice. Acta Botanica Sinica 41(6):669–671. https://doi.org/10.1360/zd-2013-43-6-1064
Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, Childs KL, Davidson RM, Lin H, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee SS, Kim J, Numa H, Itoh T, Buell CR, Matsumoto T (2013) Improvement of the Oryza sativa nipponbare reference genome using next generation sequence and optical map data. Rice 6(1):3–10. https://doi.org/10.1186/1939-8433-6-4
Kyozuka J, Shimamoto K, Ag OR (2002) Ectopic expression of OsMADS3, a rice ortholog of AGAMOUS, caused a homeotic transformation of lodicules to stamens in transgenic rice plants. Plant Cell Physiol 43(1):130–135
Lu XC, Gong HQ, Huang ML, Bai SL, He YB, Mao X, Geng Z, Li SG, Wei L, Yuwen JS, Xu ZH, Bai SN (2006) Molecular analysis of early rice stamen development using organ-specific gene expression profiling. Plant Mol Biol 61(6):845–861. https://doi.org/10.1007/s11103-006-0054-3
Ohnishi T, Takanashi H, Mogi M, Takahashi H, Kikuchi S, Yano K, Okamoto T, Fujita M, Kurata N, Tsutsumi N (2011) Distinct gene expression profiles in egg and synergid cells of rice as revealed by cell type-specific microarrays. Plant Physiol 155(2):881–891. https://doi.org/10.1104/pp.110.167502
Okada T, Endo M, Singh MB, Bhalla PL (2005) Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J 44(4):557–568. https://doi.org/10.1111/j.1365-313X.2005.02554.x
Okamoto T (2017) Analysis of proteins enriched in rice gamete. In: Schmidt A (ed) Plant germline development: methods and protocols. Springer, New York, pp 251–263. https://doi.org/10.1007/978-1-4939-7286-9_20
Russell SD, Gou XP, Wong CE, Wang X, Yuan T, Wei XP, Bhalla PL, Singh MB (2012) Genomic profiling of rice sperm cell transcripts reveals conserved and distinct elements in the flowering plant male germ lineage. New Phytol 195(3):560–573. https://doi.org/10.1111/j.1469-8137.2012.04199.x
Russell SD, Jones DS, Anderson S, Wang X, Sundaresan V, Gou X (2017) Isolation of rice sperm cells for transcriptional profiling. In: Schmidt A (ed) Plant germline development: methods and protocols. Springer, New York, pp 211–219. https://doi.org/10.1007/978-1-4939-7286-9_17
Schon MA, Nodine MD (2017) Widespread contamination of Arabidopsis embryo and endosperm transcriptome data sets. Plant Cell 29(4):608–617. https://doi.org/10.1105/tpc.16.00845
Uchiumi T, Uemura I, Okamoto T (2007) Establishment of an in vitro fertilization system in rice (Oryza sativa L.). Planta 226(3):581–589. https://doi.org/10.1007/s00425-007-0506-2
Xiao H, Wang Y, Liu D, Wang W, Li X, Zhao X, Xu J, Zhai W, Zhu L (2003) Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol Biol 52(5):957–966. https://doi.org/10.1023/A:1025401611354
Xu HP, Tsao TH (1997) Detection and immunolocalization of glycoproteins of the plasma membrane of maize sperm cells. Protoplasma 198(3–4):125–129. https://doi.org/10.1007/BF01287560
Xu H, Weterings K, Vriezen W, Feron R, Xue Y, Derksen J, Mariani C (2002) Isolation and characterization of male-germ-cell transcripts in Nicotiana tabacum. Sex Plant Reprod 14(6):339–346. https://doi.org/10.1007/s00497-002-0128-6
Zhang J, Dong WH, Galli A, Potrykus I (1999) Regeneration of fertile plants from isolated zygotes of rice (Oryza sativa). Plant Cell Rep 19(2):128–132. https://doi.org/10.1007/s002990050722
Zhang YN, Wei DM, He EM, Miao S, Tian HQ, Russell SD (2010) Isolation of male and female gametes of rice. Crop Sci 50(6):2457-2463. https://doi.org/10.2135/cropsci2010.02.0066
Acknowledgements
We thank Imtiyaz Khanday, Jonathan Gent, Sarah Anderson and Daniel Jones for helpful advice for optimizing the experimental methods. We thank Debra Skinner for assistance in artwork. This research was funded by the National Science Foundation (Award No. IOS-1547760) and the USDA Agricultural Experiment Station (Project No. CA-D-XXX-6973-H).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Thomas Dresselhaus.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A contribution to the special issue ‘Cellular Omics Methods in Plant Reproduction Research’.
Scott D. Russell and Venkatesan Sundaresan: Senior authors.
Venkatesan Sundaresan: Lead contact.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Xu, H., Russell, S.D. et al. Step-by-step protocols for rice gamete isolation. Plant Reprod 32, 5–13 (2019). https://doi.org/10.1007/s00497-019-00363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-019-00363-y