Abstract
Key message
Tree growth of Nectandra amazonum (Lauraceae) in the Central Amazonian floodplains does not respond to the annual long-term flooding but responds to variation of minimum temperature and potential evapotranspiration.
Abstract
During the last two decades, the Central Amazon region has been impacted by increasingly frequent and more severe floods and droughts and increasing temperature. Little is known about the effects of these climate trends on tree growth in floodplain forests. In this study, we analysed Nectandra amazonum (Lauraceae), an evergreen and flood-adapted tree species, dominant not only in the nutrient-rich Amazonian floodplains (várzea), but also occurring in other environments within and outside the Amazon basin. For the period from 2001 to 2017, intra- and interannual climate–growth relationships of N. amazonum were analysed applying a combination of conventional dendrochronological (cross-dating) and densiometric techniques to construct a robust tree-ring chronology. Six wood parameters were derived from the chronology (ring width, width of earlywood and latewood and corresponding wood density values) and correlated with local climate and hydrologic data. The analysed 32 trees did not show correlation between wood parameters and variation of the hydrological regime. Climate–growth relationships indicated that potential evapotranspiration and minimum temperature play an important role in tree growth mainly during the period of transition between the dry and the wet seasons, and during the aquatic phase affecting physiological processes such as photosynthesis and respiration, respectively. We discuss these results in the background of changing hydroclimatic conditions induced by climate and land-use change in the Amazon basin. Based on our findings, we emphasize the need for more dendroclimatic studies in the tropics applying a multiproxy approach. This will deepen our understanding of tree growth responses, helping to elucidate the dynamic processes of tropical forests that grow under global change impacts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last four decades, mean annual temperature increased by 0.6°–0.7 °C in the Amazon region and extreme hydroclimatic events such as droughts and floods increased in frequency and magnitude (Marengo et al. 2018). Extreme droughts were recorded in the southern region of the basin in the years 2005 and 2010 as well as in 2015/2016, when a severe El Niño drought affected approximately 43% of the area of the Brazilian Amazon, especially in the central, eastern and northern regions (Erfanian et al. 2017; Aragão et al. 2018; Espinoza et al. 2019). On the other hand, an increase in precipitation in the northwestern region mainly during the rainy season (Gloor et al. 2013; Wang et al. 2018) resulted in a significant increase of the frequency and magnitude of severe flood events in the Central Amazon region over the past 30 years (Barichivich et al. 2018; Schöngart and Junk 2020). The intensification of the hydrological cycle is primarily caused by a strong warming of the sea surface temperature (SST) of the Tropical Atlantic Ocean and simultaneously cooling of the SST of the Equatorial Pacific forcing the Walker Circulation (Barichivich et al. 2018) but also has underlying feedback mechanisms (Gouveia et al. 2019). Massive changes in the hydrological cycle are also caused by land-use changes associated with large-scale deforestation of the river catchments (Costa et al. 2003) and the implementation of hydropower plants (Timpe and Kaplan 2017).
Increasing temperature and the changing hydrological cycle in the Amazon basin is of rising concern as it affects physiology, growth and mortality of trees, which may cause changes in the dynamics and structure of tree communities and consequently affect important ecosystem services provided by the Amazon rainforest (Aleixo et al. 2019; Brando et al. 2008; Clark et al. 2010; Schöngart et al. 2021). However, climate impacts on trees can vary among different species and forest types. Severe droughts in the Amazon, which have negative impacts on tree growth in the non-flooded upland forests (terra-firme) (Foley et al. 2002; Granato-Souza et al. 2020) may result in reduced flood heights and durations in floodplain forests, potentially providing favourable growth conditions (Schöngart et al. 2004). However, depending on their leaf phenology, floodplain tree species show different responses to hydroclimatic variation (Schöngart et al. 2002).
Due to seasonal variation of precipitation in the vast catchments, large Amazonian rivers present regular and predictable flood pulses with high amplitudes inducing a distinct seasonality in the floodplains, characterized by an annual aquatic (high-water period) and a terrestrial phase (low-water period) (Junk et al. 1989). Floodplains along large rivers cover about 750,000 km2 (~ 14%) of the Amazon basin. Among these floodplains, the várzea covers about 450,000 km2 and occurs along the main stem of the Amazon river and its large white-water affluents draining the Andean region (Wittmann and Junk 2016). Várzea floodplains developed after the onset of the Amazon transcontinental river around 9.4–9.0 million years ago, bringing a new and fertile landscape element into the mainly oligotrophic Amazonian lowlands (Hoorn et al. 2017). This allowed the colonization, diversification, and speciation of the existing tree flora in the várzea, comprising more than 1000 tree species, of which about 10% are endemic to this ecosystem (Wittmann et al. 2006, 2013). Long-term relative stability of climatic conditions and the regular and predictable flood pulse (Wittmann et al. 2010a; Wittmann 2012) favoured the development of anatomical, morphological, physiological and biochemical adaptations to tolerate flooding (De Simone et al. 2002; Parolin et al. 2004; Parolin 2009; Piedade et al. 2010). Anoxic conditions as caused by flooding result in leaf shedding or exchange in many tree species that reduce or cease radial wood growth due to the secondary cambium dormancy (Schöngart et al. 2002; Parolin et al. 2010) causing the formation of annual tree rings (Worbes 1989). Earlier studies indicate that ring width reflects mainly the duration of the terrestrial phase (Schöngart et al. 2004, 2005; Batista and Schöngart 2018). Considering that climate and hydrology are important factors that influence tree growth in Amazonian floodplains, tree-ring analysis can be employed to detect the recent intensification of the hydrological cycle (Cook and Kairiukstis 1990; Speer 2010).
Traditionally, dendrochronological studies carried out in the tropics relate the total ring width to interannual hydroclimatic variables (Brienen and Zuidema 2005; Dünisch et al. 2003; Schöngart et al. 2004, 2005, 2015; Granato-Souza et al. 2020). Yet, the correlations are not very high compared to studies in temperate, boreal or semi-arid climate zones (Brienen et al. 2016). In addition to ring width, some studies analyse climate–growth relationship of earlywood and latewood, mainly from conifers outside tropical regions to enhance the understanding of the influence of different climatic factors on tree growth (Mikola 1950; Miina 2000; Schweingruber 1990; Vieira et al. 2009). Furthermore, most dendroclimatic studies in tropical moist forests (> 2000 mm annual rainfall) of equatorial lowlands do a priori not consider temperature in the analysis of climate–growth relationships (Brienen et al. 2016) due to the lack of a distinct seasonality (Worbes 2002). In this study, we developed a tree-ring chronology for the evergreen Central Amazonian tree species Nectandra amazonum Nees (Lauraceae) using traditional dendrochronological techniques (cross-dating) and X-ray densitometry, which creates intra-annual wood density profiles allowing an accurate characterization of the tree ring and the definition of earlywood (lower wood density) and latewood (higher wood density) formation (Dodd and Nancy 1988). From the exactly dated tree rings, earlywood and latewood chronologies and corresponding averages of wood density were generated. The following hypotheses were tested: (1) intra-annual variation in wood density allows an accurate definition of the annual ring boundaries avoiding dating errors induced by false rings and the development of well-replicated tree-ring chronologies; (2) As N. amazonum is a flood-adapted tree species, it responds to the variation of the flood-pulse; (3) the intensification of the hydrological cycle during the last decades is recorded in tree-ring series; (4) earlywood and latewood chronologies show stronger relationships to climate and hydrology than the ring-width chronology; (5) wood density variations of N. amazonum reveal complementary relationships between tree growth and climate in relation to those based on ring width.
Materials and methods
Study region
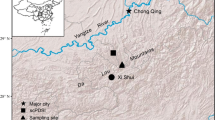
This study was conducted in the nutrient-rich and geomorphological dynamic white-water floodplains (várzea) of Catalão Lake located close to the confluence of the Negro and Solimões rivers near to the city of Manaus, in the State of Amazonas, Brazil (3°10′ S, 59°55′ W) Lake (Fig. 1). The studied floodplain forest is subject to an annual and regular flood pulse with high amplitude of more than 10 m (Junk et al. 1989; Schöngart and Junk 2007). This induces a distinct seasonality characterized by an alternation between one terrestrial and one aquatic phase during the annual cycle. Maximum annual water levels normally occur during June, followed by the receding water period, which attains the minimum water levels regularly between mid-October and mid-November, which is the period of the onset of the rainy season (Schöngart et al. 2002). The annual hydrological cycle shows a temporal shift of about three months compared to the seasonal precipitation regime, which traces back to the huge area of the Solimões and Negro rivers catchment with approximately 3 million km2 (Irion et al. 1997; Schöngart and Junk 2007) (Fig. 2).
Average hydrological conditions and climate for the study region and period (2000–2017) shown from July to June. a Mean (blue line) and standard deviation (dotted blue line) of daily water levels obtained from the Port of Manaus (data: Agência Nacional de Águas—ANA; above) in relation to the mean topography of the studied population of Nectandra amazonum (mean 24.6 SD ± 0.44 m). Based on the daily water-level record the annual duration of the aquatic phase (AP), terrestrial phase (TP), flood level (FL) and drought level (DL) have been calculated for each year. b Mean and standard deviation of monthly precipitation (P; blue bars), potential evapotranspiration (ETpot; bars in light blue), minimum (Tmin; yellow line) and maximum (Tmax; red line) monthly temperature (data for P, Tmax and Tmin: Instituto Nacional de Meteorologia—INMET; data for ETpot: Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA)
The climate in the study region is characterized by an annual precipitation (P) of 2111 mm (SD ± 339) (July 2000 to June 2017), with a distinct seasonality (Fig. 2). The rainy season comprises the period from November to May with the highest rainfall during March (mean 321 SD ± 109 mm), while the dry season occurs from July to September with monthly precipitation below 100 mm reaching its minimum in August with 57 mm (SD ± 47). Mean annual temperature (Tmean) is 28.4 °C (SD ± 1.0) with minimum monthly temperatures (Tmin) of 24.3 °C (SD ± 0.3) and maximum monthly temperatures (Tmax) of 32.6 °C (SD ± 1.2) (Fig. 2) with only small variations throughout the year. Potential evapotranspiration (ETpot) in the study region is 130.9 mm (SD ± 9.3) with higher values during the dry season and transition periods between dry and rainy season, potentially leading to water deficits during this period (ETpot exceeding monthly P) (Fig. 2).
