Abstract
Key message
In Hancornia speciosa, the latex is synthesized in the cytosol of the ground meristem cells and in plastids of the fusiform derivatives of secondary phloem. Laticifer development is related to climatic seasonality.
Hancornia speciosa is a latescent species that grows in the Brazilian Cerrado (neotropical savanna) and shows significant potential for rubber production and the extraction of bioactive compounds from its latex. We examined the development of laticifers in its stem apex and secondary phloem in relation to climate seasonality. Morphometric evaluations of elongating branches and micromorphometric evaluations of the cambial zone were carried out monthly for 1 year, with structural and ultrastructural analyses of the stem apex and secondary phloem. Laticifer development in both the stem apex and secondary phloem is related to increasing day length and maximum temperature and humidity. Laticifers are formed by anastomosis of the transverse and longitudinal walls of the ground meristem cells of the stem apex, and the fusiform derivatives of secondary phloem in the cambial zone. The process of latex secretion involves the synthesis of terpenic droplets in the cytoplasm of the ground meristem cells of the stem apex and in the plastids of the fusiform derivatives of secondary phloem. Latex is an emulsion formed by the cytosol terpenic droplets engulfed by vacuoles contaning mucilages and proteins secreted by dictyosomes and rough endoplasmic reticulum, and alkaloids secreted by the phloem cells. This work expands our knowledge concerning the development of laticifers in Apocynaceae and contributes to our better understanding of the influence of environmental factors on latex secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laticifers are important secretory structures due to their ecological roles in plant interactions with the biotic and abiotic components of their environments. They aid in deterring herbivores and phytopathogens, controlling water losses, and wound healing (Fahn 1979; Freitas et al. 2010; Milanez 1961, 1966; Ramos et al. 2019; Tan et al. 2017). Those structures are also of economic importance due to the medicinal value of bioactive compounds in the latex and their production of natural rubber precursors—which reinforce the relevance of studies related to their development (Naidoo et al. 2020; Pereira et al. 2015; Souza et al. 2016; Tan et al. 2017).
Laticifers vary widely in origin and structure (Fahn 1979). They can originate from the promeristem in the primary plant body (Gonçalves et al. 2018) and from the vascular cambium, especially from the phloem initials in the secondary structure (Lara and Marcati 2016; Leme et al. 2020; Pace et al. 2019; Rao et al. 2013; Tan et al. 2017). Latex represents a suspension composed of both hydrophilic and lipophilic compounds and maintained under pressure in the laticifers (Gama et al. 2017; Marinho and Teixeira 2019; Naidoo et al. 2020). Those secretions consist of the cell protoplast itself or accumulate in voluminous central vacuoles surrounded by a thin layer of cytoplasm rich in dictyosomes, smooth endoplasmic reticulum, ribosomes, and lipophilic bodies (Fahn 1979; Gonçalves et al 2018; Marinho and Teixeira 2019; Almeida et al. 2020). The chemical composition of latex is related to the activities of the predominant cellular components (Cai et al. 2009; Gonçalves et al. 2018; Kumar et al. 2012; Leme et al. 2020; Rajeswari et al. 2013). As such, anatomical, histochemical, and ultrastructural studies will be essential to the characterization of the secretory processes of plant species.
The development of secretory structures in the stem apex and secondary phloem is controlled by climatic factors. The breaking of apical dormancy and the resumption of cambial activity in tropical environments generally occurs at the beginning of the rainy season, and is associated with increasing temperatures and rainfall intensity (Aref et al. 2014; Krepkowski et al. 2011; Volland-Voigt et al. 2011). In subtropical environments, and in the Cerrado (Brazilian savanna) where the seasons are well defined, the resumption of meristematic activity occurs at a time of mild temperatures at the end of the austral winter, which suggests the greater relevance of day length in overcoming dormancy as compared to temperature and rainfall variations (Calle et al. 2010; Lara and Marcati 2016; Marcati et al. 2016; Oliveira et al. 2009). Studies relating to the formation of laticifers with climatic seasonality are currently restricted to just a few species.
The presence of laticifers is a universal characteristic in the family Apocynaceae (BFG 2015; APG 2016). That anastomosed structural type has been reported in Hancornia speciosa (Gonçalves et al. 2018), Tabernaemontana ventricosa (Naidoo et al. 2020) and species of Rabdadenia (Pirolla-Souza et al. 2019). The non-anastomosed type with intrusive growth has been reported in T. catharinensis (Canaveze et al. 2019) and in other various species of the family (Hagel et al. 2008; Teixeira et al. 2020). The process of cell wall lysis, which leads to anastomosis and the ontogenesis of laticiferous ducts, occurs very early and can be confused with cells elongated by intrusive growth (Gama et al. 2017; Gonçalves et al. 2018; Teixeira et al. 2020). Thus, ontogenetic studies are necessary for their correct classification.
