Abstract
The aim of this study was to evaluate the effect of seasonal heat stress (HS) on productive performance, physiological responses, metabolism, and hematological profile of hair breed male lambs finished in feedlot. Twenty Dorper × Katahdin male lambs (body weight = 34.6 ± 1.4 kg and age = 4.5 months) were housed in individual pens and exposed to environmental conditions of summer HS (n = 10, temperature = 28.3 ± 4.0 °C and THI = 77.2 ± 5.4 units) or winter thermoneutrality (n = 10, temperature = 19.2 ± 2.6 °C and THI = 64.0 ± 3.0 units). Each season, a 30-day feeding test was conducted to measure study variables. Compared to thermoneutral lambs, heat-stressed lambs had lower (P < 0.01) growth rate and feed efficiency without changing dry matter intake. Heat-stressed lambs presented higher (P < 0.01) rectal temperature and respiratory rate through the daytime than termoneutral lambs. On most sampling days, summer HS caused lower (P < 0.01) serum concentrations of glucose, cholesterol, total protein, urea, potassium, and thyroid hormones, but higher (P < 0.01) serum triglyceride and chlorine values. Overall serum concentrations of cortisol and insulin were unaffected by summer HS. The blood of heat-stressed lambs showed lower (P ≤ 0.03) erythrocyte and platelet counts, hemoglobin, and hematocrit, but higher (P ≤ 0.03) erythrocyte size and leucocyte count than the blood of thermoneutral lambs. In conclusion, hair breed male lambs in response to chronic conditions of summer HS had slow growth but avoided hyperthermia due to the activation of physiological, metabolic and endocrine adjustments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of hair breed lambs is an alternative to other ruminant sources to generate protein from animal origin in arid and semi-arid regions, since these breeds have high adaptability to hot climates and the ability to consume native vegetation (Singh et al. 2016). Under heat stress (HS) conditions, the hair lambs activate different physiological and metabolic adjustments to restore their thermal balance (Macías-Cruz et al. 2018a), being evident the redistribution of blood flow to increase body heat losses across cutaneous radiation and respiratory tract (Macías-Cruz et al. 2016, 2018b). Also, they accomplish homeothermy by increasing water intake and maintaining an adequate body water balance (Al-Dawood 2017). However, the activation of these thermoregulatory mechanisms decrease growth speed and feed efficiency in heat-stressed hair lambs because they maintain their dry matter intake (DMI) with increased energy requirements for maintenance due to thermoregulation (Macías-Cruz et al. 2013; Vicente-Pérez et al. 2020). Despite this, HS did not cause a drastic drop in the average daily gain (ADG; 28%) of these lambs during the feedlot phase, so some studies suggest that they could activate some metabolic or hematologic mechanisms to efficiently transform the available energy for growth into body mass during the hot season in arid regions (Macías-Cruz et al. 2013, 2020). However, these mechanisms have not been defined so far.
Previous studies in Afshari sheep, a hair breed adapted to HS, reported a reduction in ADG without changing DMI in response to moderate HS in fattening lambs (Mahjoubi et al. 2014); however, under severe HS, they decreased ADG due to a reduction in DMI (Mahjoubi et al. 2015). The authors indicated that HS modified the postabsorptive metabolism of theses lambs, specifically caused a metabolic ambient of hyperinsulinemia that prevented fat tissue catabolism, promoted lipid anabolism, and increased the availability cellular glucose. Additionally, it has been documented that the hyperinsulinemia promotes certain resistance in catabolic hormones (i.e., cortisol and epinephrine), which explains its antilipolytic activity (Baumgard and Rhoads 2013). Therefore, a metabolic scenario of hyperinsulinemia could explain the fact that hair breed lambs continue gaining body weight (BW) under HS. Note that some studies have suggested that heat-stressed hair lambs present high circulating serum concentration of insulin because they decrease serum metabolites concentrations associated with the energy metabolism (Macías-Cruz et al. 2016) and increase internal fat deposition (Macías-Cruz et al. 2020); however, it is necessary to demonstrate the presence of this adaptive mechanism in them.
It should be mentioned that blood cells play an important role in the physiological and metabolic adjustments experiencing sheep under HS (Al-Dawood 2017). Thus, leukocytes are necessary for a correct immune system function, while erythrocytes and hemoglobin are required to supply sufficient oxygen in the different body tissues of sheep subjected to HS (Singh et al. 2016). Correa et al. (2012) reported positive relationship among physiological variables with some hematological parameters such as erythrocytes count, hematocrit, and hemoglobin in heat-stressed hair sheep. Note that thermoregulatory mechanisms associated with the hematological profile have not been described for feedlot hair lambs under HS conditions.