As a consequence of the flood pulse, várzea forest soils are enriched through the deposition of alluvial sediments originating from the Andes. Those sediments have a higher concentration of macronutrients (P, K, Na, Ca and Mg) which increases cation-exchange capacity, resulting in higher soil fertility compared to other Amazonian forest types (Sombroek 1984; Furch 1997; Furch and Klinge 1989). Várzea soils present low or no profile development, comprising groups of Neosols, Gleysols, Organosols, and Vertisols that are classified as eutrophic or dystrophic according to the topographic location and the quality of water and sediments (Victoria et al. 1989).
Approximately 75% of the Central Amazonian várzea is covered by highly diverse floodplain forests (Wittmann et al. 2006). Tree species are partially endemic to the highly inundated low várzea, defined as flooded by more than 3 m in height (Wittmann et al. 2013). Tree species are generally well-zoned along the flood-level gradient and according to successional series (Ayres 1993; Junk 1989; Wittmann et al. 2002; Worbes et al. 1992). The várzea forests in the study region are a mosaic of natural successional stages and secondary forests, which are established on abandoned agricultural and jute lands (Chorchorus spp.), especially in the period from the 1960s to the late 1980s (WinklerPrins 2006).
The tree species Nectandra amazonum
Nectandra amazonum (Lauraceae family) is a widespread tree species that occurs mainly in Amazonia up to elevations of 300 m above average sea level (Fig. S1). In várzea forests, where it is commonly known as “louro canela”, N. amazonum is one of the most frequent tree species (Wittmann 2012), preferentially occurring in late-secondary stages, where flood levels range between 4 and 5 m (Wittmann et al. 2010b). Trees usually reach heights of up to 20 m, are relatively short-lived (mean 28 SD ± 4 years), and present high average radial growth (mean 4.84 SD ± 1.21 mm year–1) and moderate wood density (ρ) of 0.47 g cm–3 (Worbes et al. 1992). In the Central Amazonian floodplains, the species belongs to the evergreen ecotype that exchanges and replaces leaves continuously, with new leaves being flushed around the flood peak during the aquatic phase, occasionally showing a second leaf exchange during the dry season (Schöngart et al. 2002; Waldhoff and Parolin 2010). Flowering starts during the period of leaf flush in the aquatic phase while fruits reach maturity at the beginning of subsequent aquatic phase (Parolin 2000). Tree-ring formation occurs during the aquatic phase when trees face unfavourable environmental conditions induced by oxygen depletion in flooded soils (Schöngart et al. 2002). Monthly diameter increment monitored during a 26-month period (1998–2000) by Schöngart et al. (2002) shows for N. amazonum a significant correlation to monthly precipitation, but no correlation with monthly water-level variations. Many studies provide ecophysiological parameters for N. amazonum, obtained during the aquatic and terrestrial phases such as specific leaf mass, leaf area, leaf water and nitrogen content (Parolin 2002; Parolin et al. 2002), mean and maximum CO2 assimilation, stomatal conductance (Parolin 1997; Parolin et al. 2001; Piedade et al. 2000), photochemical efficiency (Fv/Fm) (Parolin 1997), sap flow and stem respiration rates (Horna et al. 2010) (more information on the ecophysiology of N. amazonum is available as Supplementary Material Fig. S2). Overall, N. amazonum shows sophisticated physiological adaptations which allow the species to maintain a carbon metabolism at low, but almost constant levels along the year, despite the anoxic conditions during the aquatic phase which lead to a reduction of the cambial activity and annual tree-ring formation.
The wood anatomical structure of tree rings in the Lauraceae family is divided into two distinct zones due to differences in wood density which result from the variation of lumen size and wall thickness of fibre cells (Worbes 1986; Worbes and Fichtler 2010). Earlywood, formed at the beginning of the growing season, comprises a brighter zone with lower wood density due to the larger lumen and thin walls of fibre cells, while the latewood has a higher wood density due to increasing thickness of cell walls and decreasing size of lumen appearing as a dark zone (Worbes 2002). In N. amazonum, tree rings are usually distinct by naked eye. However, intra-annual wood density variations, lacking an abrupt transition of the cell sizes, are frequent, indicating the occurrence of false tree rings. Vessels are visible to the naked eye, predominantly solitary without specific arrangements (diffuse-porous) (Fig. 3).
Schematic figure of obtained wood parameters from tree rings: ring width (RW), earlywood width (EW), latewood width (LW), mean wood density of ring width (ρRW), earlywood (ρEW) and latewood (ρLW). Earlywood–latewood boundary (ELB) was determined by the absolute minimum (ρmin) and maximum (ρmax) wood density as a floating threshold for each tree ring (Eq. 1). The percentage of earlywood (EW%) and latewood (LW%) was calculated to determine the period of EW and LW formation based on monthly diameter increments (Fig. S2). In the upper panel, an image of macroscopic wood anatomy of N. amazonum shows a tree ring with intra-annual density fluctuation, and a false tree ring indicated by the arrow, which results in a gradual variation of wood density
Fieldwork
Wood samples from 38 living trees were collected in September 2018 and November 2019 in the várzea secondary forests around Lake Catalão (Fig. 1). Trees were selected avoiding differences in the occurrence of their topographic elevations and assuring the inclusion of a wide range of diameter and age classes. Wood cores of 12 mm in diameter at two perpendicular relative positions were extracted from 34 trees at breast height using a power-driven increment borer. Additionally, stem discs from four trees were obtained at 1.3 m stem height in November 2019 to improve the characterization of false rings and tree-ring dating (authorization n. 124/2019—DEMUC/SEMA). The height of the maximum flood level printed on the trunk was measured to estimate the topographical elevation using daily water-level records from the nearby Port of Manaus (12.3 km in direct distance) provided by the Hydroweb platform (https://www.snirh.gov.br/hidroweb/serieshistoricas) operated by the Brazilian Water Agency (Agência Nacional de Águas—ANA). Therefore, the observed flood height in the field was subtracted from the previously recorded maximum water level allowing the estimation of the topographic elevation of the studied trees (mean 24.6 SD ± 0.44 m).
Tree-ring analyses
The wood samples were air-dried and mechanically polished with sandpaper (80–600 grains). Tree rings were identified by the intra-annual wood density variations (earlywood/latewood pattern), marked and measured to the nearest 0.01 mm under a LEICA-MS5 microscope, coupled to a digital measuring device (LINTAB, Rinntech, Germany) supported by the software TSAP-Win (Time Series Analyses and Presentation, Rinntech, Germany) to produce individual ring-width time series. These were processed using dplR package in R software (Bunn 2008) for cross-dating. Frequently occurring false tree rings in N. amazonum resulted consequently in dating errors and it was not possible to construct a reliable tree-ring chronology with robust statistics based on the macroscopically analysed wood anatomical features of growth rings.
X-ray densitometry
To improve the examination of tree-ring boundaries avoiding dating errors induced by frequently occurring false rings as observed in N. amazonum, we applied X-ray fluorescence analysis of the collected wood samples at the Department of Forest Science (ESALQ/USP) at Piracicaba, São Paulo State. To prepare the cores for densitometry analysis, a thin wood sample from each polished core was transversely cut (2.0 mm thickness) with a parallel double circular saw and conditioned in a climatic chamber at 20 °C and 60% relative humidity until reaching a stable moisture content of 12% (Tomazello Filho et al. 2008). The samples were then scanned with a calibration scale of cellulose acetate using an X-ray densitometry chamber (Faxitron MX20-DC12, Faxitron X-Ray, Illinois, USA). The images were analysed by WinDendro® software (Regent Instruments Inc.) creating microdensity profiles (interval of 1.7 μm).
Tree-ring boundaries were defined using microdensity profiles by the sharp contrast between maximum (ρmax) and minimum wood density (ρmin). Subsequently, ring width (RW) was obtained for each tree ring (Fig. 3). The earlywood-latewood boundary (ELB) was defined by the so-called relative method (Eq. 1) using ring-specific minimum (ρmin) and maximum (ρmax) wood density profile values to find the position of ELB as a floating threshold for each tree ring (Windendro® software, Regent Instruments Inc.):
where X is the percentage of ρmin related to ρmax. After fixing ELB, we obtained for every tree ring the earlywood width (EW) and latewood width (LW) as well as mean wood density for ring width (ρRW), earlywood (ρEW) and latewood (ρLW) (Fig. 3).
Climate data
We compiled a set of hydrological and climatic data, for the period between 2000 and 2017, from different sources. Monthly precipitation (P), monthly minimum (Tmin) and maximum (Tmax) temperature were obtained from INMET (Instituto Nacional de Meteorologia; station code 82331, latitude: 3°06′ S, longitude: 60°01′ W, located in Manaus). Monthly mean potential evapotranspiration (ETpot), calculated by the method proposed by Thornthwaite and Mather (1955), was obtained from EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária, latitude 2°53′ S, longitude: 59°58′ W). The Cumulative Water Deficit (CWD) defined as the sum of negative differences (P–ETpot) during consecutive months was calculated for each year (Aragão et al. 2007). From the daily water-level records, we calculated for each year (2000–2017) the duration (in days) of the aquatic (AP) and terrestrial (TP) phase for the mean topographic elevation of each tree for which the tree-ring chronology was analysed (Fig. 2). Additionally, the annual flood level (FL) and drought level (DL) (in cm), defined as the difference between the mean topography and the annual minimum water level were derived. To examine the influence of large-scale oceanic forcing of the hydrological cycle (Barichivich et al. 2018), we obtained from the NOAA website (National Oceanic Atmospheric Administration; https://psl.noaa.gov/data/climateindices/list/) monthly sea surface temperature (SST) anomalies from the Equatorial Pacific in form of three-month averages (Oceanic Niño Index—ONI) and from the tropical north (TNA) and south Atlantic (TSA) as well as for the Atlantic Meridional Mode (AMM) in form of monthly SST anomalies.