H. speciosa Gomes is a latescent tree species native to the Brazilian Cerrado (BFG 2015; Gonçalves et al. 2018; Pereira et al. 2015). Its cultivation is favored by tolerance to water deficits, which highlights the potential of the species for commercial latex production; the latex is very plastic and suitable for rubber industrialization processes (Almeida et al. 2014; Medeiros et al. 2010). Taking into account the economic and ecological value of H. speciosa, a better understanding of the structure of its laticifer system and the influence of seasonality on latex formation could contribute to the sustainable exploitation of that natural resource.
We analyzed the development of laticifers in the apical meristem as well as in the vascular cambium of the stem as a function of climatic seasonality, and sought to answer the following questions (a) How are laticifers distributed in the stem apex and secondary phloem? (b) What is the structural type of the laticifers found in H. speciosa? (c) What are the relationships between secretory cell structures, secretory processes, and the chemical composition of latex? and (d) What is the effect of seasonal growth on laticifer development?
Materials and methods
Plant material collection
The collection site was a natural area in the Cerrado biome (Brazilian savanna), in the municipality of Montes Claros, Minas Gerais State, Brazil (16°30′53.2″S, 44°04′28.0″W). The regional climate is seasonal tropical, hot, and dry (Aw, by the Köppen classification). The values of day length, rainfall, and average temperatures during the periods of plant material collections were obtained from the National Institute of Meteorology (Instituto Nacional de Meteorologia, do Ministério da Agricultura, Pecuária e Abastecimento do Brasil) (http://www.inmet.gov.br/portal/).
The plant material consisted of 16 stem tips collected monthly between January and Dec 2018, from four branches of four adult individuals in a natural population of H. speciosa (Fig. 1a–d). Each of the stem tips sampled was approximately 1 month old, and had sprouted between the collection dates; they had herbaceous aspects, were about 1 mm thick at the base, and showed no evidence of periderm development. The recent emission of the apexes was evidenced by the presence of scales (or areas of scale abscission) that surround them while dormant buds on the branches.
Stem apex of Hancornia speciosa Gomes (Apocynaceae). a Adult individual in a natural population in the Brazilian Cerrado. b Latex exudation (arrow) in the secondary phloem region in a mature branch. c, d One-month-old apex of an adult individual (arrowhead in c), and the region considered in evaluations of the thickness of the vascular cambium (arrowhead in d)
Morphometric, anatomical, micromorphometric, and histochemical evaluations
The morphometric evaluations of the 16 stem apices collected on a monthly basis, consisted of quantifying the number of internodes, and the lengths and diameters of their bases (using a ruler and digital caliper). Samples from the apical and basal regions of the stem apexes were prepared by fixing in Karnovsky’s solution (Karnovsky 1965) under vacuum conditions (560 mmHg) for 24 h, dehydrating in an ethanol series (Jensen 1962), and embedding in cold hydroxyethyl methacrylate resin (Leica Microsystems Inc., Heidenberg, Germany). Cross and longitudinal Sects. (5 μm thick) were obtained using a rotary microtome (Atako, Tokyo, Japan), stained with toluidine blue pH 4.7 (O’Brien et al. 1964 modified), and mounted on permanent slides with acrylic resin (Itacril, Itaquaquecetuba, Brasil). The micromorphometry of the vascular cambium was evaluated in cross sections of the bases of the 16 stem apexes collected each month. The region of vascular cambium was defined as the region between the vessel elements and the phloem cell elements that recently differentiated from cambium derivatives. Ten measurements were made of the thickness of the vascular cambium at equidistant points along each stem apex section. Measurements were performed using Axion Vision LE Rel.4.8.2. Version 15.0 06–2010 software (Zeiss, Jena, Germany). The morphometric and micromorphometric data were subjected to analysis of variance, and the monthly means compared by the Tukey test (P < 0.05).
Histochemical tests were performed on the sections obtained for anatomical analyzes to determine the chemical nature of the latex and locate the laticifers in differentiation among the cellular elements of the apical meristem and secondary phloem. Tannic acid (Pizzolato and Lillie 1973) was used to detect mucilage; bromophenol blue (Mazia et al. 1953) and Xylidine Ponceau (Vidal 1970) to detect proteins; Dragendorff (Svendsen and Verpoorte 1983) to detect alkaloids; Nile blue (Cain 1947) and Sudan Red IV (Pearse 1980) to detect lipids; and α-naphthol dimethyl paraphenylene diamine (David and Carde 1964) to detect terpenes. The photographic documentation of the anatomical and histochemical evaluations was performed using a digital camera coupled to an Axio Lab A.1 photomicroscope (Zeiss, