Abstract
It has been established that the synthesis of heat shock protein 70 (Hsp70) is temperature-dependent. The Hsp70 response is considered as a cellular thermometer in response to heat stress and other stimuli. The variation in Hsp70 gene expression has been positively correlated with thermotolerance in Drosophila melanogaster, Caenorhabditis elegans, rodents and human. Goats have a wide range of ecological adaptability due to their anatomical and physiological characteristics; however, the productivity of the individual declines during thermal stress. The present study was carried out to analyze the expression of heat shock proteins in different tissues and to contrast heat stress phenotypes in response to chronic heat stress. The investigation has been carried out in Jamunapari, Barbari, Jakhrana and Sirohi goats. These breeds differ in size, coat colour and production performance. The heat stress assessment in goats was carried out at a temperature humidity index (THI) ranging from 85.36–89.80 over the period. Phenotyping for heat stress susceptibility was carried out by combining respiration rate (RR) and heart rate (HR). Based on the distribution of RR and HR over the breeds in the population, individual animals were recognized as heat stress-susceptible (HSS) and heat stress-tolerant (HST). Based on their physiological responses, the selected animals were slaughtered for tissue collection during peak heat stress periods. The tissue samples from different organs such as liver, spleen, heart, testis, brain and lungs were collected and stored at −70 °C for future use. Hsp70 concentrations were analyzed from tissue extract with ELISA. mRNA expression levels were evaluated using the SYBR green method. Kidney, liver and heart had 1.5–2.0-fold higher Hsp70 concentrations as compared to other organs in the tissue extracts. Similarly, the gene expression pattern of Hsp70 in different organs indicated that the liver, spleen, brain and kidney exhibited 5.94, 4.96, 5.29 and 2.63-fold higher expression than control. Liver and brain tissues showed the highest gene expression at mRNA levels as compared to kidney, spleen and heart. HST individuals had higher levels of mRNA level expression than HSS individuals in all breeds. The Sirohi breed showed the highest (6.3-fold) mRNA expression levels as compared to the other three breeds, indicating the better heat stress regulation activity in the breed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variation in heat stress tolerance at the cellular level in response to environmental stimuli is observed in individuals in the population. Cellular tolerance to heat stress is regulated by heat shock proteins (HSPs). HSPs are released in the cell in response to various environmental and oxidative stresses (Sonna et al. 1985; Hecker et al. 2008, 2011). The regulation of HSP production is critical to cell survival. HSPs activate various regulated proteins and block apoptosis (Chirico et al. 1998; Kregel 2002). The HSP acts as a molecular chaperone by binding with other cellular proteins and facilitates intracellular transport. Among the HSPs, Hsp70 has a significant role in cell thermotolerance (Barbe et al. 1998) and animal survival (Barbe et al. 1998; King et al. 2002). HSP transcription is increased by heat shock and other stress stimuli in bovine embryos and in different tissues (Wrenzycki et al. 2001). Hsp70 concentration in blood is also a reliable indicator of chronic stress in feedlot cattle (Gaughan et al. 2013). There is considerable evidence that the synthesis of Hsp70 is temperature-dependent (Zulkifi et al. 2003) and thus Hsp70 responses could be considered as a cellular thermometer. HSPs are classified into several families based on their molecular size and amino acid sequence similarity. The most highly conserved family of HSPs is the 70 kDa family (Hsp70), which is controlled by 13 genes in human and four genes in bovine (Grosz et al. 1992; Gallagher et al. 1993; Kampinga et al. 2009).
It has been established in mouse Hsp70 gene knock out models that the cytosolic Hsp70 family members regulate the cellular stress response, while other HSPs are involved in tissue-specific and housekeeping biological tasks (Daugaard et al. 2007). Heat stress is regulated in two stages: acute (short term) and chronic (long term) (Garret et al. 2009). The acute phase includes the heat shock response at the cellular level and the chronic phase results in acclimation to the stressor and involves the reprogramming of gene expression and metabolism (Horowitz 2002; Collier et al. 2006). In ruminants, there is a loss in productivity as animals pass through the acute phase and return to productivity as they undergo acclimation to the stress (Collier et al. 2006). The differential tissue-specific expression of Hsp70 proteins is not understood in ruminants. The cellular heat stress response has been analyzed in response to high temperatures in laboratory conditions; however, limited studies have been carried out in in vivo conditions. Again, the adaptation to thermal stress should be analyzed in different physiological backgrounds to understand the interaction of several genes in different pathways.