Statistical analyses
To determine the periods of earlywood and latewood formation, we used monthly diameter increment data of N. amazonum (Fig. S2) observed for a period of 26 months (June 1998–August 2000) at a nearby várzea site (Marchantaria Island) with similar environmental conditions and tree sizes (Schöngart et al. 2002). Based on these data monthly mean diameter increments were cumulated to annual increments and transformed into relative values. Based on the determined mean EW% (67.8%) and LW% (32.2%) by densitometry analysis of those individuals used for the tree-ring chronology, the periods of EW and LW formations were estimated, indicating the occurrence of ELB in February.
To develop a RW chronology, we used the same method of statistical validation for cross-dating accuracy as for the time series based on macroscopic analysis (dplR package in R software; Bunn 2008). Cores that could not be reliably cross-dated were discarded. Based on the exactly dated RW chronology also time series for EW and LW were derived and the averages of the corresponding wood density parameters (ρRW, ρEW and ρLW) were calculated for the cross-dated tree rings. We opted for using raw wood density values and not detrended series, as the annual wood density values are composed of trees of different ages (Table S1).
To relate tree growth to climate, the individual tree-ring series were detrended by the ‘mean-value function’, fitting a horizontal line using the mean of the series to transform raw values into ring-width indices (RWI) (Cook and Kairiukstis 1990). Residual chronologies from the standardized RWI, EWI and LWI were built through a robust bi-weighted mean function. To evaluate the growth synchronism among detrended ring-width series, the intercorrelation series (r), RBAR and the expressed population signal (EPS) were calculated (Wigley et al. 1984). The mean sensitivity indicating environmental response of tree growth over time was calculated according to Speer (2010).
Pearson’s correlation was applied between wood parameters (RW, EW, LW, ρRW, ρEW and ρLW) and hydrological (AQ, TP, FL, DL) as well as local (Tmin, Tmax, P, ETpot, CWD) and large-scale (ONI, TNA, TSA, AMM) climate parameters considering a confidence level of 95% (p < 0.05). A 12-month interval was considered, starting in July coinciding with the beginning of tree-ring formation (t–1) until June of the following year when growth ring formation was concluded (t) (Schöngart et al. 2002). Spatial correlation of wood parameters and 0.5° gridded Tmin (CRU TS 4.04) were performed by the KNMI-Climate Explorer (https://climexp.knmi.nl/start.cgi).
Results
Chronologies of tree-ring parameters
From the total of 38 sampled trees, 32 individuals (84%) were used to construct a ring-width (RW) chronology based on the microdensity profiles comprising a total of 52 measured radii. The descriptive statistics of the RW chronology and the derived earlywood (EW) and latewood (LW) time series are presented in Table 1 and the chronologies in Fig. 4. The RW, EW and LW chronologies were negatively correlated with the wood density parameters (Table S2). The developed RW chronology (Fig. 4) spanned the period from 2001 to 2017 (17 years) and individual time series comprised on average 16.8 years (SD ± 5). The interseries correlation of 0.37 and RBAR of 0.28 suggest a common growth variation among trees reflected by the EPS of 0.92 which exceeded the suggested threshold of 0.85 (Wigley et al. 1984). The sensitivity of 0.52 showed that tree rings of N. amazonum were sensitive to environmental changes (Fritts 2001). The statistical parameters indicated that the chronology was successfully cross-dated, which allowed to derive the chronologies for EW and LW. Computing the dendrochronological statistics for the EW chronology showed a slight decrease in the statistical robustness compared to the RW chronology with RBAR of 0.21. However, the EPS of 0.88 (Table 1) still attained the required minimum value (Wigley et al. 1984). The LW chronology presented lower mean interseries correlation, RBAR and EPS values, below the required threshold. On the other hand, mean sensitivity of the LW chronology was higher compared to the RW and EW chronologies, indicating that there was low synchronicity between latewood width (Table 1).
Climate–growth relationships of Nectandra amazonum
From the analysed six wood parameters, EW contained the strongest climate signal, however, the most significant correlations were obtained for ρRW. In general, LW chronology and ρLW showed lower correlations with climate variables. None of the six wood parameters showed any significant correlation with the calculated hydrological variables (AP, TP, FL and DL). Negative correlations (p < 0.05) of RW and EW were observed with monthly precipitation of November, corresponding to the minimum water level at the beginning of the rainy season. Concerning wood density parameters, only ρLW indicated positive correlations with monthly precipitation of August and September (Fig. 5A). The RW, EW and LW chronologies were negatively correlated with maximum temperature (Tmax) during April, already at the beginning of the aquatic phase and showed a weak correlation in June around the maximum water level, when tree ring formation already finished (Schöngart et al. 2002). The wood density parameters presented for the same months positive correlation with Tmax (except for ρLW showing significant correlation with Tmax of August and not April) (Fig. 5b). The strongest correlations were found with Tmin (Fig. 5c) which seems to have a strong influence on tree growth of N. amazonum. Significant correlations were observed for all months, with exception of January (RW) and March (LW). The correlations were stronger during the months of the aquatic phase (April to September) reaching negative correlation of up to –0.8 (p < 0.01) for RW, EW, LW and positive correlations of even up to 0.9 (p < 0.01) for ρRW, ρEW and ρLW. The EW chronology also showed high correlations with Tmin of January and February. All wood parameters correlated with potential evapotranspiration (ETpot) (Fig. 5d), again, negative for the tree ring parameters and positive for wood density parameters. Correlations (p < 0.05) comprised the months from August to January with a gap in September at the beginning of the terrestrial phase and showed the highest correlation in November during the occurrence of the minimum water level. The period of October, November, and December (onset of the rainy season during the terrestrial phase) revealed the strongest correlations between ETpot and tree-ring parameters (RW, EW) and the corresponding wood density parameters (ρRW, ρEW). No correlation was observed between the six wood parameters and CWD. The correlations among wood parameters and large-scale climate drivers from the surrounding tropical ocean sectors (ONI, TNA, TSA and AMM), showed only weak positive relationships (p < 0.05) between TNA-SST anomalies of October and ρRW and ρEW (Fig. 5e).
Correlation (Pearson coefficient; p < 0.05) values between ring width (RW), earlywood (EW), latewood (LW) chronologies, mean wood density of ring width (ρRW), earlywood (ρEW) and latewood (ρLW) with monthly climate data: a precipitation (P), b maximum temperature (Tmax), c minimum temperature (Tmin), d potential evapotranspiration (ETpot), and e tropical North Atlantic SST anomalies (TNA), during the period ranging from July 2000 to June 2017
Spatial correlation of 0.5° gridded Tmin (CRU TS 4.04) with RW and ρRW for three-month averages spanning the dry season (July–September), transition period (October–December) and rainy season (January–March) showed congruent patterns for both wood parameters, however, with opposite signals (negative for RW and positive for ρRW) (Fig. 6). For the dry season, both wood parameters indicated large-scale correlations (p < 0.05) covering not only most of the Amazon but also NW-South America and NE and SE-Brazil. For the transition period between dry and rainy seasons, correlations were almost absent and appeared again for the rainy season covering mainly the central and eastern part of the Amazon basin.
The spatial correlation pattern (p < 0.05) computed by the KNMI-Climate Explorer between the ring-width chronology (a–c) and mean ring wood density (d–f) with CRU TS 4.04 0.5° gridded minimum temperature (Tmin) for the dry season (July–September, a and d), the transition period from the dry to the rainy season (October–December, b and e) and the rainy season (January–March, c and f) from 2001 to 2017
Discussion
Nectandra amazonum forms distinct annual growth rings (Schöngart et al. 2002; Worbes 1986), as many other Lauraceae tree species occurring in tropical (Schöngart et al. 2017) and tropical–subtropical regions of the Coastal Atlantic rainforests (Mata Atlântica) in Brazil (Alves and Angyolossy-Alfonso 2000; Barros et al. 2006; Granato-Souza et al. 2017; Spathelf et al. 2010). However, in this study, it was not possible to develop a reliable tree-ring chronology for N. amazonum based on macroscopic analyses of the earlywood/latewood patterns, due to the high frequency of false tree ring occurrences, visible as intra-annual wood density variation, typical for tropical trees (Brienen et al. 2016; Worbes 2002). In these cases, other methods must be employed to enhance the visualization of tree-ring boundaries, especially when these structures are hard to distinguish, like in tropical trees. Such methods comprise autofluorescence (Godoy-Veiga et al. 2019), intra-annual stable isotope patterns (e.g., Evans and Schrag 2004; Ohashi et al. 2009; Pons and Helle 2011; Verheyden et al. 2004,), intra-annual variation in wood density highlighted by X-ray (e.g., Nepveu 1976; Vetter 1995) or high-frequency (HF) densitometry (e.g., Schnackenburg et al. 2008). Tree species from the Lauraceae family have generally tree rings defined by intra-annual wood density variations (earlywood and latewood pattern) (Fontana et al. 2019; Reis-Avila and Oliveira 2017; Worbes 1986) with high potential for densitometry analysis. Hansen (2004) demonstrated for some Lauraceae tree species (N. megapotamica and Ocotea pulchella) from the subtropical southern Brazilian region promising results applying HF-densitometry, reflecting well the intra-annual wood density variations. The application of densitometry was essential in this study to define the ring boundaries by the sharp contrast between ρmax of the latewood and ρmin of the earlywood of the subsequent tree ring (Fig. 3). The majority of intra-annual wood density variation was eliminated by the wood density profile, as false tree-rings are reflected by gradual changes in the intra-annual wood density spectrum (e.g., Pagotto et al. 2017). This technique improved the individual series dating and allowed the construction of a tree-ring chronology with a robust sample size including more than 80% of the sampled trees, meeting the statistical requirements for climate–growth analyses (Wigley et al. 1984).
Although considered a valuable technique widely applied in studies of temperate and boreal climates (e.g., Helama et al. 2012; Klusek et al. 2015; Schweingruber et al. 1978; Wang et al. 2001), densitometry has found so far limited application in the tropics. Most studies in tropical regions applied densitometry with the aim to detect indistinct ring boundaries by the intra-annual wood density profiles (e.g., Lisi et al. 2008; Pagotto et al. 2017). X-ray densitometry has been successfully applied for the African tree species Terminalia ivorensis (Combretaceae) demonstrating a good congruence between tree rings and wood density patterns (Nepveu 1976). However, it was not possible to match interannual wood density variations with visible tree rings from the African species Aucoumea klaineana (Burseraceae) (Mariaux 1967) or Scleronema micranthum (Malvaceae) in Central Amazonian terra-firme (Lisi et al. 2008). Over time, methods of densitometry have been improved, through more sophisticated X-ray densitometry techniques (Tomazello Filho et al. 2008) and HF-densitometry (Schinker et al. 2003) enhancing the application of this method for tropical tree species.
However, only a few studies in the tropics have achieved successful results in relating wood density profiles to climate variation. Worbes et al. (1995) built chronologies of maximum wood densities from Macrolobium acaciifolium and Swartzia polyphylla (both Fabaceae) from the Central Amazonian floodplains and observed a positive relation between the duration of the terrestrial phase on wood density variations. Another study performed with Rhizophora mangle (Rhizophoraceae) in mangroves of northeast Colombia, revealed a negative correlation between minimum wood densities and rainfall (Ramírez Correa et al. 2010). One reason for the limited application of densitometry in tropical tree species is related to the anatomy of tree rings. Tree species with tree rings delimited by fine marginal parenchyma bands or alternating fibre and parenchyma tissues appear less promising for densitometry analysis (Eshete and Ståhl 1999; Lisi et al. 2008; Vetter 1995). Naturally, tree species that present tree rings with intra-annual density variations, like conifer species (Araucaria angustifolia, Prumnopitys montana), Lauraceae (N. amazonum, N. megapotamica, Ocotea pulchella) and others (Worbes 2002) are suitable for densitometry (Hansen 2004; Lisi et al. 2008; Schnackenburg et al. 2008). In this sense, our study confirms the potential of applying densitometry analysis to assist the construction of chronologies in tropical trees. Densitometry additionally provides a variety of wood density parameters (ρRW, ρEW, ρLW, ρmin, ρmax) (Fig. 3), which can be used to build chronologies of specific wood features and proxies (e.g., Ferreira and Tomazello Filho 2009; Klusek et al. 2015). This opens new insights into several functional traits, which are important to understand the functional ecology of tropical tree species and dynamical processes of ecosystems, considering natural and anthropogenic drivers (e.g., Seidl et al. 2017). Although the majority of tree‐ring data sets are based on ring width, the measurements of wood density at annual or even higher resolution (i.e., microdensitometry) can provide additional knowledge on climate–growth relationships, paleoclimatology, tree physiology and ecophysiological processes, especially those related to the hydrological cycle and carbon dynamics (Sullivan et al. 2020).
Despite the construction of only a short RW chronology, it was robust enough to provide an exactly dated time series for EW and LW and the corresponding wood density parameters (ρRW, ρEW and ρLW). This is, to the best of our knowledge, the first time that EW and LW parameters have been provided for a tropical angiosperm species. However, since N. amazonum hardly achieves ages above 30 years (Schöngart 2003; Worbes et al. 1992), the produced chronologies were short, which limits to some degree the extrapolation of the obtained results. Only the EW chronology presented robust statistical parameters, while the LW chronology did not achieve the statistic requirements presenting a high sensitivity (Table 1) which might be the reason for weak correlations with the tested climate and hydrological data (Fig. 5). The wood density parameters achieved higher correlations with Tmin as the corresponding ring-width parameters (Fig. 5c). Ring-width parameters showed a strong negative correlation with its wood density counterparts (Table S2). Across species, wood density is positively associated with survival and lifespan, but mostly negatively associated with diameter growth rates (Poorter et al. 2010; Schöngart et al. 2010; Siefert et al. 2015), explaining the opposite trend of ring width (RW, EW, LW) and wood density parameters (ρRW, ρEW, ρLW). The strong negative correlation between ring-width and wood density parameters might also be caused by strong ontogenetic effects, as N. amazonum presents short lifespans and high diameter increment rates that rapidly decline with increasing tree age (Schöngart et al. 2010).
Annual tree-ring formation in N. amazonum is triggered by the anoxic conditions induced by the annual flood pulse (Worbes 1997) and the species grows mainly during the terrestrial phase (Schöngart et al. 2002). For this reason, we expected strong relationships of ring width and wood density parameters (Schöngart et al. 2004, 2005; Worbes et al. 1995) with hydrological variables (AP, TP, FL, DL), especially considering the differences between EW and LW and the wood density counterparts. However, no correlation was found, which also has been observed by Schöngart et al. (2002), who related monthly diameter increment rates of N. amazonum to monthly water levels for a period of 26 months. However, the authors observed weak, but still significant correlations between monthly diameter increment and precipitation. On the interannual scale weak correlations with monthly precipitation were observed only for some months during dry season.
Our postulated hypothesis that the intensification of the hydrological cycle affects tree growth of N. amazonum was not corroborated. To test this hypothesis, there is probably no better site in the Amazon region than the Central Amazonian floodplains, where daily water-level records are available for more than 100 years, evidencing significant changes in the hydrological regime resulting in an increase of the flood amplitude by 1.5 m during the last 30 years compared to the period 1903–1990 (Schöngart and Junk 2020). The intensification of the hydrological cycle is mainly driven by large-scale climate anomalies originating from a warming tropical Atlantic and simultaneously cooling equatorial Pacific during the last 2–3 decades (Barichivich et al. 2018). Large-scale SST anomalies from these oceanographic regions influence rainfall regimes and hydrological cycles across the Amazon basin with a high spatiotemporal variability (Aragão et al. 2018; Gloor et al. 2015; Marengo and Espinoza 2016; Marengo et al. 2012; Yoon and Zeng 2010), and were detected in the tree growth of many tree species (e.g., Brienen et al. 2012; Granato-Souza et al. 2020; Schöngart et al. 2004, 2005). However, as the analysed six wood parameters of N. amazonum are not sensitive to the precipitation regime and hydrological cycle, it is not surprising that no relations with SST anomalies are observed (ONI, AMM, TNA, TSA) (except a weak correlation between SST of the TNA for October with ρRW and ρEW).
Especially the period from October to December seems to be critical for tree growth of N. amazonum with ring width responding negatively (positively with ρ) to ETpot (Fig. 5d). This time of the year corresponds to the low-water period and the transition from the dry to the rainy season (Fig. 2). In years of a shortened aquatic phase after El Niño occurrence (Schöngart and Junk 2007) and a delayed onset of rainfall leading to enhanced maximum temperatures, ETpot might impact growth performance of N. amazonum. Photosynthetic rates in these months are low, even in comparison to those measured during the aquatic phase (Parolin 1997; Piedade et al. 2000). Trees of N. amazonum often present a small, second leaf exchange during this period (Schöngart et al. 2002). In consequence, monthly diameter increments are often reduced in this period (Fig. S2) and possibly lead to the frequently observed formation of false tree rings in form of intra-annual wood density variations, which turns EW more sensitive against ETpot compared to RW (Fig. 5d). Horna et al. (2010) observe a linear increase in xylem sap flux (Js) with increasing vapour pressure deficit (VPD) suggesting no stomata control. Periods of water deficit result in reduced CO2 assimilation, a second leaf exchange, reduced diameter growth and frequent formation of false tree rings, which impeded the development of a tree-ring chronology based on macroscopic wood anatomy.
Our results show further a strong influence of Tmin throughout the year, especially during the aquatic phase. Although several studies carried out in tropical forests have shown similar results (Anderegg et al. 2015; Ballantyne et al. 2017; Clark et al. 2003; Fontana et al. 2019; Sullivan et al. 2020), this was no expected for a tree growing in the Amazon várzea floodplain. Minimum temperature is associated with night-time temperatures. Clark et al. (2010) found that an increase of 1–2 °C in night-time temperatures severely reduced radial growth in six species in Costa Rica, due to a rise in stem respiration. Rahman et al. (2018) also showed that strong negative correlation between RW and minimum temperature indicated respiration-related growth reduction during the growing season. The minimum temperature of our study region is 24.3 °C (SD ± 0.3) during the period 2000–2017 showing almost no seasonal variation (Fig. 2). However, these data refer to non-flooded sites which have stable night-time temperatures throughout the year (Góes Ribeiro 1976). In floodplains, however, minimum temperatures at night can be up to 4 °C higher during the aquatic phase compared to the terrestrial phase as the warm water acts as a buffer (Irmler 1986). It is likely, that the higher night-time temperature during the aquatic phase increases stem respiration leading to reduced diameter growth of N. amazonum (Anderegg et al. 2015; Ballantyne et al. 2017; Clark et al. 2003) particularly at the beginning and the ending of the growing season (Fig. S2). Further, it is notable that minimum annual temperature in the Manaus region increased by more than 0.3 °C since 1980 with reference to the long-term period (1910–1979) (Schöngart and Junk 2020), especially during the last decade. The increased frequency and magnitude of floods during this period (2009, 2012–2015) (Barichivich et al. 2018) might have increased night-time temperatures even more during the prolonged aquatic phase and extensive flooded areas in the várzea.
