Abstract
The goal of this study was to evaluate the daily rhythmicity of the thermoregulatory responses of Morada Nova ewes that were raised in a semiarid environment. The experiment was conducted during the dry season. Data were collected from 5:00 a.m. to 4:00 a.m.. Samples were taken over the course of 8 days, with a 1-week interval between sampling periods. During each day that the data were collected, animals were measured once an hour for 24 h in an area directly exposed to solar radiation. The environment was characterized by measuring the following variables: air temperature (TA), relative humidity (RH), Black Globe Humidity Index (BGHI), radiant heat load (RHL), and wind speed (WS). Physiological variables that were measured included rectal temperature (RT, °C), respiratory rate (RR, breaths/min), surface temperature (ST, °C), and sweating rate (SR, g m2 h−1). We observed that RT, RR, and ST increased as environmental conditions became more stressful. Specifically, environmental conditions became more stressful as RHL, air temperature, and BGHI increased, while RH decreased. All physiological variables of the animals were strongly affected by the time of the day: environmental variables changed drastically between nighttime and noon. Physiological parameters increased sharply from the morning (7:00 a.m.–10:00 a.m.) until noon (11:00 a.m.–2:00 p.m.), except for sweating rate. After noon, these variables began to drop until nighttime (11:00 p.m.–6:00 am), and values of the main physiological indexes were stable during this period. The Morada Nova breed exhibited daily cyclic variations in thermoregulatory responses. Evaporative heat loss mechanisms were triggered during the most stressful times of the day. The first mechanism that animals used was panting, which was an immediate response to environmental heat stress. Cutaneous evaporation had a slower response mechanism to environmental heat stress. Homeothermy conditions were restored to the animals at approximately 5:00 p.m.; however, these findings confirm the importance of providing environmental protection during critical periods of the day, even for locally adapted breeds. These responses suggest that the use of thermal storage allowed the animals to achieve equilibrium with the environment and maintain a stable body temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The heat tolerance and adaptability of various breeds ensure their survival and performance in harsh environments. The Morada Nova, a locally adapted sheep breed, is an important genetic resource that is utilized in meat production systems in Brazil, especially in northeastern regions where small ruminant production is an important activity. This breed is highly prolific, has a fertility index, and has a robust maternal capacity, even under adverse weather conditions and low food availability.
Although it is considered a small breed, their adult size favors heat exchange, an important characteristic for animals raised in low-latitude regions, which reflects their high degree of adaptation to local weather conditions. For this reason, this breed is commonly used in the extensive production systems in Brazil’s semiarid region. According to Paim et al. (2013), a production system needs to characterize genetic resources so that the ability of the breed to adapt to environmental conditions can be ameliorated.
Despite the recognized heat tolerance of non-wooled sheep, the thermal environment is an important determinant of suitable management strategies. Therefore, more detailed studies are needed, especially in hot regions, as few studies have been conducted in such regions (McManus et al. 2011). Even animals with attributes that allow them to acclimate to stressful conditions might suffer under environmental pressures at certain times of the year, especially during the hottest hours of the day. A strategic solution for coping with these environmental challenges is to identify the most stressful time of year, the most stressful seasons, and the most stressful parts of day. Selection of better-adapted animals together with environmental management practices to reduce the total heat load on animals can optimize reproductive performance and production.
Circadian physiology addresses the temporal organization of vital processes over the course of a day. Circadian clocks allow animals to adapt their physiology and behavior in an anticipatory way to rhythmic changes in their environment (Delaunay and Laudet 2002). The biological clock controls circadian patterns of body temperature, metabolic rate, and state of arousal as well as the amplitude of other variables and functions (Brown and Schibler 1999). It combines functions in both the spatial and temporal dimensions. Findings in circadian physiology have implications for all other areas of knowledge and provide essential information about the temporal structure of biological cycles, such as thermoregulatory patterns.
Many organisms, including animals, have developmental processes that are affected by genetic inheritance and environmental factors. Circadian rhythmicity is generated endogenously, but it has been shown that different environmental inputs influence body temperature at different rates, creating their own pattern for a particular environment.
The loss of latent and sensible heat, through radiation, skin and respiratory changes in surface evaporation, and blood flow, contributes to homeostasis, which can be achieved, inter alia, by the maintenance of normal body temperature, normal concentrations of calorigenic and metabolic hormones, and standard hematological and biochemical values and references for the species. Moreover, the same hormones related to thermogenesis can act directly or synergistically with hormones linked to productive aspects, such as growth, fattening, and lactation. Façanha et al. (2010) realized that another factor that contributes to heat balance in these animals comprises outer cover characteristics, as they are directly connected to heat exchange by radiation and interfere in homeothermy. This phenomenon is especially the case in animals that are managed in low-latitude environments, due to their frequent exposure to high levels of solar radiation.
Our hypothesis is that Morada Nova, as a locally adapted hair breed from the Brazilian semiarid region, is resistant to heat stress and triggers efficiently the thermoregulatory mechanisms during the most stressful time of the day aiming to recover in few hours the homoeothermic patterns. However, studies concerning circadian physiology and thermoregulatory traits are scarce. This scarcity is concerning given the impact that daily rhythmicity under natural environmental conditions can have on biological cycles. For this reason, the goal of the present study was to evaluate the daily rhythmicity of the thermoregulatory responses of Morada Nova ewes that were raised in a semiarid environment under natural weather conditions.
Materials and methods
This study was approved by the Ethics Committee CEUA-UFERSA (number 23091003895/2014–71) and was conducted at the Center of Studies and Research in Small Ruminants at the Federal Rural University of Semiarid-UFERSA, Campus Mossoró-RN, with the geographic coordinates 5° 12′ south, 37° 19′ west. The climate in the region is tropical semiarid, with two seasons throughout the year: the rainy season from January to May and the dry season from June to December. The annual average temperature and humidity are 27.2 °C and 70%, respectively, with a mean annual rainfall of 414.7 mm (IDEMA 2003; INEMET 2009).
The experimental period was the dry season. Data were collected from 5:00 a.m. to 4:00 a.m., throughout the day. Samples were taken over 8 days, with a 1-week interval between sampling periods. On each day that the data were collected, the animals were evaluated once an hour over 24 h, totaling 1728 observations. For the statistical analysis, the contiguous hours were sorted into six groups according to similarities in environmental features.
Data for this study were collected from nine adult Red Morada Nova hair ewes, aged between 2 and 4 years, that were not lactating and not pregnant. According to Leite (2011), these sheep are a locally adapted hair breed and have coat characteristics that allow them to cope with environments with high levels of radiation. Some of the traits include less thick fur, low hair density, and short hair length. The animals were selected after clinical and laboratorial examinations confirmed that they were free of any infectious diseases.
During the sampling period, the animals were kept in native pasture, where they are directly exposed to solar radiation, similar to the management systems used in commercial farms located in the study region. However, because the native pasture during the dry season does not typically provide enough food, the animals receive a food supply composed of Cynodon dactylon hay and a concentrate mix composed by corn grain, soybean meal, mineral, and vitamin complex. The forage/concentrate ratio was 70:30. According to Gonzaga Neto et al. (2005), this ratio can provide 2.01 Mcal/day of metabolizable energy, which is compatible with the requirements of Morada Nova sheep.
The environment was characterized using the following meteorological variables: air temperature (TA), relative humidity (RH) (both measured with a portable digital thermohygrometer positioned approximately 0.5 m above the animals). Black globe temperature (Tbg) and wind speed (WS) were also measured with a digital anemometer and were recorded in a spreadsheet at the same time that they were collected. Measurements of Tbg and WS were important to estimate environmental comfort indexes, especially when animals are exposed to high levels of solar radiation. Thus, knowledge of daily WS patterns is important for identifying the least stressful period of the day, since the wind can alleviate heat stress (Façanha et al. 2008).
The Black Globe Humidity Index (BGHI) was calculated using the formula of Buffington et al. (1981), and radiant heat load (RHL) was calculated using the formula of Esmay (1969).
where
where
Physiological data that were collected included rectal temperature (RT, °C), which was measured with a digital thermometer (Omron Flex Temp Digital Thermometer, China) that was inserted into the animal’s rectum. The respiratory rate (RR, breaths/min) was recorded by counting the number of flank movements for 1 min. Body surface temperature (ST, °C) was measured using an infrared thermometer (Scan Temp ST-600, scale—60 a 500 °C, Incoterm®, Mult Temp Portatil) in three body regions: the neck, the flank, and the haunches. The average surface temperature was then calculated.
Sweating rate (SR, g m2 h−1) was estimated using the colorimetric method of Schleger and Turner (1965). However, to improve the precision of the measurements, we used the device developed by Pereira et al. (2010), which was built from two transparent acrylic plastic plates and provided better adhesion of the paper discs to the skin and so that lateral slide movements are minimized. Higher precision and a lower repeatability of SR measurements were achieved using the approach described above. This approach is also advantageous in that several animals can be simultaneously measured. In addition, measurements can be made a variety times in large-scale studies where animals are kept under field conditions. In cattle, the sacral area can be used for sampling because this part of the body does not interfere with their social interactions with other animals. However, in goats, Baker (1989) suggests that the rate of sweating can be measured securely on the side of the neck, a spot that gives an average value compared to other regions of the body. Thus, this approach is recommended in addition to excluding the extreme values of SR.
Statistical tests performed included multivariate analysis and correlations. To determine the physiological response of the animals throughout the day, principal component analysis (PCA) was used. We performed a distance-based permutational-repeated measure MANOVA (Anderson et al. 2008) to test the physiological response of the animals to environmental conditions throughout the day. Differences among hour groups were analyzed using posteriori pairwise comparisons with a t statistic. A similarity percentage (SIMPER) procedure based on Euclidean distance (Anderson et al. 2008) was used to identify the physiological variables that contributed most to differences among day hour groups (P < 0.05). Variable contributions were calculated as percentages of distance of each variable with respect to total Euclidean distance among day hour groups, since whole distance is the sum of distances of each variable. All statistical analyses were conducted using PRIMER 6 and PERMANOVA + (PRIMER-E Ltd. Plymouth, UK).
The statistical model used was the following:
where Y ijkl is the n-th observation of the physiological response to environmental conditions throughout the day, HG i represents the fixed effects of the i-th hour group (i = 1, 2, 3…6), S j represents the fixed effects of the j-th sampling (j = 1, 2, 3…8), A k represents the fixed effects of the k-th animal (k = 1, 2, 3…9), HG S ij represents the interaction effects between the i-th hour group and the j-th sampling, HG A ik represents the interaction effects between the hour group and animals, and ε ijkl is random error.
Results
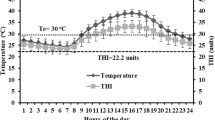
Average values for the environmental variables within each hour group are shown in Table 1. Environmental conditions changed significantly throughout the day. RHL, BGHI, and AT increased sharply from group 1 to group 3. Variables then decreased from group 4 to group 6 (Table 1). However, RH evolved inversely to these variables. Group 3 (11:00 a.m.–2:00 p.m.) showed the greatest TA (33.12 °C), RHL (747.14 W m−2), and BGHI (94.45) and a low RH (43.62%). Therefore, this period could be considered the hottest and most stressful for the animals in this study.
All of the physiological variables of the animals considered were affected by the time of the day: environmental variables changed from nighttime to noon. All parameter values, except sweat rate, increased sharply from morning (7:00 a.m.–10:00 a.m.) until noon (11:00 a.m.−2:00 p.m.). After noon, these variables began to decrease until nighttime (11:00 p.m.–6:00 a.m.), and values of physiological indexes were stable during this period (Fig. 1a). In axis 1, rectal temperature, respiratory rate, and surface temperature increased as environmental conditions became more stressful (RHL, air temperature, and BGHI increased whereas humidity decreased) (Fig. 1b). Axis 2 displays an inverse relationship between sweating rate and wind speed, where the animals tended to sweat more when the wind speed was lower. Sweating rate and relative humidity were not correlated; however, axis 2 explains only 25.1% of the variation in the data (Fig. 1b).
PCA plot based on physiological and environment variables of animals. a Average and standard error of PCA 1 and PCA 2 scores for each hour group (1: 3:00–6:00; 2: 7:00–10:00; 3: 11:00–14:00; 4: 15:00–18:00; 5: 19:00–22:00; 6: 23:00–2:00). b Scores of each variable included in PCA analysis (RR respiratory rate, RT rectal temperature, ST surface temperature, SR sweating rate, RH relative humidity; Tbg Black globe temperature, TA air temperature, WS wind speed). Total explained variance is 93.8% (axis I and axis II explained variance are 68.7 and 25.1%, respectively)
Sweating rate was not directly related to environmental conditions but was affected by the time of the day. Despite this result, the range of values for sweating rate was greater during the nighttime than in the daytime. Variability in sweating rate tended to decrease as environmental conditions were more stressful, and the animals used respiratory evaporation more intensively, thereby increasing respiratory rate (Fig. 1). Sweating rate was highly variable during the nighttime (hour groups 1, 5, and 6), but it was more homogeneous among animals as environmental conditions became more stressful (group 3).
This finding was confirmed by the results of the ANOVA analysis (Table 2). Physiological responses changed sharply until noon (11:00 a.m.–2:00 p.m.). Most of the change between the first and second group was due to increases in rectal temperature (27.35%) and surface temperature (33.86%) and decreases in respiratory rate (16.60%) (Table 2). This response was exceptionally homogeneous among animals (squared distance by standard deviation of >1). From the second to the third hour group, variation was primarily explained by respiratory rate (38.38%) and rectal temperature (27.66%). This period was the period when the animals more severely felt the effects of heat stress. From the third to the fourth group, there was a change in this trend as RR decreased at the same rate (35.61%) as ST (32.67%). Therefore, animals began to normalize their physiological variables in response to more comfortable conditions. From the fourth hour group onward, most of the differences among hour groups were related to sweat rate. Although sweat rate played an important role in this change, the response of the animals was uneven (squared distance by standard deviation of <1). Therefore, although respiratory rate, surface temperature, and rectal temperature played a major role in the response of the animals throughout the day, sweat rate explained more than 80% of the differences among animals in the nighttime, but this response was uneven compared to the daytime.
It is possible that the Morada Nova sheep uses a thermal storage mechanism to retain heat from the hottest times of the day as a way to balance the warm environment and release heat during the night or early morning. The interpretation that sweating provides a heat dissipation mechanism is based on the delayed response to increased environmental stress. With respect to the homogeneity of the animals’ responses, respiratory rate seemed to be used most by the animals during the day. However, sweat rate and rectal temperature values were more uneven, suggesting that these variables are more individually determined.
The Pearson correlation showed that there was a strong relationship between most of the studied variables that describe physiological responses to environmental conditions. Animals increased rectal temperature and triggered heat dissipation mechanisms, especially respiratory rate, as a response to increasing TA, BGHI, and RHL (Table 3). Sweating rate showed a significant negative correlation with RR, RT, and ST (−0.201, −0.087, and −0.076, respectively), although these relationships were weak. Sweating rate was not significantly correlated with environmental variables.
Respiratory rate was negatively correlated with sweating rates, rectal temperature, and surface temperature, indicating that when respiratory thermolysis is insufficient to compensate for body warming, the animals turn to cutaneous evaporative thermolysis, which is usually observed in animals in high radiation regions. BGHI was not correlated with any mechanism of evaporative thermolysis (RR and SR), probably because of the low humidity registered during sampling, which was not enough to inhibit the latent heat flux from the animal to the environment (Table 3).
Analysis of the relationship between rectal temperature, respiratory rate, and body surface temperature with respect to RHL (Fig. 2) showed that, between 10:00 a.m. and 2:00 p.m., the highest RHL (related to raising solar radiation) increased RT, ST, and RR. Although this is a well-known response, animals triggered physiological mechanisms for dissipating received heat.
Relationship between radiant heat load and thermoregulatory characteristics of Morada Nova ewes during a 24-h period, under natural environmental conditions in a semiarid region. RR respiratory rate (breaths/min), RT rectal temperature (°C), ST skin temperature (°C), RHL radiant heat load (W/m2); SR sweating rate (g/m2/h)
Figure 2 shows that radiation is low until 5:00 a.m. and then it increases, reaching its peak between 12:00 a.m. and 1:00 p.m., after which it decreases to the same low values registered during the nighttime.
These three variables behaved in the same way to a RHL rise, as increasing ST and then RT triggers RR in order to dissipate surplus heat.
However, the sweating rate did not vary with RHL, and it was uneven throughout the day. Therefore, sweating rate was not the mechanism that was responsible for most of the heat loss during the daytime. However, sweating was used between night and dawn (Fig. 2). Therefore, respiratory rate was the mechanism most used during the period of highest heat stress by the animals in this study. Although Morada Nova is a type of hair sheep, it behaves as a wool breed; therefore, it used respiratory thermolysis more intensively than cutaneous evaporation.
Changes in RR and RT throughout the day show that these variables are strongly positively correlated. When RT begins to increase due to rising environmental temperatures, RR nearly always increases almost instantly. During the hottest hours of the day (10:00 a.m. to 2:00 p.m.), RT and RR values were high. At lower ambient temperatures, increases in RR rapidly decreased, showing that RR responds quickly, while RT decreases gradually until they were balanced in the evening (Fig. 3).
Relationship between body temperatures and evaporative thermoregulatory mechanisms of Morada Nova ewes during a 24-h period, under natural environmental conditions in a semiarid region. RR respiratory rate (breaths/min), RT rectal temperature (°C), ST skin temperature (°C), SR sweating rate (g/m2/h)
Changes in RT, ST, and RR relative to sweating rate throughout the day were similar. Sweating was used by the animals throughout the day but was not very efficient at dissipating heat during the hottest hours that caused the greatest thermal discomfort for the animals. During the hottest hours, the animals maintained higher ST, RT, and RR values than sweating rate values. At night, animals tended to decrease ST, RT, and RR. During the day, animals maintained a constant, high sweating rate; however, higher values of these variables were observed during the night and at dawn. The most perceptible response of the animals to heat stress was elevated RT and ST and the intense use of RR during the most stressful hours.
Discussion
The effect of climate on animals occurs mainly through the influence of air temperature, solar radiation, and relative humidity combined with temperature. This mainly impacts the organic functions involved in maintaining homeothermy. At higher ambient temperatures, when the thermal gradient between the animal and the medium decreases, the animals often found it more difficult to maintain body temperature at normal levels. Although animals can adapt to hot weather, these adaptive responses can be harmful to production and reproductive performance (Kumar and De 2013).
Tropical regions are characterized by high levels of solar radiation and temperature, which are known to negatively affect livestock production (McManus et al. 2009; Naqvi and Sejian 2010). A ruminant’s ability to regulate body temperature depends on species and breed identity (Bernabucci et al. 2010).
According to the National Weather Service—USA, BGHI values of up to 74 indicate comfortable conditions, 74 to 78 indicate alert conditions, 79 to 84 indicate dangerous conditions, and 84 and above indicate emergency conditions. As air temperature increases throughout the day, indexes of thermal comfort for the animals also increased. Animals in group 3 (11:00 a.m. to 2:00 p.m.) certainly felt the effects of heat stress more severely as, according to Baêta and Souza (2010), the thermal comfort zone for sheep is between 20 and 30 °C, and the temperature during this time slot exceeded this value; therefore, this range of hours was considered more critical to the environmental conditions. This finding was supported by the high BGHI values (94.45 ± 00:53) and RHL values (747.14 ± 8:35) within the same time group (Table 1).
In the present study, an inverse relationship was observed between sweating rate and wind speed, where the animals tended to sweat more when the wind speed was lower. According to Silva et al. (2007), wind speed mitigates the adverse effects of high temperatures, and high humidity reduces the potential for skin and respiratory evaporation by the animal (Fig. 1b).
Santos et al. (2011) found values similar to the present study for BGHI and RHL between 1:00 p.m. and 3:00 p.m., BGHI (86.2 and 88.8) and RHL (754.2 and 810.1 W m−2), respectively, and thus considered this time period to be the most stressful for heat. In a hot environment, efficient heat loss is essential for maintaining homeothermy (Bligh 1998). An increase in core temperature is the main factor that influences heat loss by evaporation (Finch 1986; Hahn et al. 1997). When there is an increase in air temperature, RT increases, triggering an increase in RR, which is the first visible symptom of an animal’s response to heat stress (Fig. 3). Based on this assumption, the present study shows that respiration rate appears to be the way in which animals dissipate heat during the day. An increase in RR increases heat loss through the respiratory tract and contribute to a reduction in body temperature. Similar results were also found by Gomes et al. (2008) and Perissinotto et al. (2009), who observed RR increases in an attempt to dissipate heat and maintain homeothermy. This study confirmed that respiratory evaporation is a superior heat loss mechanism in sheep and that higher respiratory rates tend to closely follow the loss of heat by evaporation (Marai et al. 2007). These findings support previous studies of other sheep breeds (Marai et al. 2009; McManus et al. 2009).
The magnitude of evaporative heat loss is an indicator of the degree of discomfort of the animal experiencing thermal stress; however, the anatomical and physiological characteristics of species or breeds can lead to different levels of thermostability (Bligh 1998; Gatenby Ruth 1986; Gebremedhin et al. 2008). At high temperatures, evaporation becomes the major pathway for thermal energy dissipation in animals (Finch 1985; Gebremedhin et al. 1981), which occurs in the respiratory tract by increased respiratory rates (Stevens 1981; Silva et al. 2002; Maia et al. 2005a) and on the epidermal surface by sweating (Mclean 1963; Taneja 1958; Silva and Staling 2003; Maia et al. 2005b). Studies have shown that, in sheep with wool, transpiration was a slower response is an important means of heat loss (Silva et al. 2003). In contrast, in hair sheep, respiration is important (McManus et al. 2011).
According to Silanikove (2000), the rate of respiration can quantify the severity of heat stress in ruminants, with an RR of 40–60, 60–80, and 80–120 mov/min representing low stress, medium-high stress, and high stress, respectively, and over 200 mov/min, representing severe stress in sheep. In Table 2, periods 2, 3, and 4, respectively, were when the animals experienced the highest degree of thermal discomfort, as shown by the high internal and surface temperatures. Thus, there was a greater need for evaporative heat dissipation to be triggered. In several studies (Cezar et al. 2004; Cordão et al. 2010), animals proved to be under severe stress or in danger in the afternoon.
Alamer and Al-Hozab (2004) indicated that respiration rate can be used as a heat stress indicator and to estimate the adverse effects of environmental temperatures. Respiratory rate is an efficient and immediate mechanism for responding to high radiation, as was observed in the animal responses in groups 2, 3, and 4 in this study (Table 2). When air humidity was lower than at other times of day, evaporative losses were facilitated. Silva and Starling (2003) concluded that Corriedale sheep used respiratory evaporation, which is a functionally appropriate mechanism for intense conditions over short periods. Our findings are consistent with the results of Silva and Starling (2003). Although the hair cover of the Morada Nova breed provided less thermal insulation, the animals followed the typical pattern of their species since they used panting as the principal mechanism of thermolysis.
However, Silva and Starling (2003) warned that continued elevation of RR for long periods of time may cause a reduction in blood pressure as well as substantial increases in heat stored in tissues due to the increased work of the respiratory muscles. Sweating was also an efficient method for the animals to maintain homeothermy in this study, as the amounts of sweating recorded in this study were higher than those reported by Costa et al. (2014), Jiang et al. (2005), and Finch (1985). In addition, the ability to sweat can be considered a great advantage and potentially adaptive in sheep.
Based on the RT and RR (Fig. 3), it can be concluded that the hours of greatest heat stress for the animals were between 11:00 a.m. and 2:00 p.m. (group 3). We also observed an increase in RT followed by RR in an attempt to dissipate heat. A similar result was also reported by Santos et al. (2011).
The elevation of RR indicates that extra energy is expended by the animals to maintain body temperature, but the animals in this study demonstrated a superb ability to control body temperature at the end of the day. As air temperature decreased, the animals were able to reduce their physiological rhythm, even stabilizing and reaching a balance again.
Luz et al. (2014) studied sheep thermoregulatory characteristics in the dry and rainy seasons, using the method of Schleger and Turner (1965) to quantify sweating rate. Their study did not detect a significant difference in the sweating rate of animals during both periods. The values found by Luz et al. (2014) were all below those found in this study (Table 2) in all periods. The direct exposure to the sun of the animals in this study is probably responsible for the higher sweating rate values. Costa et al. (2014) found that the loss of latent heat through dermal average evaporation was significantly greater in animals exposed to the sun than animals in the shade. These differences are certainly produced by direct solar radiation, which can cause an acceleration in evaporation from the skin surface, suggesting there is a positive relationship between skin evaporation and solar radiation.
Some studies that have been conducted with hair sheep under Brazilian semiarid environmental conditions have reported large differences in SR among times of data collection and sun and shade exposure (Costa et al. 2014; Souza Junior et al. 2008). Nevertheless, in the present work, although the difference in SR during the daytime and the nighttime was statistically significant, it was not large relative to the difference observed in RR. For example, when the environmental factors represented the peak of heat stress (approximately 2:00 p.m.), RR was increased nearly 556.2% from the nighttime to the daytime. On the other hand, SR increased by only 18.03% during the same period (Table 2). This behavior can be reinforced by the particular pattern of sheep, which is considered a panting species (Collier and Gebremdhin 2015). The animals recovered the homeothermy conditions at approximately 5:00 p.m. (Fig. 2).
These findings suggest that Morada Nova ewes used RR more intensively during the daytime, when they were directly exposed to short wave solar radiation and, through this mechanism, were able to reach homeothermy. The increase of respiratory thermolysis allowed a substantial amount of heat to be lost, and, possibly for that reason, the animals did not sweat intensively during the daytime. In the case of RR, the maximum value was 6.6 times higher than the minimum value, whereas the maximum SR was only 1.2 times higher than the minimum value. This finding demonstrates the relative importance of each mechanism in maintaining homeothermy. On the other hand, the major environmental trigger of RR was absent at night when radiation was only short wave. Thus, the animals used a low proportion of cutaneous thermolysis to dissipate heat that was stored during the day. This observation can explain why the sweating rate was higher during the night. The low range of variation in SR explains its low correlation with other environmental variables (Table 3). These findings are consistent with those of Dmi’el and Robertsshaw (1983), who observed that the onset of sweating occurs later than panting when animals are exposed to direct solar radiation. These observations have an important adaptive value because large-scale sweating may have additional deleterious effects, such as alterations in fluid balance, which may lead to intense dehydration and losses of electrolytes (Olsson and Dahlborn 1989). Nevertheless, prolonged increases in RR can disturb acid-base equilibrium; however, this disturbance can be avoided by providing shade on the pasture during the more stressful times of day, which, in the present study, occurred from 11:00 a.m. to 2:00 p.m. (Table 1), when the highest mean RR was recorded.
However, in this study, the highest sweating rate was observed at night and cutaneous evaporation occurred with greater shifts in intensity between five and one, respectively (Table 2), suggesting that the use of thermal storage acts as a balancing mechanism between the animals and the environment. The findings of this study are consistent with those of Bligh (1961) and Robertshaw (1968). Bligh (1961) and Robertshaw (1968) showed that goats and sheep are not able to sustain a continuous flow of fluid from their sweat glands and thus discharge the glandular content briefly and synchronously from time to time. However, the rate of a particular process may vary to the extent necessary to produce a continuous discharge when the animals are exposed to heat. Mader et al. (2010) have shown that animals can accumulate heat during the hottest hours of the day (an increase in body temperature), and this heat is then dissipated when the temperature decreases.
Conclusions
The Morada Nova breed exhibits daily cyclic variations in thermoregulatory responses, which trigger mechanisms of evaporative heat loss during the most stressful times of day. The first mechanism that the animals used was panting, which was an immediate response to environmental heat stress whereas cutaneous evaporation was a slower response mechanism that was only triggered after substantial exposure to environmental heat stress. The animals recovered homeothermic conditions at approximately 5:00 p.m.; however, these findings confirm the importance of providing environmental protection during critical periods of the day, even for locally adapted breeds. These responses suggest that the use of thermal storage allows the animals to achieve equilibrium with the environment and maintain a stable body temperature.
References
Alamer M, Al-Hozab A (2004) Effect of water deprivation and season on feed intake, body weight and thermoregulation in Awassi and Najdi sheep breeds in Saudi Arabia. J Arid Environ 59:71–84
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E Ltd, Plymouth
Baêta FC, Souza CF (2010) Ambiência em edificações rurais: conforto animal, 2.ed edn. UFV, Viçosa 269
Baker MA (1989) Effects of dehydration and rehydration on thermoregulatory sweating in goats. J Physiol 417:421–435
Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A (2010) Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4:1167–1183
Bligh J (1961) The synchronous discharge of apocrine sweat glands of the Welsh Mountain sheep. Nat Lond 189:582–583
Bligh J (1998) Mammalian homeothermy: an integrative thesis. J Therm Biol 23:143–258
Brown SA, Schibler U (1999) The ins and outs of circadian timekeeping. Curr Opin Genet Dev 9:588–594
Buffington DE, Collazo-Arocho A, Canton GH, Pitt D, Thatcher WW, Collier RJ (1981) Black globe-humidity index (BGHI) as comfort equation for dairy cows. Trans ASABE 24:711–714
Cezar MF, Souza BB, Souza WH, Pimenta Filho EC, Tavares GP, Medeiros GX (2004) Avaliação de parâmetros fisiológicos de ovinos Dorper, Santa Inês e seus mestiços perante condições climáticas do trópico semi-árido nordestino. Ciênc Agrotec 28:614–620
Collier RJ, Gebremdhin (2015) Thermal biology of domestic animals. Annu Rev Anim Biosci 3:513–532
Cordão MA, Souza BB, Pereira GM, Bakke OA, Silva AMA, Lopes JJ (2010) Respostas fisiológicas de cordeiros Santa Inês em confinamento à dieta e ao ambiente físico no trópico semiárido. Agropecuária Científica no Semi-Árido 6:47–51
Costa CCM, Maia ASC, Fontenele Neto JD, Oliveira SEO, Queiroz JPAF (2014) Latent heat loss and sweat gland histology of male goats in an equatorial semi-arid environment. Int J Biometeorol. doi:10.1007/s00484-013-0642 2
Delaunay F, Laudet V (2002) Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet 18:595–597
Dmi’el R, Robertsshaw D (1983) The control of panting and sweating in the black Bedouin goat: a comparison of two modes of imposing a heat load. Physiol Zool 56:404–411
Esmay ML (1969) Principles of animal environment, 2ed edn. AVI, Wastport 325
Façanha DAE, Maia ASC, Silva RG, Vasconcelos AM, Lima PO, Guilhermino MM (2008) Variação anual de hormônios tireoideanos e características termorreguladoras de vacas leiteiras em ambiente quente. R Bras Zootec 37:538–545
Façanha DAE, Silva RG, Maia ASC, Guilhermino MM, Vasconcelos AM (2010) Variação Anual de características morfológicas e da temperatura de superficie do pelame de vacas da raça Holandesa em ambiente semiárido. R Bras Zootec 39:837–844
Finch VA (1985) Comparison of non-evaporative heat transfer in different cattle breeds. Aust J Agric Res 36:497–508
Finch VA (1986) Body temperature in beef cattle: its control and relevance to production in the tropics. J Anim Sci 62:531–542
Gatenby Ruth M (1986) Exponential relation between sweat rate and skin temperature in hot climates. J Agric Sci 106:175–183
Gebremedhin KG, Cramer CO, Porter WP (1981) Predictions and measurements of heat production and food and water requirements of Holstein calves in different environments. Trans ASAE 3:715–720
Gebremedhin KG, Hillman PE, Lee CN, Collier RJ, Willard ST, Arthington JD, Brown-Brandl TM (2008) Sweating rates of dairy cows and beef heifers in hot conditions. Trans ASABE 51(6):2167–2178. doi:10.13031/2013.25397
Gomes CAV, Furtado DA, Medeiros NA, Silva DS, Pimenta Filho EC, Lima Júnior V (2008) Efeito do ambiente térmico e níveis de suplementação nos parâmetros fisiológicos de caprinos Moxotó. R Bras Eng Agríc Ambiental 12:213–219
Gonzaga Neto S, Silva Sobrinho AG, Resende KT, Zeola NMBL, Silva AMA, Marques CAT, Leão AG (2005) Body composition and nutritional requeriments of protein and energy for Morada Nova lambs. R Bras de Zootec 34:2446–2456 supl.
Hahn GL, Parkhurst AM, Gaughan JB (1997) Cattle respiration rate as a function of ambient temperature. ASAE Paper no. MC 97–121. ASAE St. Joseph, Mich
IDEMA 2003 Instituto de Defesa do Meio Ambiente
INEMET 2009 Instituto Nacional de Meteorologia
Jiang M, Gebremedhin KG, Albright LD (2005) Simulation of skin temperature and sensible and latent heat losses through fur layers. Tran ASAE 48(2):767–775. doi:10.13031/2013.18319
Kumar D, De K (2013) Extreme climatic variables affecting male reproduction in sheep. In: Sahoo A, Kumar D, SMK N (eds) Climate resilient small ruminant production. National Initiative on Climate Resilient Agriculture (NICRA). Central Sheep and Wool Research Institute, Izatnagar, pp 1–106
Leite JHGM (2011) Caracterização de atributos adaptativos de ovinos da raça Morada Nova. Mossoro:UFERSA. 93 p, Dissertação de Mestrado
Luz CSM, Fonseca WJL, Barros Junior CP, Terto E, Sousa GG, Amorim RB, Silva LA, Lima LA, Sousa Júnior SC, Santos KR (2014) Characteristics estimates of thermoregulatory sheep in rainy and dry seasons created in the region Gurguéia Valley, south of the state of Piaui. Acta Veterinaria Brasilica 8:19–24
Mader TL, Johnson LJ, Gaughan JB (2010) Components of the comprehensive climate index. J Anim Sci. doi:10.2527/jas.2009-2586
Maia ASC, Silva RG, Battiston CM (2005a) Respiratory heat loss of Holstein cows in a tropical environment. Int J Biometeorol 49:332–336. doi:10.1007/s00484-004-0244-0
Maia ASC, Silva RG, Battiston CM (2005b) Sensible and latent heat loss from body surface of Holstein cows in a tropical environment. Int J Biometeorol (in press) 50:17–22. doi:10.1007/s00484-005-0267-1
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep—a review. Small Rumin Res 71:1–12
Marai IFM, El-Darawany AA, Abou-Fandoud EI, Abdel-Hafez MAM (2009) Reproductive and physiological traits of Egyptian Suffolk rams as affected by selenium dietary supplementation during the sub-tropical environment of Egypt. Livest Res Rural Dev 21:1–12
McLean JA (1963) The partition of insensible losses of body weight in heat from cattle under various climatic conditions. J Physiol 167:427–447
McManus C, Paludo GR, Louvandini H, Gugel R, Sasaki LCB, Paiva SR (2009) Heat tolerance in Brazilian sheep: physiological and blood parameters. Trop Anim Health Prod 41:95–101
McManus C, Louvandini H, Gugel R, Sasaki LCB, Bianchini E, Bernal FEM, Paiva SR, Paim TP (2011) Skin and coat traits in sheep in Brazil and their relation with heat tolerance. Trop Anim Health Prod 43:121–126. doi:10.1007/s11250-010-9663-6
Naqvi SMK, Sejian V (2010) Physiological adaptation of sheep to hot environmental conditions with special reference to climate change. In: Karim SA, Joshi A, Sankhyan SK, Shinde AK, Shakyawar DB, Naqvi SMK, Tripathi BN (eds) Climate change and stress management: sheep and goat production, 1st edn. Satish, Delhi, pp 259–282
Olsson K, Dahlborn K (1989) Fluid balance during heat stress in lactation goats. Q J Exp Physiol 74:645–659
Paim TP, Borges BA, Lima PMT, Gomes EF, Dallago BSL, Fadel R, Menezes AM, Louvandini H, Canozzi MEA, Barcellos JOJ, McManus C (2013) Thermographic evaluation of climatic conditions on lambs from different genetic groups. Int J Biometeorol 57:59–66
Pereira AMF, Alves A, Infante P, Titto EAL, Baccari F Jr, Almeida JAA (2010) A device to improve the Schleger and Turner method for sweating rate measurements. Int J Biometeorol 54:37–43
Perissinotto M, Moura DJ, Cruz VF, Souza SRL, Lima KAO, Mendes AS (2009) Conforto térmico de bovinos leiteiros confinados em clima subtropical e mediterrâneo pela análise de parâmetros fisiológicos utilizando a teoria dos conjuntos fuzzy. Ciência Rural 39:1492–1498
Robertshaw D (1968) The pattern and control of sweating in the sheep and goat. J. Physiol Lond 198(3):531–539
Santos MM, Azevedo M, Costa LAB, Silva Filho FP, Modesto EC, Lana AMQ (2011) Comportamento de ovinos da raça Santa Inês, de diferentes pelagens, em pastejo. Acta Scientiarum. Anim Sci 33:287–294
Schleger AV, Turner HG (1965) Sweating rates of cattle in the field and their reaction to diurnal and seasonal changes. Aust J Agric Res, East Melbourne 16:92–106
Silanikove N (2000) Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest Prod Sci 67:1–18
Silva RG, Starling JMC (2003) Evaporação Cutânea e Respiratória em Ovinos sob Altas Temperaturas Ambientes. R Bras de Zootec 32(Supl. 2):1956–1961
Silva RG, LaScala N Jr, Lima Filho AE, Catharin MC (2002) Respiratory heat loss in the sheep: a comprehensive model. Int J Biometeorol 46:136–140
Silva RG, Scala Junior NLA, Tonhati H (2003) Radiative properties of the skin and haircoat of cattle and other animals. Trans ASABE 46:913–918
Silva RG, Façanha-Morais DAE, Guilhermino MM (2007) Evaluation of thermal stress indexes for dairy cows in tropical regions. R Bras de Zootec 36(supl):1192–1198
Souza Junior SC, Façanha DAE, Vasconceos AM, Nery KM, Morais JH, Guilhermino MM (2008) Características termorreguladoras de caprinos, ovinos e bovinos em diferentes épocas do ano em região Semi-Árida. Rev Cient de Prod Anim 10:127–137
Stevens DG (1981) A model of respiratory vapor loss in Holstein dairy cattle. Trans ASABE 24:151–158
Taneja GC (1958) Cutaneous evaporative losses in calves and its relationship with respiratory evaporative loss and skin and rectal temperatures. J Agric Sci 50:73–85
Acknowledgements
The authors acknowledge EMBRAPA—Goat and Sheep and Capes. Luis Alberto Bermejo Asensio was funded by the Ciência sem Fronteiras Program under Special Visiting Researcher project n° 88881030352/2013-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee CEUA-UFERSA (number 23091003895/2014–71) and was conducted at the Center of Studies and Research in Small Ruminants at the Federal Rural University of Semiarid-UFERSA, Campus Mossoró-RN, with the geographic coordinates 5° 12′ South, 37° 19′ West.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
da Silva, W.E., Leite, J.H.G.M., de Sousa, J.E.R. et al. Daily rhythmicity of the thermoregulatory responses of locally adapted Brazilian sheep in a semiarid environment. Int J Biometeorol 61, 1221–1231 (2017). https://doi.org/10.1007/s00484-016-1300-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-016-1300-2