Genetic differences in thermotolerance at the physiological and cellular levels are analyzed in Bos indicus and Bos taurus (Paula-Lopes et al. 2003; Hansen 2004; Lacetera et al. 2006). The polymorphic pattern of the Hsp70 gene is associated with thermotolerance in dairy cattle (Basirico et al. 2011). The loss of productivity in livestock due to heat stress has been documented in cattle (Bernabucci et al. 2010). Goats have broad ecological adaptability and are more productive in harsh environments than other ruminants, including sheep (Devendra 1990; King 1983). However, the productivity of goats declines during thermal stress (Al-Tamimi 2007). Goats are highly adaptable to heat stress due to specific anatomical and physiological characteristics. The morphological characteristics that result in better adaptation are large salivary glands, a higher surface area of absorptive mucosa and the capacity to increase the volume of the foregut with high-fibrous foods (Silanikove and Koluman 2015). The anatomical structure of goats favours better regulation of body temperature during periods of extreme heat. Short hair, abundant sweat glands and minimal subcutaneous fat allow goats to regulate body temperature in an efficient manner. Information is available on the physiological changes that occur in goats during heat stress, but the regulation of heat stress at the cellular and genetic level is not understood properly. Understanding the regulation of heat stress at the cellular level and the expression pattern of Hsp70 gene will throw light on the mechanism of heat stress adaptation in goats. Moreover, as individual animals respond differently to heat stress, it is interesting to observe gene expression in differing physiological response backgrounds. The population was selected based on contrasting physiological responses, and Hsp70 expression patterns were observed in heat stress-tolerant and heat stress-susceptible phenotypes. Characterization of the cellular heat stress response in terms of the expression of HSPs has been carried out in response to high temperatures in laboratory conditions, whereas only limited studies have been carried out to analyze messenger RNA (mRNA) levels in different organs. Hsp70 gene expression has been positively correlated with variations in thermotolerance in different organisms. As Hsp70 plays a multifarious role at the cellular and tissue levels, the present study was carried out to analyze heat shock protein 70 gene expression in different tissues and to contrast phenotypes in response to peak heat stress in vivo conditions.
Material and methods
Animal, management, physiological response and plasma collection
The investigation has been carried out on Jamunapari, Barbari, Jakhrana and Sirohi goats. We included large size breeds and medium size breed with contrasting coat colours. The observations were recorded on Barbari, Jamunapari, Jakhrana and Sirohi goats maintained at the CIRG. The Barbari goat is a medium-sized, dual-purpose breed and is known for its adaptability over a wide range of agro-climatic situations. The Jamunapari is a large white breed, distributed in semi-arid regions and is known for milk production (Rout et al. 2000). The Jakhrana is a black breed, is known for milk production and is distributed in semi-arid and arid regions of Rajasthan. The goats were maintained under a semi-intensive management system with 6–7 h of grazing and stall feeding with seasonally available green fodder, supplemented with concentrated mixtures depending on the age of the animals. Generally, animals were housed separately according to their ages, sex, physiological status and health status. The Sirohi goat is a large, brown breed and is distributed in semi-arid and arid regions of Rajasthan.
Rectal temperatures (RT), respiration rate (RR) and heart rate (HR) were recorded as physiological indicators of heat stress. The animals were allowed to graze (exposed to sunlight) from 9.00 AM to 13.30 PM and then they were brought back to their shed at 13.30. RT, RR and HR were recorded during the highest temperature of the day ranging from 13.30 to 14.30. The physiological response was recorded in each group three times during the period (May–June) for 10–12 days. RT was measured by a digital clinical thermometer (accuracy, ±0.1 °C). The number of inhalations and exhalations during 60 s indicated the RR and the heartbeat per min were measured with a stethoscope.
During peak heat stress periods, blood samples (6–9 ml) were collected from animals by jugular vein puncture in EDTA-coated vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA) under aseptic conditions and were transported to the laboratory under refrigeration (4 °C). In brief, vacutainer tubes containing blood were centrifuged at 1200×g for 25 min at 4 °C to separate the plasma. Non-lactating females were included in the experiment. Animals did not exhibit any apparent discomfort, nasal discharge or distress and were apparently healthy. During peak heat stress periods, the temperature varied from 45.0 to 49.4 °C and relative humidity (RH) varied from 14.33–27.0 and animals were exposed to radiation for 4–5 h for 45 days.