According to the aforementioned, it was hypothesized that hair male lambs grow moderately under summer HS because they adjust their metabolism to maintain homeothermy and to prevent catabolism of body tissues. Therefore, the aim of this study was to evaluate the effects of summer HS prevailing in an arid region on productive performance, and physiological, metabolic, and hematological variables of hair breed male lambs finished in feedlot.
Materials and methods
All procedures that involved sheep were carried out under the guidelines of the Official Mexican Standard for production, use and care of laboratory animals (NOM-062-ZOO-1999). Additionally, the Animal Care Committee of the Universidad Autónoma de Baja California (UABC) approved the experimental procedures.
Experimental location
The study was conducted in the Mexicali Valley, Baja California, northwestern México, specifically in the sheep farm facilities of the Instituto de Ciencias Agrícolas (ICA), UABC (32.4° N and 115.4° W). The prevailing climate in this region is desert (Bwh), with minimum and maximum temperatures in winter (≤ 0 ° C) and summer (≥ 48 ° C) respectively, and it hardly rainfall (85 mm/year) (INEGI 2017).
Experimental design and animal handling
The study was designed to expose hair male lambs (n = 10) to an outdoor environment of HS or thermoneutral over a 30-day experimental period. So, environmental conditions of a desert region were used to generate treatments, specifically the second half of summer (heat-stressed lambs) and winter (thermoneutral lambs). Each season, 10 Dorper × Katahdin male lambs with initial BW of 33.9 ± 0.4 kg and age of 4.5 months old were selected based on their BW and were housed in 1.5 m2 individual pens; these pens were equipped with two buckets of 4 L, one for feeding and the second for drinking. All individual pens had a shade made of galvanized sheet metal placed at height of 2.5 m and has dimensions of 72 m2 (6 × 12 m) and with N-S orientation. The floor of the pens was provided with loose sand for the absorption of urine; it was inspected daily; and when there was excess moisture or feces, it was replaced by clean sand.
Also, they were treated with vitamins A-D-E (Vigantol; Bayer, México City, México; 1 mL/lamb) and against internal and external parasites (Ivermectin; Sanfer Laboratory, México City, México; 0.5 mL/lamb) the first day of the adaptation period. Feed (2 kg on average) and water were offered two-fold ad libitum every day (0600 and 1800 h). Additionally, water was offered again at 1200 h when its availability was considered insufficient. Lambs’ health was visually monitored during the morning and afternoon feeding. Similar feeding, water, and health managements were carried out during the adaptation and experimental periods. Basal diet had 16% of crude protein and 2.8 Mcal of metabolizable energy/kg dry matter (DM), coinciding with nutritional requirements suggested for finishing lambs (NRC 2007). Ingredients and chemical composition of the basal diet are shown in Table 1.
Measurement of study variables
All study variables were measured only during the experimental period of each season. Environmental data of ambient temperature (Ta, °C), relative humidity (HR, %), and wind speed (WS, m/s) were collected from the meteorological station belonging to the ICA-UABC (Model Wireless Vantage Pro2, Davis Instruments, CA, USA). The station was programmed to record daily data at 15-min intervals; it was located less than 1 km from the pens. The temperature-humidity index (THI) was calculated using the formula proposed by Hahn (1999): THI = 0.81 × Te + HR/100 × (Ta − 14.40) + 46.4. From these data, daily minimum and maximum averages and overall daily averages for climatic variables were calculated.
Productive performance was evaluated by measuring initial and final BW, DMI, daily water intake, total weight gain (TWG), ADG, and feed efficiency. The individual BW was recorded before the morning feeding at the beginning (initial BW) and end (final BW) of the feedlot phase, and these data was used to calculate TWG (final BW – initial BW) and ADG (TWG/30 days). Daily, offered and refused amounts of feed and water were also measured to calculate DMI and daily water intake. The feed efficiency was obtained establishing the ADG:DMI ratio.
Physiological variables evaluated were rectal temperature (RT) and respiratory rate (RR), which were measured at 0600, 1200, and 1800 h every 3 days. A rectal thermometer (DeltaTrack Pleasanton, CA, USA) was introduced rectally during one minute to determinate RT, while RR was measured by counting the number of breaths per minute (bpm) with the help of a manual counter and a stopwatch.