Overall, our results indicate that tree growth and variation of the analysed wood parameters of N. amazonum are mainly triggered by Tmin, especially during the aquatic phase and ETpot, mainly during the low-water period (transition from dry to rainy season). This has a strong influence on respiration (Tmin) and photosynthesis (ETpot) affecting tree growth. The species is adapted to the regular occurring anoxic conditions through long-term flooding. It maintains throughout the year a photochemical efficient canopy formed by leaves with comparatively small leaf area, but high specific leaf mass (Parolin 1997, 2002) with almost constant stomatal conductance (Parolin 1997) and no control of xylem sap flux (Horna et al. 2010). This allows the species to maintain photosynthesis active throughout the year, even under unfavourable conditions (Parolin et al. 2001; Piedade et al. 2000). The anoxic conditions during flooding, however, lead to a reduction of cambial activity which coincides with leaf flush and flowering (Schöngart et al. 2002). In contrast to other floodplain tree species analysed by previous studies (Schöngart et al. 2002, 2004, 2005; Worbes et al. 1995), N. amazonum presents climate–growth relationships which are not related to the hydrological regime and associated oceanic forcing.
Our results suggest that N. amazonum is more resilient to disturbances of the hydrological regime than those tree species responding to variations of the flood pulse. In floodplains downstream of the Balbina hydropower dam, which has been implemented in the 1980s in the Uatumã River (located about 140 km in the northeast direction from this study site), N. amazonum is a dominating tree species (Targhetta et al. 2015; Schöngart et al. 2021) growing at similar topographical levels compared to this study site. Due to the operation of the hydropower dam, the flood pulse was strongly affected (Assahira et al. 2017) resulting at these topographic elevations to changes in the timing of annual flood and drought events and an increasing alternation of water deficit and anoxic conditions. A tree-ring study performed by Neves et al. (2019) indicated that N. amazonum was established at the disturbed floodplains after the implementation of the Balbina dam. Schöngart et al. (2021) postulated the hypothesis that the anthropogenic alteration of the flood pulse at these topographic elevations set up new environmental filters inducing trade-offs leading to the dominance of tree species adapted to the disturbance regime. This hypothesis is sustained by the results obtained from this study, suggesting that N. amazonum is more resilient to flood pulse disturbances compared to other floodplain tree species.
Conclusions
Our findings indicate that tree growth of N. amazonum responds to variations in potential evapotranspiration and minimum temperature but not to the flood pulse. This result is in contradiction with earlier studies which have shown that the flood pulse is the main trigger of tree growth of many species in Central Amazonian floodplains. We suggest that growth responses to potential evapotranspiration and minimum temperature turns N. amazonum also more resilient to disturbances of the hydrological cycle compared to those tree species showing an intrinsic relationship with the flood pulse. On the other hand, future climate scenarios of increased temperatures and severe dry seasons as already observed in the southern Amazon basin (Marengo et al. 2018) might impact tree growth of this species in that region. These are important findings in the background of ongoing climate and land-use changes in the Amazon floodplains such as the intensification of the hydrological cycle (Gloor et al. 2013; Barichivich et al. 2018) or disturbances of the flood pulse by the implementation of several dozen hydropower dams (Assahira et al. 2017; Resende et al. 2020; Schöngart et al. 2021).
In this study, we combined the analysis of different components of tree growth rings with densitometry parameters that provided additional information on several important functional traits to understand the ecology of tropical tree species. Based on our results, we emphasize the need to conduct further dendroclimatic studies, establishing several proxies derived from dated tree rings for tropical species. The combination of different proxies, such as ring width, wood density parameters, stable isotope compounds and wood anatomical parameters, can result in stronger climate–growth relationships compared to the traditional approach based on the total ring width. This innovative approach provides a greater understanding of tree physiology and ecophysiological processes related to the hydrological cycle and carbon dynamics, climate–growth relationships, and dynamic ecosystem processes under global change scenarios.
Author contribution statement
Conceptualization: JS, FW, and MTFP. Field measurements: JQG. Lab processing of X-ray densitometry: JQB, DROR, and MTF. Data analysis: JQG, DROR, FMD, and JS. Supervision: FMD and JS. Writing: JQG, JS, FMD, FW, DROR, MTF and PP. All authors read and commented on this manuscript.
References
Aleixo I, Norris D, Hemerik L, Barbosa A, Prata E, Costa F, Poorter L (2019) Amazonian rainforest tree mortality driven by climate and functional traits. Nat Clim Chang 9:384–388. https://doi.org/10.1038/s41558-019-0458-0
Alves ES, Angyolossy-Alfonso V (2000) Ecological trends in the wood anatomy of some Brazilian species, I: growth rings and vessels. IAWA J 21:3–30. https://doi.org/10.1163/22941932-90000233
Anderegg WRL, Schwalm C, Biondi F, Camarero JJ, Koch G, Litvak M, Ogle K, Shaw JD, Shevliakova E, Williams AP, Wolf A, Ziaco E, Pacala S (2015) Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349:528–532. https://doi.org/10.1126/science.aab1833
Aragão LEOC, Malhi Y, Roman-Cuesta RM, Saatchi S, Anderson LO, Shimabukuro YE (2007) Spatial patterns and fire response of recent Amazonian droughts. Geophys Res Lett 34:L07701. https://doi.org/10.1029/2006GL028946
Aragão LEOC, Anderson LO, Fonseca MG et al (2018) 21st Century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat Commun 9:536. https://doi.org/10.1038/s41467-017-02771-y
Assahira C, Piedade MTF, Trumbore SE, Wittmann F, Cintra BBL, Batista ES, Resende AF, Schöngart J (2017) Tree mortality of a flood-adapted species in response of hydrographic changes caused by an Amazonian river dam. Forest Ecol Manag 396:113–123. https://doi.org/10.1016/j.foreco.2017.04.016
Ayres JMC (1993) As matas de várzea do Mamirauá: Médio Rio Solimões. Estudos Mamiraua, CNPq, Sociedade Civil de Mamirauá, Brasília
Ballantyne A, Smith W, Anderegg W, Kauppi P, Sarmiento J, Tans P, Shevliakova E, Pan Y, Poulter B, Anav A, Friedlingstein P, Houghton R, Running S (2017) Accelerating net terrestrial carbon uptake during the warming hiatus due to reduced respiration. Nat Clim Change 7:148–152. https://doi.org/10.1038/nclimate3204
Barichivich J, Gloor E, Peylin P, Brienen R, Schöngart J, Espinoza JC, Pattnayak KC (2018) Recent intensification of Amazon flooding extremes driven by strengthened Walker circulation. Sci Adv 4:2375–2548. https://doi.org/10.1126/sciadv.aat8785
Barros CF, Ferreira-Marcon ML, Callado CH, Lima HRP, Cunha M, Marquete O, Costa CG (2006) Tendências ecológicas na anatomia da madeira de espécie da comunidade arbórea da Reserva Biológica de Poço das Antas, Rio de Janeiro. Brasil Rodriguésia 57(3):443–460. https://doi.org/10.2307/23498739
Batista ES, Schöngart J (2018) Dendroecology of Macrolobium acaciifolium (Fabaceae) in Central Amazonian floodplain forests. Acta Amazon 48:311–320. https://doi.org/10.1590/1809-4392201800302
Brando PM, Nepstad DC, Davidson EA, Trumbore SE, Ray D, Camargo P (2008) Drought effects on litterfall, wood production and belowground carbon cycling in an Amazon forest: results of a throughfall reduction experiment. Philos Trans R Soc Lond B Biol Sci 363:1839–1848. https://doi.org/10.1098/rstb.2007.0019
Brienen RJW, Zuidema PA (2005) Relating tree growth rainfall in Bolivian rain forest: a test for six species using tree-ring analysis. Oecologia 146:1–12. https://doi.org/10.1007/s00442-005-0160-y
Brienen RJW, Helle G, Pons TL, Guyot J-L, Gloor E (2012) Oxygen isotopes in tree rings are a good proxy for Amazon precipitation and El Niño-Southern Oscillation variability. PNAS 109(42):16957–16962. https://doi.org/10.1073/pnas.1205977109
Brienen RJW, Schöngart J, Zuidema PA (2016) Tree rings in the tropics: insights into the ecology and climate sensitivity of tropical trees. In: Goldstein G, Santiago LS (eds) Tropical tree physiology: adaptations and responses in a changing environment. Springer International Publishing, pp 439–461
Bunn A (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26:115–124. https://doi.org/10.1016/j.dendro.2008.01.002
Clark DA, Piper SC, Keeling CD, Clark DB (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. PNAS 100:5852–5857. https://doi.org/10.1073/pnas.0935903100
Clark DB, Clark DA, Oberbauer SF (2010) Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Global Change Bio 16:747–759. https://doi.org/10.1111/j.1365-2486.2009.02004.x
Cook ER, Kairiukstis LA (1990) Methods of dendrochronology: applications in the environmental sciences. Springer, Netherlands
Costa MH, Botta A, Cardille J (2003) Effects of large-scale change in land cover on the discharge of the Tocantins River, Amazonia. J Hydrol 283:206–217. https://doi.org/10.1016/S0022-1694(03)00267-1
De Simone O, Haase K, Müller E, Junk WJ, Gonsior GA, Schmidt W (2002) Impact of root morphology on metabolism and oxygen distribution in roots and rhizosphere from two Central Amazon floodplain tree species. Funct Plant Biol 29:1025–1035. https://doi.org/10.1071/PP01239
Dodd SR, Nancy KW (1988) Cyclophytic effects on wood structure in Pinus radiata D. Don. I. Densitometry and grain angle. Can J For Res 18:406–412. https://doi.org/10.1139/x88-060
Dünisch O, Montóia VR, Bauch J (2003) Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 17:244–250. https://doi.org/10.1007/s00468-002-0230-2
Erfanian A, Wang G, Fomenko L (2017) Unprecedented drought over tropical South America in 2016: significantly under-predicted by tropical SST. Sci Rep 7:5811. https://doi.org/10.1038/s41598-017-05373-2
Eshete G, Stahl G (1999) Tree rings as indicator of growth periodicity of acacias in the Rift Valley of Ethiopia. Forest Ecol Manag 116:107–117. https://doi.org/10.1016/S0378-1127(98)00442-3
Espinoza JC, Ronchail J, Marengo JA, Segura H (2019) Contrasting North-South changes in Amazon wet-day and dry-day frequency and related atmospheric features (1981–2017). Clim Dyn 52:5413–5430. https://doi.org/10.1007/s00382-018-4462-2x
Evans MN, Schrag DP (2004) A stable isotope-based approach to tropical dendroclimatology. Geochim Cosmochim Acta 68:3295–3305. https://doi.org/10.1016/j.gca.2004.01.006
Ferreira ATB, Tomazello Filho M (2009) Caracterização dos anéis de crescimento de árvores de Pinus caribaea var. hondurensis Barr. et Golf. por densitometria de raios X. Sci Forest 37(83):287–298
Foley JA, Botta A, Coe MT, Costa MH (2002) El Niño-Southern Oscillation and the climate, ecosystems and rivers of Amazonia. Global Biogeochem Cycles 16:1132. https://doi.org/10.1029/2002GB001872
Fontana C, Santini-Junior L, Olmedo GM, Botosso PC, Tomazello-Filho M, Oliveira JM (2019) Assessment of the dendrochronological potential of Licaria bahiana Kurz, an endemic laurel of lowland Atlantic forests in Brazil. Acta Bot Bras 33:454–464. https://doi.org/10.1590/0102-33062019abb0028
Furch K (1997) Chemistry of várzea and igapó soils and nutrient inventory of their floodplain forests. In: Junk WJ (ed) The Central Amazon floodplain: ecology of a pulsing system. Ecological studies, 126. Springer Verlag, Berlin, pp 47–68
Furch K, Klinge H (1989) Chemical relationship between vegetation, soil and water in contrasting inundation areas of Amazonia. In: Proctor J (ed) Mineral nutrients in tropical forest and savanna ecosystems. Blackwell, Oxford, pp 189–204
Gloor M, Brienen RJW, Galbraith D, Feldpausch TR, Schöngart J, Guyot J-L, Espinoza JC, Lloyd J, Phillips OL (2013) Intensification of the Amazon hydrological cycle over the last two decades. Geophys Res Lett 40:1729–1733. https://doi.org/10.1002/grl.50377
Gloor M, Barichivich J, Ziv G, Brienen R, Schöngart J, Peylin P, Cintra BBL, Feldpausch T, Phillips O, Baker J (2015) Recent Amazon climate as background for possible ongoing and future changes of Amazon humid forests. Global Biogeochem Cycles 29:1384–1399. https://doi.org/10.1002/2014GB005080
Godoy-Veiga M, Slotta F, Alecio PC, Ceccantini G, Buckeridge MS, Locosselli GM (2019) Improved tree-ring visualization using autofluorescence. Dendrochronologia 55:33–42. https://doi.org/10.1016/j.dendro.2019.03.003
Goes Ribeiro MN (1976) Aspectos climatológicos de Manaus. Acta Amazónica 6(2):229–233
Gouveia NA, Gherardi DFM, Aragão LEOC (2019) The role of the Amazon river plume on the intensification of the hydrological cycle. Geophys Res Lett 46:12221–12229. https://doi.org/10.1029/2019GL084302
Granato-Souza D, Adenesky-Filho E, Esemann-Quadros K (2017) Dendrochronology and climatic signals in the wood of Nectandra oppositifolia from a dense rain forest in southern Brazil. J For Res 30:545–553. https://doi.org/10.1007/s11676-018-0687-5
Granato-Souza D, Stahle DW, Torbenson MCA, Howard IM, Barbosa AMC, Feng S, Fernandes K, Schöngart J (2020) Multidecadal changes in wet season precipitation totals over the eastern amazon. Geophys Res Lett 47(8):e2020GL087478. https://doi.org/10.1029/2020GL087478
Hansen N (2004) Zur retrospektiven Lokalisation von Zuwachszonen in subtropischen Laubhölzern mittels der Methode der Hochfrequenz-Densitometrie. Freiburger forstliche Forschung, Schriftenreihe 27
Helama S, Bégin Y, Vartiainen M, Peltola H, Kolström T, Meriläinen J (2012) Quantifications of dendrochronological information from contrasting microdensitometric measuring circumstances of experimental wood samples. Appl Radiat Isot 70(6):1014–1023. https://doi.org/10.1016/j.apradiso.2012.03.025
Hoorn C, Bogotá GR, Romero-Baez M, Lammertsma EI, Flantua SGA, Dantas EL, Dino R, Carmo DA, Chemale F Jr (2017) The Amazon at sea: onset and stages of the Amazon River froma marine record, with special reference to Neogene plant turnover in the drainage basin. Glob Planet Change 153:51–65. https://doi.org/10.1016/j.gloplacha.2017.02.005
Horna V, Zimmermann R, Müller E, Parolin P (2010) Sap flow and stem respiration. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological studies, vol 210. Springer Verlag, Dordrecht, pp 223–242
Irion G, Junk W, Mello JN (1997) The large Central Amazonian River floodplains near Manaus: geological, climatological, hydrological and geomorphological aspects. In: The Central Amazon Floodplain, Springer, Berlin, pp 23–46
Irmler U (1986) Temperature dependent generation cycle for the cicindelid beetle Pentacomia egregia Chaud. (Coleoptera, Carabidae, Cicindelidae) of the Amazon valley. Amazoniana 9(3):431–439
Junk WJ (1989) Flood tolerance and tree distribution in Central Amazonian floodplains. In: Nielsen L, Nielsen IC, Baisley H (eds) Tropical forests: botanical dynamics, speciation and diversity. Academic Press, London, pp 47–64
Junk WJ, Bayley PB, Sparks RE (1989) The Flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the International Large River Symposium. Can Spec Publ of Fish and Aquat Sci 106, pp 110–127
Klusek M, Melvin TM, Grabner M (2015) Multi-century long density chronology of living and sub-fossil trees from Lake Schwarzensee, Austria. Dendrochronologia 33:42–53. https://doi.org/10.1016/j.dendro.2014.11.004
Lisi CS, Tomazello Filho M, Botosso PC, Roig FA, Maria VRB, Ferreira-Fedele L, Voigt ARA (2008) Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA J 29:189–207. https://doi.org/10.1163/22941932-90000179
Marengo JA, Espinoza JC (2016) Extreme seasonal droughts and floods in Amazonia: causes, trends and impacts. Intern J Climatol 36:1033–1050. https://doi.org/10.1002/joc.4420
Marengo JA, Tomasella J, Soares WR, Alves LM, Nobre CA (2012) Extreme climatic events in the Amazon basin—climatological and hydrological context of recent floods. Theor Appl Climatol 107:73–85. https://doi.org/10.1007/s00704-011-0465-1
Marengo JA, Souza CA Jr, Thonicke K, Burton C, Halladay K, Betts RA, Alves LM, Soares WR (2018) Changes in climate and land use over the Amazon Region: current and future variability and trends. Front Earth Sci 6:228. https://doi.org/10.3389/feart.2018.00228
Mariaux A (1967) Les cernes dans les bois tropicaux africains, nature et périodicité. Bois et Forêts des Tropiques 113:3–14
Miina P (2000) Dependence of tree-ring, earlywood and latewood indices of Scots pine and Norway spruce on climatic factors in eastern Finland. Ecol Modell 132(5):259–273. https://doi.org/10.1016/S0304-3800(00)00296-9
Mikola P (1950) On variations in tree growth and their significance to growth studies. Commun Inst For Fenn 38(5):1–131
Nepveu G (1976) Croissance et qualité du bois de Framiré. Evolution de la largeur de cerne et composantes densitométriques en function de l’âge. Bois et Fôrets des Tropiques 165:39–58
Neves JRD, Piedade MTF, Resende AF, Feitosa YO, Schöngart J (2019) Impact of climatic and hydrological disturbances on black-water floodplain forests in Central Amazonia. Biotropica 51:484–489. https://doi.org/10.1111/btp.12667
Ohashi S, Okada N, Nobuchi T, Siripatanadilok S, Veenin T (2009) Detecting invisible growth rings of trees in seasonally dry forests in Thailand: isotopic and wood anatomical approaches. Trees 23:813–822. https://doi.org/10.1007/s00468-009-0322-3
Pagotto MA, DeSoto L, Carvalho A, Nabais C, Tomazello Filho M, Ribeiro A, Lisi CS (2017) Evaluation of X-ray densitometry to identify tree-ring boundaries of two deciduous species from semi-arid forests in Brazil. Dendrochronologia 42:94–103. https://doi.org/10.1016/j.dendro.2017.01.007
Parolin P (1997) Auswirkungen periodischer Vernässung und Überflutung auf Phänologie, Photosynthese und Blattphysiologie von Baumarten unterschiedlicher Wachstumsstrategie in zentralamazonischen Überschwemmungsgebieten. Ph.D. thesis, University of Hamburg
Parolin P (2002) Seasonal changes of specific leaf mass and leaf size in trees of Amazonian floodplains. Phyton 42:169–185
Parolin P (2009) Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Annal Bot 103:359–376. https://doi.org/10.1093/aob/mcn216
Parolin P, Junk WJ, Piedade MTF (2001) Gas exchange of six tree species from Central Amazonian floodplains. Trop Ecol 42(1):15–24
Parolin P, Armbrüster N, Junk WJ (2002) Seasonal changes of leaf nitrogen content in trees of Amazonian floodplains. Acta Amazon 32(2):231–240
Parolin P, De Simone O, Haase K, Waldhoff D, Rottenberger S, Kuhn U, Kesselmeier J, Schmidt W, Piedade MTF, Junk W (2004) Central Amazon floodplain forests: tree survival in a pulsing system. Bot Rev 70:357–380
Parolin P, Wittmann F, Schöngart J (2010) Tree phenology in Amazonian floodplain forests. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological studies, vol 210. Springer Verlag, Dordrecht, pp 105–126
Piedade MTF, Junk WJ, Parolin P (2000) The flood pulse and photosynthetic response of trees in white water floodplain (várzea) of the Central Amazon. Brazil Verh Internt Verein Limnol 27(4):1734–1739
Piedade MTF, Ferreira CS, Oliveira Wittmann A, Buckeride M, Parolin P (2010) Biochemistry of Amazonian floodplain trees. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological studies, vol 210. Springer Verlag, Dordrecht, pp 127–140
Pons TL, Helle G (2011) Identification of anatomically non-distinct annual rings in tropical trees using stable isotopes. Trees 25:83–93. https://doi.org/10.1007/s00468-010-0527-5
Poorter L, McDonald I, Alarcón A, Fichtler E, Licona J-C, Peña-Claros M, Sterck F, Villegas Z, Saas-Klaasen U (2010) The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rain forest tree species. New Phytol 185:481–492. https://doi.org/10.1111/j.1469-8137.2009.03092.x
Rahman M, Islam M, Bräuning A (2018) Tree radial growth is projected to decline in South Asian moist forest trees under climate change. Glob Planet Change 170:106–119. https://doi.org/10.1016/j.gloplacha.2018.08.008
Ramírez Correa JA, Grajales ECM, Escobar MB (2010) Anillos Anuales y Clima en Rhizophora mangle L. de la Bahía de Cispatá, Colombia. Revista Facultad Nacional Agronomía, Medellín 63(2):5639–5650
Reis-Avila G, Oliveira JM (2017) Lauraceae: a promising family for the advance of neotropical dendrochronology. Dendrochronologia 44:103–116. https://doi.org/10.1016/j.dendro.2017.04.002
Resende AF, Piedade MTF, Feitosa YO, Andrade VHF, Trumbore SE, Durgante FM, Macedo MO, Schöngart J (2020) Flood-pulse disturbances as a threat for long-living Amazonian trees. New Phytol. https://doi.org/10.1111/nph.16665
Schinker M, Hansen N, Spiecker H (2003) High-frequency densitometry—a new method for the rapid evaluation of wood density variations. IAWA J 24(3):231–239. https://doi.org/10.1163/22941932-90001592
Schnackenburg P, Bräuning A, Helle G (2008) Detecting annual growth rhythms from high-frequency densitometry and carbon isotopes in tropical mountain rain forest trees in southern Ecuador. In: Elferts D, Brumelis G, Gärtner H, Helle G, Schleser G (eds). TRACE—tree rings in archaeology, climatology and ecology, Vol 6: Proceedings of the Dendrosymposium 2007, Riga, Latvia. GFZ Potsdam, Scientific Technical Report STR 08/05, Potsdam, pp 96–99
Schöngart J (2003) Dendrochronologische Untersuchungen in Überschwemmungs-wäldern der Várzea Zentralamazoniens. Göttinger Beiträge zur Land- und Forstwirtschaft in den Tropen und Subtropen 149, Erich Goltze Verlag, Göttingen
Schöngart J, Junk WJ (2007) Forecasting the flood-pulse in Central Amazonia by ENSO-indices. J Hydrol 335:124–132. https://doi.org/10.1016/j.jhydrol.2006.11.005
Schöngart J, Junk WJ (2020) Clima e hidrologia nas várzeas da Amazônia Central. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J (eds) Várzeas Amazônicas: Desafios para um Manejo Sustentável. Editora INPA, Manaus, pp 44–65
Schöngart J, Piedade MTF, Ludwigshausen S, Horna V, Worbes M (2002) Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J Trop Ecol 18:581–597. https://doi.org/10.1017/S0266467402002389
Schöngart J, Junk WJ, Piedade MTF, Ayres JM, Hüttermann A, Worbes M (2004) Teleconnection between tree growth in the Amazonian floodplains and the El Niño-Southern Oscillation effect. Glob Chang Bio 10:683–692. https://doi.org/10.1111/j.1529-8817.2003.00754.x
Schöngart J, Piedade MTF, Wittmann F, Junk WJ, Worbes M (2005) Wood growth patterns of Macrolobium acaciifolium (Benth.) Benth. (Fabaceae) in Amazonian black-water and white-water floodplain forests. Oecologia 145:454–461. https://doi.org/10.1007/s00442-005-0147-8
Schöngart J, Wittmann F, Worbes M (2010) Biomass and NPP of Central Amazonian floodplain forests. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological Studies, vol 210. Springer Verlag, Dordrecht, pp 347–388
Schöngart J, Gribel R, Ferreira da Fonseca-Junior S, Haugaasen T, Group PP, Cient D, Management NR (2015) Age and growth patterns of Brazil Nut Trees (Bertholletia excelsa Bonpl.) in Amazonia, Brazil. Biotropica 47:550–558. https://doi.org/10.1111/btp.12243
Schöngart J, Bräuning A, Barbosa ACMC, Lisi SG, Oliveira JM (2017) Dendroecological studies in the neotropics: history, status and future challenges. In: Amoroso MM, Daniels LD, Baker PJ, Camarero JJ (eds) Dendroecology. Ecological Studies (Analysis and Synthesis), vol 231. Springer, Cham, pp 35–73
Schöngart J, Wittmann F, Resende AF, Assahira C, Lobo GS, Neves JRD, Rocha M, Mori GB, Quaresma AC, Demarchi LO, Albuquerque BW, Feitosa YO, Costa GS, Feitoza GV, Durgante FM, Lopes A, Trumbore SE, Silva TSF, ter Steege H, Val AL, Junk WJ, Piedade MTF (2021) The shadow of the Balbina dam—a synthesis of over 35 years of downstream impacts on floodplain forests in Central Amazonia. Aquat Conserv. https://doi.org/10.1002/aqc.3526
Schweingruber FH (1990) Radiodensitometry. In: Cook ER, Kairiukstis LA (eds) Methods of dendrochronology: applications in the environmental sciences. Springer, Netherlands, pp 55–63
Schweingruber FH, Bräker OU, Schär E (1978) X-ray densitometric results for subalpine conifers and their relationship to climate. In: Fletcher J (ed) Dendrochronology in Europe. British Archaeological Reports, International Series 51, pp 89–100
Seidl R, Thom D, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer CPO (2017) Forest disturbances under climate change. Nature Clim Change 7:395–402. https://doi.org/10.1038/nclimate3303
Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A et al (2015) A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol Lett 18:1406–1419
Sombroek WG (1984) Soils of the Amazon Region. In: Sioli H (ed) The Amazon,limnology and landscape ecology of amighty tropical river and its basin. Dr. W. Junk Publishers, Dordrecht, pp 521–536
Spathelf P, Tomazello Filho M, Tonini H (2010) Dendroecological analysis of Ocotea pulchella and Nectandra maegapotamica on two sites near Santa Maria, Rio Grande do Sul (Brazil). Floresta 40(4):777–788
Speer J (2010) Fundamentals of tree ring research. The University of Arizona Press, Tucson
Sullivan MJP, Lewis SL, Affum-Baffoe K, Castilho C, Costa F, Sanchez AC, Ewango CEN, Hubau W, Marimon B, Monteagudo-Mendoza A, Qie L, Sonké B, Martinez RV, Baker TR, Brienen RJW, Feldpausch TR, Galbraith D, Gloor M, Malhi Y, Aiba S-I, Alexiades MN, Almeida EC, de Oliveira EA, Dávila EÁ, Loayza PA, Andrade A, Vieira SA, Aragão LEOC, Araujo-Murakami A, Arets EJMM, Arroyo L, Ashton P, Aymard CG, Baccaro FB, Banin LF, Baraloto C, Camargo PB, Barlow J, Barroso J, Bastin J-F, Batterman SA, Beeckman H, Begne SK, Bennett AC, Berenguer E, Berry N, Blanc L, Boeckx P, Bogaert J, Bonal D, Bongers F, Bradford M, Brearley FQ, Brncic T, Brown F, Burban B, Camargo JL, Castro W, Céron C, Ribeiro SC, Moscoso VC, Chave J, Chezeaux E, Clark CJ, de Souza FC, Collins M, Comiskey JA, Valverde FC, Medina MC, da Costa L, Dancák M, Dargie GC, Davies S, Cardozo ND, de Haulleville T, de Medeiros MB, del Aguila PJ, Derroire G, Di Fiore A, Doucet J-L, Dourdain A, Droissant V, Duque LF, Ekoungoulou R, Elias F, Erwin T, Esquivel-Muelbert A, Fauset S, Ferreira J, Llampazo GF, Foli E, Ford A, Gilpin M, Hall JS, Hamer KC, Hamilton AC, Dj H, Hart TB, Hédl R, Herault B, Herrera R, Higuchi N, Hladik A, Coronado EH, Huamantupa-Chuquimaco I, Huasco WH, Jeffery KJ, Jimenez-Rojas E, Kalamandeen M, Djuikouo MNK, Kearsley E, Umetsu RK, Kho LK, Killeen T, Kitayama K, Klitgaard B, Koch A, Labrière N, Laurance W, Laurance S, Leal ME, Levesley A, Lima AJN, Lisingo J, Lopes AP, Lopez-Gonzalez G, Lovejoy T, Lovett JC, Lowe R, Magnusson WE, Malumbres-Olarte J, Manzatto ÂG, Marimon BH, Marshall AR, Marthews T, de Almeida Reis SM, Maycock C, Melgaço K, Mendoza C, Metali F, Mihindou V, Milliken W, Mitchard ETA, Morandi PS, Mossman HL, Nagy L, Nascimento H, Neill D, Nilus R, Vargas PN, Palacios W, Camacho NP, Peacock J, Pendry C, Peñuela Mora MC, Pickavance GC, Pipoly J, Pitman N, Playfair M, Poorter L, Poulsen JR, Poulsen AD, Preziosi R, Prieto A, Primack RB, Ramírez-Angulo H, Reitsma J, Réjou-Méchain M, Correa ZR, de Sousa TR, Bayona LR, Roopsind A, Rudas A, Rutishauser E, Abu Salim K, Salomão RP, Schietti J, Sheil D, Silva RC, Espejo JS, Valeria CS, Silveira M, Simo-Droissart M, Simon MF, Singh J, Soto Shareva YC, Stahl C, Stropp J, Sukri R, Sunderland TCH, Svátek M, Swaine MD, Swamy V, Taedoumg H, Talbot J, Taplin J, Taylor D, Ter Steege H, Terborgh J, Thomas R, Thomas SC, Torres-Lezama A, Umunay P, Gamarra LV, Van Der Heijden G, van der Hout P, Van der Meer P, van Nieuwstadt M, Verbeeck H, Vernimmen R, Vicentini A, Vieira ICG, Torre EV, Vleminckx J, Vos V, Wang O, White LJT, Willcock S, Woods JT, Wortel V, Young K, Zagt R, Zemagho L, Zuidema PA, Zwerts JA, Phillips OL (2020) Long-term thermal sensitivity of Earth’s tropical forests. Science 368:869–874. https://doi.org/10.1126/science.aaw7578
Targhetta N, Kesselmeier J, Wittmann F (2015) Effects of the hydroedaphic gradient on tree species composition and aboveground wood biomass of oligotrophic forest ecosystems in the central Amazon basin. Folia Geobot 50:185–205. https://doi.org/10.1007/s12224-015-9225-9
Thornthwaite CW, Mather JR (1955) The water balance. Johns Hopkins Univ, Laboratory in Climatology, Publ in Climat 8(1)
Timpe K, Kaplan D (2017) The changing hydrology of a dammed Amazon. Sci Adv 3:e1700611. https://doi.org/10.1126/sciadv.1700611
Tomazello Filho M, Brazolin S, Chagas MP, Oliveira JTS, Ballarin AW, Benjamin CA (2008) Application of X-ray technique in nondestructiveevaluation of Eucalypt wood. Maderas Ciência Tecnologia 10:139–145. https://doi.org/10.4067/S0718-221X2008000200006
Verheyden A, Helle G, Schleser GH, Dehairs F, Beeckman H, Koedam N (2004) Annual cyclicity in high-resolution stable carbon and oxygen isotope ratios in the wood of the mangrove tree Rhizophora mucronata. Plant Cell Environ 27:1525–1536. https://doi.org/10.1111/j.1365-3040.2004.01258.x
Vetter RE (1995) Untersuchungen über Zuwachsrhythmen an tropischen Bäumen in Amazonien. PhD Thesis, University of Freiburg
Victoria RL, Martinelli LA, Richey JE, Forsberg BR (1989) Spatial and temporal variations in soil chemistry on the Amazon Floodplain. GeoJournal 19:45–52. https://doi.org/10.1007/BF00620548
Vieira J, Campelo F, Nabais C (2009) Age-dependent responses of tree-ring growth and intra-annual density fluctuations of Pinus pinaster to Mediterranean climate. Trees 23:257–265. https://doi.org/10.1007/s00468-008-0273-0
Waldhoff D, Parolin P (2010) Morphology and anatomy of leafs. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological studies, vol 210. Springer Verlag, Dordrecht, pp 179–202
Wang L, Payetete S, Bégin Y (2001) 1300-year tree-ring width and density series based on living, dead and subfossil black spruce at tree-line in Subartic Québec. Canada Holocene 11(3):333–341. https://doi.org/10.1191/095968301674769686
Wang X, Li X, Zhu J, Tanajuta CAS (2018) The strengthening of Amazonian precipitation during the wet season driven by tropical sea surface temperature forcing. Environ Res Lett 13:094015. https://doi.org/10.1088/1748-9326/aadbb9
Wigley TML, Briffa KR, Jones PD (1984) On the average value of correlated time series with applications in dendroclimatology and hydrometeorology. J Climate Appl Meteorol 23:201–221. https://doi.org/10.1175/1520-0450(1984)023%3c0201:OTAVOC%3e2.0.CO;2
WinklerPrins AMGA (2006) Jute cultivation in the lower Amazon, 1940–1990: an ethnographic account from Santarém, Pará, Brazil. J Hist Geogr 32:818–838. https://doi.org/10.1016/j.jhg.2005.09.028
Wittmann F (2012) Tree species composition and diversity in Brazilian freshwater floodplains. In: Pagano M (ed) Mycorrhiza: occurrence and role in aquatic and riparian environments. Nova Science, New York, pp 223–263
Wittmann F, Junk WJ (2016) The Amazon River basin. In: Finlayson CM, Milton GR, Prentice RC, Davidson NC (eds) The wetland book II: distribution, description and conservation. Springer Verlag, New York, pp 1–20
Wittmann F, Anhuf D, Junk WJ (2002) Tree species distribution and community structure of Central Amazonian várrzea forests by remote sensing techniques. J Trop Ecol 18:805–820. https://doi.org/10.1017/S0266467402002523
Wittmann F, Schöngart J, Montero JC, Motzer T, Junk WJ, Piedade MTF, Queiroz HL, Worbes M (2006) Tree species composition and diversity gradients in white-water forests across the Amazon Basin. J Biogeogr 33:1334–1347. https://doi.org/10.1111/j.1365-2699.2006.01495.x
Wittmann F, Schöngart J, Junk WJ (2010a) Phytogeography, species diversity, community structure and dynamics of central Amazonian floodplain forests. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. ecological studies, vol 210. Springer Verlag, Dordrecht, pp 61–102
Wittmann F, Schöngart J, Brito JM, Oliveira Wittmann A, Piedade MTF, Parolin P, Junk WJ, Guillaumet JL (2010b) Manual of trees from central Amazonian várzea floodplains: taxonomy, ecology, and use—Manual de Árvores de Várzea da Amazônia Central. Taxonomia, Ecologia e Uso. Editora INPA, Manaus
Wittmann F, Householder E, Piedade MTF, Assis RLJ, Schöngart J, Parolin P, Junk WJ (2013) Habitat specificity, endemism and the neotropical distribution of Amazon white-water floodplain trees. Ecography 36:690–707. https://doi.org/10.1111/j.1600-0587.2012.07723.x
Worbes M (1986) Lebensbedingungen und Holzwachstum in zentralamazonischen Überschwemmungswäldern. Erich Goltze, Göttingen. Scripta Geobotanica 17:1–112
Worbes M (1989) Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the neotropics. IAWA J 10:109–122. https://doi.org/10.1163/22941932-90000479
Worbes M (1997) The forest ecosystem of the floodplains. In: Junk WJ (ed) The central amazon floodplain: ecology of a pulsating system. Ecological studies, vol 126. Springer Verlag, Berlin, pp 223–265
Worbes M (2002) One hundred years of tree-ring research in the tropics—a brief history and an outlook to future challenges. Dendrochronologia 20:217–231. https://doi.org/10.1078/1125-7865-00018
Worbes M, Fichtler E (2010) Wood anatomy and tree-ring structure and their importance for tropical dendrochronology. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds) Central Amazonian Floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological studies, vol 210. Springer Verlag, Dordrecht, pp 329–346
Worbes M, Klinge H, Revilla JD, Martius C (1992) On the dynamics, floristic subdivision and geographical distríbutíon of várzea forests in Central Amazônia. J Veg Sci 3:553–564. https://doi.org/10.2307/3235812
Worbes M, Klosa D, Lewark S (1995) Rohdichtestruktur von Jahresringen tropischer Hölzer aus zentralamazonischen Überschwemmungswäldern. Holz als Roh- und Werkstoff 53:63–67
Yoon JH, Zeng N (2010) An Atlantic influence on Amazon rainfall. Clim Dyn 34:249–264. https://doi.org/10.1007/s00382-009-0551-6
Acknowledgements
This contribution is part of the Master thesis of JQG at the Instituto Nacional de Pesquisas da Amazônia (INPA, PPG-ECO), which was supported by a fellowship from the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM). This research was made possible by the German-Brazilian project ATTO (Amazon Tall Tower Observatory), supported by the German Federal Ministry of Education and Research (BMBF contracts 01LB1001A and 01LK1602) and the Brazilian Ministério da Ciência, Tecnologia e Inovação (MCTI/FINEP contract 01.11.01248.00). We, furthermore, acknowledge the support by the Amazon State University (UEA), FAPEAM, LBA/INPA, and SDS/CEUC/RDS-Uatumã. We thank the Brazilian National Council for Scientific and Technological Development (CNPq) for support by the Long-term Ecological Research Program–PELD (MCTI/CNPq/FAPs; Grant number: 403792/2012-6) and the Technical/Scientific Cooperation between INPA and the Max-Planck Society for financial support. DROR acknowledges the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for research fellowship (Grant number 2018/22914-8). JS acknowledges support from CNPq (Grant number: 311874/2017-7). We thank the Wood Anatomy and Tree-Ring Laboratory (LAIM) (FAPESP; Grant umber 2009/53951-7) and PIRE-project (FAPESP; Grant number 2017/50085-3). We thank EMBRAPA (Brazilian Agricultural Research Corporation) Amazônia Ocidental for providing climate data; Aparecido Cândido Siqueira for technical support at the laboratory of USP/ESALQ; Celso Rabelo Costa, Mario Luiz Picanço Marinho, Gildo Feitoza Vieira, Alberto Fialho (Cunha) for field work assistance and Anderson de Araújo Reis for creating the map of Catalão Lake location.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical statement
This manuscript is not currently being considered for publication in another journal.
Additional information
Communicated by S. Vospernik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gonçalves, J.Q., Durgante, F.M., Wittmann, F. et al. Minimum temperature and evapotranspiration in Central Amazonian floodplains limit tree growth of Nectandra amazonum (Lauraceae). Trees 35, 1367–1384 (2021). https://doi.org/10.1007/s00468-021-02126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-021-02126-7