Temperature humidity index
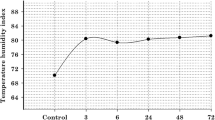
The study area has a semi-arid climate and average annual rainfall of about 400 mm. The temperature–humidity index (THI) could be used as an indicator of thermal climatic conditions (McDowell et al. 1976). The THI is calculated from the wet and dry bulb air temperatures for a particular day according to the following formula:
THI = 0.72(W + D) + 40.6, where W is wet bulb and D is dry bulb temperature in degree centigrade. The heat stress assessment in goats was carried out in THI ranging from 85.36–89.80.
Recognition of heat stress-tolerant and heat stress-susceptible phenotypes
Phenotyping for heat stress susceptibility was carried out based on respiration rate (RR) and heart rate (HR). Based on the distribution of RR and HR over the breeds in the population, it was observed that individuals having RR ≥50 and HR ≥130 would be recognized as heat stress-susceptible (HSS) phenotype and RR ≤30 and HR ≤100 would be recognized as heat stress-tolerant (HST) individual in adult goats.
Tissue preparation
Based on their physiological responses all the selected animals were slaughtered for tissue collection during peak heat stress periods. The necessary permission was obtained from the Institute’s animal ethics committee. Animals were slaughtered according to the Halal method at the Institute’s experimental slaughterhouse and tissue samples were collected aseptically. Tissue samples were washed in PBS twice followed by RNA later. The tissue samples were collected from different organs such as liver, spleen, heart, testis, brain and lungs. Subsequently, tissue samples were stored at −70 °C for future use.
RNA extraction and cDNA preparation
Total RNA was isolated from tissues by the TRI reagent method. Two milligrams TRI reagent (2 ml) was added to 200 mg tissue, and the tissue was homogenized in a homogenizer for 5 min at room temperature. Subsequently, 0.6 ml chloroform was added to each tube. It was mixed thoroughly at room temperature for 5 min and centrifuged at 10,000 rpm for 35 min. The aqueous phase was collected and precipitated with an equal volume of isopropanol. The centrifugation was carried out at 10000 rpm for 35 min, and the supernatant was discarded to obtain an RNA pellet. The RNA pellet was washed with 2 ml of 75 % ethanol and centrifuged at 10000 rpm for 10 min. The ethanol was discarded, and a pure RNA pellet was obtained. One hundred microlitres DEPC-treated TE buffer was added and stored at −70 °C for further use. RNA quality and quantity were assessed by Biophotometer (Eppendorf) by using OD260 for concentration and the ratios 260/280 and 260/230 to assess the purity of the sample. The integrity of the RNA was checked in 1.4 % agarose gel.
Subsequently, 1 μg of RNA was treated with DNase (1 IU/μl) and incubated at 37 °C for 15 min. One microgram of total RNA was reverse transcribed with a transcriptor first strand complementary DNA (cDNA) synthesis kit (www.roche-applied-science.com) in a total volume of 20 μl reaction and was incubated at 55 °C for 30 min in a thermal cycler (Bio-Rad). The cDNA was stored at −70 °C for further use. Controls without reverse transcriptase were carried out to exclude the possibility of DNA contamination. To exclude genomic DNA contamination, total RNA was treated with RNase free DNAse I (Fermentas Life Sciences, Canada). The absence of contaminant genomic DNA in RNA preparations was tested further using RNA as a template in real-time PCR assays.
Gene expression analysis
SYBR intercalating dye was used to analyze mRNA level expression. Real-time PCR was carried out for Hsp70 and housekeeping gene (GADPH). Real-time PCR was performed in the Light Cycler 480 (Roche Applied Science, Indianapolis, IL, USA) using SYBR green detection. Each reaction contained 10 μl of SYBR green I master mix (containing Fast startTaq DNA Polymerase, dNTP mix, SYBR green I dye, MgCl2 (Roche Applied Science, Indianapolis, IL, USA)), 20 pmole of the sense and antisense specific primers, and 5 μl of cDNA in a final volume of 20 μl. A negative control without cDNA template was run in each assay.