Blood analyte concentrations and hematological profiles were evaluated on the same days as physiological variables (10 sampling days). Blood samples were taken by venipuncture of the jugular vein before the morning feeding; the blood for quantification of analytes was collected into 10-mL vacutainer tubes coated with silica, while blood for hematological analysis was deposited into 4-mL vacutainer tubes coated with EDTA-K2 anticoagulant. Tubes containing the blood for analytes were centrifuged at 3500×g for 15 min at 10 °C, and then, the serum was stored by duplicate in 2-mL vials at − 20 °C. Analytes measured in serum were metabolites (i.e., glucose, cholesterol, triglycerides, total protein, and urea [BUN]), electrolytes (i.e., sodium, potassium, and chlorine), and metabolic hormones (i.e., triiodothyronine and thyroxine hormones, insulin and cortisol). Metabolite serum concentrations were determined using a blood auto-analyzer of liquid phase (EasyVet; KrontroLab, Morelia, Mich., México), electrolyte serum concentrations with an electrolyte analyzer (LW E60A, LandWind, Shenzhen, China), and hormones serum concentrations with commercial kits (Monobind Inc., Lake Forest, CA, USA) in a fully automated ELISA analyzer (Thunderbolt, Gold Standard Diagnostics, CA, USA). The intra- and inter-assay coefficients of variation were 5.4 and 6.7% for triiodothyronine (T3), 1.6 and 6.1% for thyroxine (T4), 4.9 and 5.6% for insulin, and 6.4 and 7.0% for cortisol. Moreover, the blood collected for hematology was directly analyzed using an automated hematology equipment (MINDRAY, BC-2800 Vet, Shenzhen, China), which determined the following hematological parameters: leukocyte and erythrocyte counts, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), erythrocyte distribution width (RDW), platelets count, plateletcrit, mean platelet volume (MPV), and platelet distribution width (PDW).
Statistical analysis
Descriptive statistics were used to summarize climatic variables. Data of feedlot performance were subjected to analysis of variance under a completely randomized design (CRD) using initial BW as a covariate and season as treatment. Physiological variables were analyzed as a CRD with repeated measurements over time (sampling day), and the model included the covariate, season, time (sampling day), and the interaction season x time. The rest of the study variables (i.e., blood analytes and hematological parameters) were analyzed as a CRD, where models included the covariate, and fixed effects of treatment, time (sampling days), and hour (hour of the day), as well as their interactions. Lamb was used as random effect, and the variance-covariance structure with the best fit was unstructured (showed the lowest BIC, AIC, and AICC information criteria). All analyses of variance were developed applying the PROC MIXED procedure of the SAS program (SAS 2004). Means were compared using the LSMEANS/PDIFF option, considering significance at P ≤ 0.05.
Results
Climatic conditions during the experimental winter and summer periods are shown in Table 2. Averages in winter for Ta, RH, and THI were 19.2 ± 2.6 °C, 41.7 ± 11.0%, and 64.0 ± 3.0 units, respectively, while in summer were 28.3 ± 4.0 °C, 55.2 ± 18.1%, and 77.2 ± 5.4 units, respectively. The average WS was 1.5 m/s in both seasons. According to the diurnal pattern, the winter Ta and THI ranged from 11.5 to 26.9 °C and from 53.9 to 70.8 units, respectively, while the summer Ta and THI varied from 21.3 to 35.1 °C and 69.6 to 81.6 units, respectively (Fig. 1). In both seasons, the maximum Ta and THI were recorded between 1400 and 1900 h, and minimum values for these same climatic variables were recorded between 0600 and 0700 h.
Results of feedlot performance and physiological responses are shown in Table 3 and Fig. 2, respectively. Thermoneutral lambs showed higher (P ≤ 0.02) final BW, TWG, ADG and feed efficiency than heat-stressed lambs. The DMI was unaffected (P = 0.71) by the summer HS, but water intake was 49 % higher (P < 0.01) in heat-stressed lambs than their counterpart. The environmental treatment × hour of the day interaction affected physiological variables, being RT and RR higher (P < 0.01) at 0600, 1200, and 1800 h in heat-stressed lambs than thermoneutral lambs.
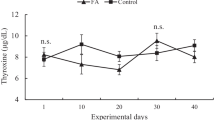
Results of serum concentrations of the analytes are shown in Table 4. The interaction treatment × sampling day affected (P ≤ 0.02) serum concentrations of all metabolites, potassium, chloride, and T4. Serum analytes as sodium, T3, cortisol, and insulin were not altered (P ≥ 0.11) by the interaction. Compared to thermoneutral lambs, heat-stressed lambs had lower (P < 0.01) serum concentrations of glucose, cholesterol, total protein, BUN, potassium, and T4, but higher (P < 0.01) serum concentration of chlorine on most sampling days. For its part, the HS effect on serum concentration of triglyceride was very inconsistent across the sampling days, leading to that heat-stressed and thermoneutral lambs had similar (P = 0.22) serum concentrations of this metabolite. Additionally, summer HS alone decreased (P < 0.01) serum concentrations of T3, but did not modified (P ≥ 0.33) serum concentrations of sodium, cortisol, and insulin. Sampling day affected (P = 0.02) only serum concentrations of T3.
Results of hematological parameters are shown in Table 5. The environmental treatment × sampling day interaction altered (P ≤ 0.05) some hematological parameters (i.e., leukocytes, hemoglobin, MCH, and MCHC). Compared with termoneutral lambs, heat-stressed lambs had higher (P ≤ 0.05) leukocyte count and MCH but lower (P ≤ 0.05) hemoglobin and MCHC on most sampling days. Considering environmental treatment as a main effect, summer HS decreased (P ≤ 0.03) erythrocyte and platelet counts, hematocrit, and plateletcrit, but increased (P = 0.03) MCV, without affecting (P ≥ 0.19) RDW, MVP, and PDW. For their part, sampling day affected (P < 0.01) only platelet count and plateletcrit.
Discussion
Arid regions are characterized by extreme temperatures, being for sheep very hot during summer and relatively thermoneutral in the second half of the winter season and first half of the spring season. In fact, the summer ambient in late night and early morning becomes thermoneutral for sheep, so they will partially recover from HS acquired during daytime hours (Sejian et al. 2017a). The thermoneutral zone for hair breed sheep is located between 15 and 30 °C, whereby they experience HS when Ta ≥ 30 °C and THI ≥ 78 units (Vicente-Pérez et al. 2020). Therefore, our fattening male lambs were exposed mainly to thermoneutral conditions in winter while in summer to moderate HS (Hahn 1999). Notice that summer Ta was ≥ 30 °C from 0900 to 2300 h and then dropped until 21.3 °C at 0700 h, so heat-stressed lambs recovered between midnight and early morning. The WS was considered mild (1.5 m/s) and was similar in both seasons; however, given that hair sheep lose significant body heat load by non-evaporative means (Macías-Cruz et al. 2016), the presence of air during summer is necessary to prevent them from hyperthermia.
Thermoregulation mechanisms
Summer HS increased RT in hair male lambs during the daytime; however, their mean values do not suggest hyperthermia conditions as they remained within normal range (38.3 to 39.9 °C; Al-Dawood 2017). This agrees with previous reports in the same study site using hair breed sheep (Macías-Cruz et al. 2016, 2018). So, our heat-stressed male lamb avoided hyperthermia because they activated different thermoregulation mechanisms (higher RR, lower thyroid hormone levels, and adequate water balance) combined with the drop in summer Ta during late and early hours of the nights and mornings, respectively. Under high Ta conditions, hair breed sheep can dissipate up to 90% of their heat load by increasing RR (Fonseca et al. 2017); furthermore, they regulate the thyroid gland activity to decrease T3 and T4 production and release, which in turn causes a reduction in its metabolism, endogenous heat production, and, consequently, body heat load (Sejian et al. 2017a; Vicente-Pérez et al. 2020). Likewise, it has been reported that sheep experiencing HS in the sunlight time can lose the heat gained with 3 or 6 h of thermoneutral conditions (Moslemipur and Golzar-Adabi 2017), and our lambs had around 9 h with Ta < 30 °C.
Notice that increased RR in heat-stressed lambs causes excessive body water losses through the respiratory tract, which in turn alters their electrolytic balance and promotes dehydration (Sejian et al. 2017b). So increased water intake along with other adjustments at kidney level to reduce water loss through urine and fecal are also important thermoregulatory mechanisms to avoid hyperthermia and dehydration in hair lambs experiencing HS (Macías-Cruz et al. 2016; Vicente-Pérez et al. 2020), which is supported in the current study by results of water intake (Table 3) and serum concentration of chlorine (Table 4). Additionally, reduction in serum concentration of potassium in heat-stressed male lambs in arid regions during summer is indicative of a rise in sweating rate (El-Zeiny 2011), another physiological mechanism that also contributed to maintain normothermia. Likewise, the increased of RR during summer causes an excessive loss of carbonic acid and carbon dioxide in water form, resulting in respiratory alkalosis (Chauhan et al. 2015); consequently, the kidney performs an exchange of K+ for hydrogen ions to reestablish the acid-base balance (Korde et al. 2007). This adaptation mechanism at renal level could explain the decrease in K+ levels in heat-stressed lambs of the present study. Finally, as in other studies (Macías-Cruz et al. 2016; Vicente-Pérez et al. 2020), there were not changes in the serum sodium concentrations due to HS, demonstrating that our lambs did not suffer dehydration in summer.
Growth and metabolism
Heat stress conditions decreased growth rate and feed efficiency without affecting DMI in hair male lambs, and these findings agree with reports published for Dorper × Pelibuey ewe lambs (Macías-Cruz et al. 2013) and Afshari male lambs (Mahjoubi et al. 2014). In those studies, DMI was unaffected by HS, which was associated with high adaptation level to hot climates of these sheep breeds; in addition, the decreased of Ta overnight was attributed to explain why DMI did not decrease. However, the point that summer HS retarded growth rate without altering DMI could be the result of increased redirection of dietary nutrients for maintenance due to thermoregulation (Mahjoubi et al. 2015). In congruence with this elucidation, we estimated that the maintenance energy costs in heat-stressed lambs increased about 32 % compared to thermoneutral lambs. This was calculated considering requirements of 0.83 Mcal for 100 g ADG (Cannas et al. 2004) and 2.25 Mcal/day for maintenance according to the DMI and dietary ME. Given that HS did not affect DMI, the extra expenditure of energy for thermoregulation was obtained directly from the dietary energy destined for growth.
Interestingly, previous studies demonstrated that HS causes slow growth during the fattening period without affecting DMI and carcass weight in hair breed ewe and male lambs (Macías-Cruz et al. 2013, 2020). Hence, heat-stressed lambs were able to deposit similar carcass tissue mass as non-stressed lambs, but with less dietary energy available for growth. So, our feedlot results and those published suggest that heat-stressed hair lambs have the ability to transform dietary energy into carcass mass more efficiently. Metabolism results revealed two possible mechanisms that could explain this finding and, in consequence, the capacity of hair lambs to grow under HS. First, heat-stressed lambs were able to maintain a metabolic environment of anabolism, even when HS can naturally induce a catabolic environment, leading to continue their growth but to a lesser degree. This metabolic change was evident taking into consideration that they maintained blood insulin and cortisol concentrations as thermoneutral lambs, but decreased their serum concentrations of thyroid hormone, as well as metabolites associated with the catabolism of fat (triglycerides and cholesterol) and muscle (total protein and BUN) tissue. Second, unaffected blood insulin and cortisol concentrations combined with decreased circulating glucose concentrations due to HS suggest that the available glucose had high insulin sensitivity and cortisol resistance. This improved insulin sensitivity in heat-stressed lambs could made them more efficient in converting dietary nutrients for growth into carcass mass. Therefore, all aforementioned confirms the initial hypothesis that hair male lambs grow moderately under summer HS because they adjust their metabolism to maintain homeothermy and prevent catabolism of body tissues (Singh et al. 2016; Vicente-Pérez et al. 2020).
It should be mentioned that this is the first study identifying the metabolic mechanisms promoting weight gain in hair male lambs under outdoor HS environment. In partial congruence with our metabolite results, heat-stressed hair ewe lambs showed decreased serum concentrations of glucose, cholesterol, and triglycerides, but increased BUN concentration with no change in total protein (Macías-Cruz et al. 2016). However, the serum concentrations of the metabolites remained within reference levels (Da Cruz et al. 2017). The authors indicated that, while the BUN increase resulted from a high renal fluid recycling, the metabolite concentrations associated with energy metabolism decreased due to the high-energy cost involved in keeping the physiological mechanisms of thermoregulation active, mainly the increase in RR. Moreover, that study is also evidencing that the energy metabolism observed in ewe lambs in response to seasonal HS is closely connected with changes in the insulin-regulated postabsorptive metabolism. We elucidate that the same mechanism was present in our male lambs.
As in this study, Mahjoubi et al. (2014, 2015) reported in Afshari breed sheep that male lambs subjected to induced HS had similar blood insulin concentrations than thermoneutral male lambs fed ad libitum, but higher levels compared to pair-fed lambs with nutritional restriction under thermoneutral conditions. Additionally, DMI was similar between heat-stressed and pair-fed lambs, but the latter decreased their levels of non-esterified fatty (NEFA). In contrast, HS conditions in sheep have also shown to increase blood cortisol and epinephrine concentrations, catabolic hormones that inhibit pancreatic insulin secretion and promote glycolysis, gluconeogenesis, and NEFA production (Mayorga et al. 2020). Meanwhile, insulin is an anabolic hormone increasing cellular glucose intake, glycogenesis, glycolysis, lipogenesis, and protein synthesis, and reducing at the same time glycogenolysis, gluconeogenesis, lipolysis, and proteolysis; so, insulin increases the deposition of hepatic and muscle glycogen, body reserves, and muscle tissue (Qaid and Abdelrahman 2016). A hyperinsulinemia condition in heat-stressed sheep is somewhat contradictory, and to date, there is no clear explanation for this; however, this metabolic scenario in animals experiencing HS is effective in increasing cellular glucose availability, promoting cortisol resistance and reducing catabolism in fat and muscle tissue (Baumgard and Rhoads 2013). Therefore, this explains why our heat-stressed lambs had low serum concentrations in their metabolites despite having similar serum insulin and cortisol concentrations as thermoneutral lambs.
It seems that heat-stressed hair male lambs showed high insulin sensitivity (lower glucose with similar DMI and blood insulin level) compared to their counterpart. Similarly, studies in pigs (Sanz-Fernandez et al. 2015) and mice (Morera et al. 2012) have documented increased insulin sensitivity by the HS effect. This finding is highly relevant since high insulin sensitivity could lead to greater muscle development by promoting better glucose intake in myocytes and their oxidation within muscle (Qaid and Abdelrahman 2016). Future studies could be directed to determine the effect of this high insulin sensitivity on muscle hypertrophy of heat-stressed male lambs.
Hematological profile
In our hair male lambs, HS decreased the number of erythrocytes and, consequently, hemoglobin and hematocrit values, which coincide with previous reports in sheep (Singh et al. 2016; Sejian et al. 2017b). Although these hematological results could be explained by a simple hemodilution, keep in mind that HS also increases arterial partial pressure of oxygen due to increased RR, a situation that causes erythrocyte lysis, low erythropoiesis, and, therefore, a reduction in erythrocyte count, hemoglobin, and hematocrit (Singh et al. 2016; Habibu et al. 2018). Surprisingly, heat-stressed hair male lambs compensated for this problem by increasing size (MPV) and hemoglobin concentration (MCH) in erythrocytes, representing an adaptive mechanism of thermal resilience that was previously suggested in Nellore sheep (Reddy et al. 2019). Possibly, this mechanism allowed to supply adequate levels of oxygen and nutrients to tissues, considering the slight deficit of erythrocytes. On the other hand, HS also increased leukocyte count, a hematological parameter that has an inverse relationship with blood cortisol concentrations (Correa et al. 2012), but this was not the case in our study. Then, leukocyte results suggest that hair male lambs do not undergo immunosuppression due to HS, and their increase could be a response to some inflammatory process (Habibu et al. 2018). Finally, HS only modified count of platelet cells and plateletcrits, particularly decreased both number and total mass of platelets in hair male lambs due to a hemodilution, which was consistent with results in ruminants (Habibu et al. 2018).
Conclusions
In conclusion, hair male lambs adequately tolerated moderate HS conditions as they maintained normothermia and continued to gain weight, although at a slower rate. Increased respiratory frequency, decreased metabolic heat production, and adjusted water metabolism were responsible in preventing hyperthermia. Meanwhile, these lambs continued to gain weight under HS because they changed their insulin-regulated postabsorptive metabolism to reduce tissue catabolism and increase insulin sensitivity. Hematological profile also evidenced that summer HS did not compromised the health status of hair male lambs, since they had a correct functioning of their immune system and oxygen transport toward body tissues.
References
Al-Dawood A (2017) Towards heat stress management in small ruminants - a review. Ann Anim Sci 17:59–88. https://doi.org/10.1515/aoas-2016-0068
Baumgard LH, Rhoads RP (2013) Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci 1:311–337. https://doi.org/10.1146/annurev-animal-031412-103644
Cannas A, Tedeschi LO, Fox DG, Pell AN, Van Soest PJ (2004) A mechanism model for predicting the nutrient requirements and feed biological values for sheep. J Anim Sci 82:149–169. https://doi.org/10.2527/2004.821149x
Chauhan SS, Celi P, Leury BJ, Dunshea FR (2015) High dietary selenium and vitamin E supplementation ameliorates the impacts of heat load on oxidative status and acid-base balance in sheep. J Anim Sci 93:3342–3354. https://doi.org/10.2527/jas2014-8731
Correa MPC, Cardoso MT, Castanheira M, Landim AV, Dallago BSL, Louvandini H, McManus C (2012) Heat tolerance in three genetic groups of lambs in central Brazil. Small Rumin Res 104:70–77. https://doi.org/10.1016/j.smallrumres.2011.11.001
Da Cruz RES, Rocha FM, Sena CVB, Noleto PG, Guimarães EC, Galo JA, Mundim AV (2017) Effects of age and sex on blood biochemistry of Dorper lambs. Semina Cien Agrar 38:3085–3093. https://doi.org/10.5433/1679-0359.2017v38n5p3085
El-Zeiny WT (2011) Sweating losses of urea, sodium and potassium in Barki sheep under the environmental stress of semi-arid coastal desert conditions. J Anim Poultry Prod 2:393–409. https://doi.org/10.21608/JAPPMU.2011.83402
Fonseca VC, Saraiva EP, Maia ASC, Nagib NCC, Araújo J, Pereira WE, Pimenta FEC, Vieira AM (2017) Models to predict both sensible and latent heat transfer in the respiratory tract of Morada Nova sheep under semiarid tropical environment. Int J Biometeorol 61:777–784. https://doi.org/10.1007/s00484-016-1255-3
Habibu B, Dzenda T, Ayo JO, Yaqub LS, Kawu MU (2018) Haematological changes and plasma fluid dynamics in livestock during thermal stress, and response to mitigative measures. Livest Sci 214:189–201. https://doi.org/10.1016/j.livsci.2018.05.023
Hahn GL (1999) Dynamic responses of cattle to thermal heat loads. J Anim Sci 77(Suppl 2):10–20. https://doi.org/10.2527/1997.77suppl_210x
INEGI (2017) Anuario estadístico y geográfico de Baja California. Available in: https://www.datatur.sectur.gob.mx/ITxEF_Docs/BCN_ANUARIO_PDF.pdf. Accessed 14 May 2020
Korde JP, Singh G, Varshney VP, Shukla DC (2007) Effects of long-term heat exposure on adaptive mechanism of blood acid-base in buffalo calves. Asian Australas J Anim Sci 20:742–747. https://doi.org/10.5713/ajas.2007.742
Macías-Cruz U, Avendaño-Reyes L, Álvarez-Valenzuela FD, Torrentera-Olivera NG, Meza-Herrera C, Mellado-Bosque M, Correa-Calderón A (2013) Growth and carcass characteristics of ewe lambs treated with zilpaterol hydrochloride during spring and summer. Rev Mex Cienc Pecu 4(1):1–12
Macías-Cruz U, López-Baca MA, Vicente R, Mejía A, Álvarez FD, Correa-Calderón A, Meza-Herrera CA, Mellado M, Guerra-Liera JE, Avendaño-Reyes L (2016) Effects of seasonal ambient heat stress (spring vs. summer) on physiological and metabolic variables in hair sheep located in an arid region. Int J Biometeorol 60:1279–1286. https://doi.org/10.1007/s00484-015-1123-6
Macías-Cruz U, Correa-Calderón A, Meza-Herrera C, Mellado M, Aréchiga CF, Avendaño-Reyes L (2018a) Thermoregulatory response to outdoor heat stress of hair sheep females at different physiological stage. Int J Biometeorol 62(12):2151-2160. https://doi.org/10.1007/s00484-018-1615-2
Macías-Cruz U, Gastélum M, Avendaño-Reyes L, Correa-Calderón A, Mellado M, Chay-Canul A (2018b) Variaciones en las respuestas termoregulatorias de ovejas de pelo durante los meses de verano en un clima desértico Variations in the thermoregulatory responses of hair ewes during the summer months in a desert climate. Rev Mex Cienc Pecu 9:738–753
Macías-Cruz U, Saavedra R, Correa-Calderón A, Mellado M, Torrentera NG, Chay-Canul A, López-Baca MA, Avendaño-Reyes L (2020) Feedlot growth, carcass characteristics and meat quality of hair breed male lambs exposed to seasonal heat stress (winter vs. summer) in an arid climate. Meat Sci 169:108202. https://doi.org/10.1016/j.meatsci.2020.108202
Mahjoubi E, Amanlou H, Mirzaei-Alamouti HR, Aghaziarati N, Hossein MY, Noori GR, Yuan K, Baumgard LH (2014) The effect of cyclical and mild heat stress on productivity and metabolism in Afshari lambs. J Anim Sci 92:1007–1014. https://doi.org/10.2527/jas.2013-7153
Mahjoubi E, Hossein Y, Aghaziarati N, Noori GR, Afsarian O, Baumgard LH (2015) The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs. J Anim Sci 93:1632–1640. https://doi.org/10.2527/jas.2014-8641
Mayorga EJ, Ross JW, Keating AF, Rhoads RP, Baumgard LH (2020) Biology to heat stress; the nexus between intestinal hyperpermeability and swine reproduction. Theriogenology 154:73–83. https://doi.org/10.1016/j.theriogenology.2020.05.023
Morera P, Basirico L, Hosoda K, Bernabucci U (2012) Chronic heat stressup-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J Mol Endocrinol 48:129–138. https://doi.org/10.1530/JME-11-0054
Moslemipur F, Golzar-Adabi S (2017) Physiological and growth parameters of fattening lambs after shearing under heat-stress conditions. Anim Prod Sci 57:569–575. https://doi.org/10.1071/AN15001
NRC (2007) Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids
Qaid MM, Abdelrahman (2016) Role of insuline and other related hormones in energy metabolism –a review. Cogent Food Agric 2:1–18
Reddy PR, Kumar BR, Prasad CS, Venkataseshiah C, Hyder I (2019) Erythrocyte fragility based assessment of true thermal resilience in tropical small ruminants. Biol Rhythm Res 00:1–12. https://doi.org/10.1080/09291016.2019.1629087
Sanz-Fernandez MV, Stoakes SK, Abuajamieh M, Seibert JT, Johnson JS, Horst EA, Rhoads RP, Baumgard LH (2015) Heats stress increases insulin sensitivity in pigs. Physiol Rep 08:1–12. https://doi.org/10.14814/phy2.12478
SAS (ed) (2004) SAS/STAT: User´s guide stadistics released 9.1, 2nd edn. SAS Institute, Inc., Cary
Sejian V, Bhatta R, Gaughan J, Malik PK, Naqvi SMK, Lal R (2017a) Adapting sheep production to climate change. In: Sejian V, Bhatta R, Gaughan J, Malik PK, Naqvi SMK, Lal R (eds) Sheep Production Adapting to Climate Change, 1st edn. Springer Nature, Singapure, pp 1–29
Sejian V, Hyder I, Maurya VP, Bagath M, Krishnan G, Aleena J, Archana PR, Lees AM, Kumar D, Bhatta R, Naqvi SMK (2017b) Adaptive mechanisms of sheep to climate change. In: Sejian V, Bhatta R, Gaughan J, Malik PK, Naqvi SMK, Lal R (eds) Sheep Production Adapting to Climate Change, 1st edn. Springer Nature, Singapure, pp 117–147
Singh KM, Singh S, Ganguly I, Ganguly A, Nachiappan RK, Chopra A, Narula HK (2016) Evaluation of Indian sheep breeds of arid zone under heat stress condition. Small Rumin Res 141:113–117. https://doi.org/10.1016/j.smallrumres.2016.07.008
Vicente-Pérez R, Macías-Cruz U, Avendaño-Reyes, Correa-Calderón A, López-Baca MA, Lara-Rivera AL (2020) Impacto del estrés por calor en la produción de ovinos de pelo. Revisión. Rev Mex Cienc Pecu 11:205–222
Acknowledgements
This research is part of the following projects approved by the Universidad Autónoma de Baja California: 200/2460 (departmental project) and 2237 (21th Internal Call). This article is part of the research project of the first author, who thanks CONACYT-México for the scholarship received to carry out his doctoral studies. Finally, thanks to Karen Valadez, Romario Saavedra, Marco A. Silva, Arnulfo Vicente, and other graduate students who helped in the field phase.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nicolás-López, P., Macías-Cruz, U., Mellado, M. et al. Growth performance and changes in physiological, metabolic and hematological parameters due to outdoor heat stress in hair breed male lambs finished in feedlot. Int J Biometeorol 65, 1451–1459 (2021). https://doi.org/10.1007/s00484-021-02116-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-021-02116-x