The primers of Hsp70 (Gene Bank accession number JN833720.1) were (forward 5′-TCATCGGAGATGCAGCCAAGAA-3′ and reverse 5′- AGATCTCCTCGGGGAAGAAGGT - 3′) used with an annealing temperature of 62 °C to amplify a 210 bp fragment from position 22 to 476. The GAPDH primers were (forward 5′GTGATGCTGGTGCTGAGTAC3′ and reverse 5′GTAGAAGAGTGAGTGTCGC-3′) used with an annealing temperature of 62 °C to amplify a 62-bp fragment. PCR products were subjected to melting curve analysis in the Light cycler 480 and subsequently 2 % agarose gel electrophoresis to confirm amplification specificity and amplicon size. The amplicon size was 210 bp. PCR product was sequenced and was observed as Hsp70.3 gene A2 allele by blast.
Absolute quantification was carried out after PCR to analyze the whole set of data. The crossing point (Cp) readings for each unknown sample were then used to calculate the amount of either the target or housekeeping gene using the second derivative maximum method with the Light cycler 480 analysis software version 1.5 (Roche Applied Science, Indianapolis, IL, USA). GADPH was used to normalize gene expression. The susceptible individual was used as positive calibrator to obtain normalized gene expression.
Measurement of Hsp70 protein in different tissue by ELISA
Frozen tissue was used for protein extraction. Briefly, 100 mg tissue was taken and protein was extracted according to the manufacturer’s instructions (StressMarq Biosciences, Canada). Extraction buffer was added to frozen tissue and homogenized properly. Subsequently, it was centrifuged at 1200 rpm for 10 min at 4 °C. The supernatant (tissue extract) was transferred to a fresh Eppendorf tube for further processing. Subsequently, the tissue lysate was diluted with diluent buffer and used for enzyme linked immune sorbent assay (ELISA). Similarly, the standards were prepared and ELISA was carried out as per instruction (StressMarq Biosciences, Canada). The observance was taken at 450 nm (SpectraMax, Canada). The assay was carried out for Hsp70 protein concentration in tissue extract.
Statistical analysis
All the experiments were replicated three times. Results were expressed as the means ± SEM. The differences between the various group means were determined by student’s t test using the SPSS, Version 16.0.1 software (SPSS, Inc., Chicago, IL, USA). Least squares analysis of variance was carried out to observe the breed and sex effect on Hsp concentration (Harvey 1990).
Gene expression levels were quantified with Cp (cycle threshold) values; Cp value is the number of PCR cycles required for the fluorescence signal to cross threshold line. Cp values are inversely proportional to the amount of target nucleic acid (the greater the Cp value the lower the amount of target nucleic acid in the sample). All analyses were performed on mean Cp values, which were calculated from the three sample replicates used in the real-time PCR. Fold-change in expression levels of the target genes (Hsp70) were presented using the 2–ΔΔCt method and by E-method (Livak and Schmittgen 2001).
Results
Hsp70 protein concentration in tissue extract of different organs
Hsp70 protein concentrations in tissue extracts of different organs were analyzed. Organs from 22 different individuals belonging to four breeds were collected, and the concentration of Hsp70 protein concentration was estimated by ELISA. Measurable concentrations of Hsp70 were detected in 36 samples. Hsp70 concentration was found to be significantly higher (P < 0.01) in liver, kidney and heart followed by brain, spleen, lungs and testis. Kidney, liver and heart had 1.5–2.0-fold higher Hsp70 concentrations than other organs (Fig. 1). The least squares means of Hsp70 concentration in tissue extract varied from 98.0 to 101 ng/ml in different breeds. Breed and sex had no significant effect (P > 0.05) on Hsp70 protein concentration in tissue extracted during peak heat stress periods.
Gene expression analysis in different tissues in response to heat stress
Individual animals belonging to four breeds (96) were phenotyped based on respiration rate and heart rate. The contrasting phenotypes were identified as HST and HSS, and 22 different individuals were slaughtered during peak heat periods and high THI (85.36–89.80). The differential mRNA level expression was analyzed for the Hsp70 gene in goat. Gene expression patterns of the Hsp70 gene in different organs indicated that the liver, spleen, brain and kidney exhibited 5.94, 4.96, 5.29 and 2.63-fold higher mRNA level expressions than the control (Table 1). Liver and brain tissues showed the highest gene expression levels as compared to kidney, spleen and heart. The Sirohi breed showed the highest level at 6.3-fold mRNA level as compared to the Jakhrana. In addition, the Sirohi showed a 4.2-fold and 1.97-fold higher mRNA level than the Jamunapari and Barbari goats, respectively (Table 2). The distribution of HSS and HST over the population was 41.82 and 26.08 %, respectively. The differential mRNA level expression between contrasting phenotypes indicated that HST individuals showed higher Hsp expression than HSS individuals. The differential mRNA level expression of HST individuals was 22.3, 22.1, 24.3 and 95.6-fold higher than HSS individuals in the Jamunapari, Barbari, Jakhrana and Sirohi breeds, respectively (Fig. 2).
Differential mRNA level expression in heat stress-susceptible and heat stress-tolerant phenotypes within breeds during peak heat stress period. The environmental conditions of the heat stress period were THI (85.36–89.80), temperature (45–49.4ºC), relative humidity (RH) (%) (14.33–27) and sunshine (hr) (8–12)
Discussion
It is necessary to analyze heat stress regulation and thermal tolerance in different environmental conditions at the cellular and tissue level. HSPs protect cells from the negative effects of heat stress by synthesizing HSP at the cellular level. In addition to their response to heat stress, HSPs are also synthesized by cells in response to a variety of stimuli, including oxidative, metabolic and chemical stress (Welch 1992; Morimoto et al. 1994; Airaksinen et al. 2003). It has been suggested that the expression of Hsp70 was significantly higher during the summer season as compared to the winter season in tropical region goats, which might play an important role in thermal stress tolerance against harsh environmental conditions (Dangi et al. 2012). Thermal stress induces differential gene expression and biochemical response at the cellular level. Individuals exposed to stress elicit HSP response in the cells of various organs. In the present study, Hsp70 concentration was higher in the liver and kidney. Higher expression of HSP 70 at the tissue level provides protection to cells during chronic heat stress (Latchman 2001). Similarly, Zulkifli et al. (2010) also reported a higher expression of Hsp70 in the heart and kidney of goats during transportation stress. The protective effect of Hsp70 in different organs such as the heart and kidney tissue has been established (Latchman 2001). Higher Hsp70 expression at the cellular level is a contributing factor for better meat quality in goat as Hsp70 provides protection to muscle glycogen content and thereby influences meat quality in ruminants (Zulkifli et al. 2008, 2010). Further study is necessary to establish the association of Hsp70 expression with meat quality.
It has been observed that the mean Hsp expression at the protein level did not vary significantly (P > 0.01) among the breeds indicating a differential inducible response to heat stress within breeds. However, the Hsp expression pattern at the mRNA level differed significantly (P < 0.01) among the breeds. Heat shock (41 °C) causes an increase in HSP synthesis and a decrease in protein synthesis. Collier et al. (2006) reported the direct effect of thermal stress on cellular growth and ductal branching of bovine mammary epithelial cells (BMECs) and showed a downregulation of genes associated with protein synthesis and cellular metabolism. Banerjee et al (2014) reported that Sirohi and Barbari showed an almost similar pattern of expression, but the expression level in Sirohi goats was observed to be relatively lower. Sirohi is a breed of arid regions and is highly adapted to heat stress whereas Barbari is a breed of semi-arid regions and is comparatively less well adapted to heat stress conditions. Similarly, the Sirohi breed exhibited a higher mRNA level of Hsp70 gene indicating that it was better at regulating heat stress compared to the other three breeds. Moreover, the four breeds were compared under similar climatic conditions, feeding patterns and housing conditions. Again, the variation in observation within the present study can be attributed to the use of a control gene for normalization (Derveaux et al. 2010).
The variation in Hsp70 gene expression has been positively correlated with thermotolerance in Drosophila melanogaster, Caenorhabditis elegans, rodents and humans (Hashmi et al. 1997). In farm animals, the association between differential gene expression pattern and thermal tolerance has been less studied. However, in the present study, it was observed that heat stress-tolerant (HST) individuals exhibited higher Hsp70 expression than heat stress-susceptible (HSS) individuals during peak heat stress periods. HSP genes are highly conserved between different species, but the HSP gene expression is breed specific and species specific. The species–specific difference in HSP70 isoforms are due to variation in thermal tolerance (Yamashita et al. 2004) and isoform expression may vary with regard to thermotolerance (Hightower et al. 1999). Stress tolerance is a complex mechanism. The mechanism of stress tolerance by HSPs has not been well understood; however, HSPs play major roles in processing of stress-denatured proteins (Mizzen and Welch 1988). HST phenotypes regulate heat stress in an efficient manner exhibiting higher Hsp70 at the cellular level. However, it is necessary to associate the stress phenotype with adaptation, fitness and production parameters during growth, lactation and dry periods.
Conclusion
HST individuals exhibited higher Hsp70 mRNA level expression than HSS individuals in different breeds. The Sirohi breed exhibited higher Hsp70 mRNA level expression than all the other breeds. The tissue-specific expression was observed during peak heat stress periods. The liver and kidney exhibited higher HSP concentrations than the other organs. The higher levels of Hsp70 mRNA expression during peak heat stress periods indicate that productivity will be affected by heat stress, and it is necessary to manage stress at cellular level. However, it is also necessary to associate the stress phenotype with adaptation, fitness, production parameters and feed consumption at different ages and different physiological stages.
References
Airaksinen S, Jokilehto T, Rabergh CMI, Nikinmaa M (2003) Heat- and cold-inducible regulation of HSP70 expression in zebrafish ZF4. Comp Biochem Physiol 36:275–282
Al-Tamimi HJ (2007) Thermoregulatory response of goat kids subjected to heat stress. Small Rumin Res 71:280–285
Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh GJM, Polley S, Mukherjee A, Das TK, De S (2014) Seasonal variation in expression pattern of genes under HSP70. Cell Stress Chaperones 19:401–408
Barbe MF, Tytell M, Gower DJ, Welch WJ (1998) Hyperthermia protects against light damage in the rat retina. Science 241:1817–1820
Basirico L, Morera P, Primi V, Lacetera N, Nardone A, Bernabucci U (2011) Cellular thermotolerance is associated with heat shock protein 70.1 genetic polymorphisms in Holstein lactating cows. Cell Stress Chaperones 16(4):441–448
Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A (2010) Metabolic and hormonal adaptations to heat stress in domesticated ruminants. Animal 4:1167–1183
Chirico WJ, Waters MG, Blobel G (1998) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810
Collier RJ, Stiening CM, Pollard BC, VanBaale MJ, Baumgard LH, Gentry PC, Coussens PM (2006) Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J Anim Sci 84(E Suppl):E1–E13
Dangi SS, Gupta M, Maurya D, Yadav VP, Panda RP, Singh G, Mohan NH, Bhure SK, Das BC, Bag S, Mahapatra R, Sharma GT, Sarkar M (2012) Expression profile of HSP genes during different seasons in goats (Capra hircus). Trop Anim Health Prod 44(8):1905–1912
Daugaard M, Rohde M, Jäättelä M (2007) The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett 581:3702–3710
Derveaux S, Vandesompele J, Hellemans J (2010) How to do successful gene expression analysis using real-time PCR. Methods 50:227–230
Devendra C, Devendra C, Imazumi E (1990) Comparative aspects of digestive physiology and nutrition in goats and sheep. In: Ruminant nutrition and physiology in Asia. Japan Society of Zootechnical Science Ed, Sendai, pp 45–60
Gallagher DS, Grosz MD, Womack JE, Skow LC (1993) Chromosomal localization of HSP70 genes in cattle. Mamm Genome 4:388–390
Garret AT, Goosen NG, Rehrer NG, Rehrer NG, Patterson MJ, Cotter JD (2009) Induction and decay of short-term heat acclimation. Eur J Appl Physiol 107:659–670
Gaughan JB, Bonner SL, Loxton I, Mader TL (2013) Effects of chronic heat stress on plasma concentration of secreted heat shock protein 70 in growing feedlot cattle. J Anim Sci 91(1):120–129
Grosz MD, Womack JE, Skow LC (1992) Syntenic conservation of HSP70 genes in cattle and humans. Genomics 14:863–868
Hansen PJ (2004) Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci 82:349–360
Harvey WR (1990) User’s guide for LSMLMW. PC-Version 2, mixed model least squares and maximum likelihood computer program, mimeograph. Ohio State University press, Columbus
Hashmi G, Hashmi S, Selvan S, Grewal P, Gaugler R (1997) Polymorphism in heat shock protein gene (Hsp70) in entomopathogenic nematodes (rhabditida). J Therm Biol 22:143–149
Hecker JG, McGarvey M (2011) Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones 16:119–131
Hecker JG, Sundram H, Zou S, Praestgaard A, Bavaria JE, Ramchandren S, McGarvey M (2008) Heat shock proteins HSP70 and HSP27 in the cerebral spinal fluid of patients undergoing thoracic aneurysm repair correlate with the probability of postoperative paralysis. Cell Stress Chaperones 13(4):435–446
Hightower LE, Norris CE, di Iorio PJ, Fielding E (1999) Heat shock responses of closely related species of tropical and desert fish. Integr Comp Biol 39:877–888
Horowitz M (2002) From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol Part A, Mol Intergr Physiol 131:475–483
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
King JM (1983) Livestock water needs in pastoral Africa in relation to climate and forage. Res report no. 7. Int. Lives Center Africa (ILCA), Addis Ababa
King YT, Lin CS, Lin JH, Lee WC (2002) Whole-body hyperthermia-induced thermo tolerance is associated with the induction of heat shock protein 70 in mice. J Exp Biol 205:273–278
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermo tolerance. J Appl Physiol 92:2177–2186
Lacetera N, Bernabucci U, Scalia D, Basiricò L, Morera P, Nardone A (2006) Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J Dairy Sci 89:4606–4612
Latchman DS (2001) Heat shock protein and cardiac protection. Cardiovasc Res 51:637–646
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−_ʌʌCT method. Methods 25:402–408
McDowell RE, Hooven NW, Camoens JK (1976) Effects of climate on performance of Holsteins in first lactation. J Dairy Sci 59:965–973
Mizzen L, Welch W (1988) Effects on protein synthesis activity and the regulation of heat shock protein 70 expression. J Cell Biol 106:1105–1116
Morimoto RI, Tissieres A, Georgopoulos C (1994) Progress and perspectives on the biology of heat shock proteins and molecular chaperones In: Morimoto, R.I., Tissieres, A, Georgopoulos, C. (Eds.), The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbour Laboratory Press, Cold Spring Harbour 1–30
Paula-Lopes FF, Chase CC Jr, Al-Katanani YM, Krininger CE 3rd, Rivera RM, Tekin S, Majewski AC, Ocon OM, Olson TA, Hansen PJ (2003) Genetic divergence in cellular resistance to heat shock in cattle: differences between breeds developed in temperate versus hot climates in responses of preimplantation embryos, reproductive tract tissues and lymphocytes to increased culture temperatures. Reproduction 125(2):285–294
Rout PK, Saxena VK, Khan BU, Roy R, Mandal A, Singh SK, Singh LB (2000) Characterization of Jamunapari goats in their home tract. Anim Genet Resource Inf 27:43–52
Silanikove N, Koluman N (2015) Impact of climate change on the dairy industry in temperate zones: predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin Res 123:27–34
Sonna LA, Fujita J, Gaffin SL, Lilly CM (1985) Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92(4):1725–1742
Welch WJ (1992) Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72:1063–1081
Wrenzycki C, Wells D, Herrmann D, Miller A, Oliver J, Tervit R, Niemann H (2001) Nuclear transfer protocol affects messenger RNA expression patterns in cloned bovine blastocysts. Biol Reprod 65:309–317
Yamashita M, Hirayoshi K, Nagata K (2004) Characterization of multiple members of the HSP70 family in platy fish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene 336:207–218
Zulkifi I, Liew PK, Israf DA, Omar AR, Hair-Bejo M (2003) Effect of early age feed restriction and thermal conditioning on heterophil/lymphocyte ratio, heat shock 70 and body temperature of male broiler chickens subjected to acute heat stress. J Therm Biol 28:217–222
Zulkifli I, Al-Aqil A, Omar AR, Sazili AQ, Rajion MA (2008) Housing system affects stress and fear reaction and meat quality of broiler chickens subjected to road transportation. In proceeding of XXIII world’s poultry congress. World’s Poultry Science Association, Odijk, The Netherlands 380
Zulkifli I, Norbaiyah B, Cheah YW, Soleimani AF, Sazli AQ, Goh YM, Rajion MA (2010) A note on heat shock protein 70 expression in goats subjected to road transportation hot, humid tropical conditions. Animal 4:973–976
Acknowledgments
The work was funded through project no. C4/C-30033(comp-IV) by the NAIP, Indian Council of Agricultural Research, New Delhi, India. The authors thank Helen Neumann of the Cell Stress & Chaperones editorial office for the English language help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institute Animal Ethics Committee (IAEC), Central Institute for Research on Goats (CIRG), Makhdoom, approved the experimental procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rout, P.K., Kaushik, R. & Ramachandran, N. Differential expression pattern of heat shock protein 70 gene in tissues and heat stress phenotypes in goats during peak heat stress period. Cell Stress and Chaperones 21, 645–651 (2016). https://doi.org/10.1007/s12192-016-0689-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0689-1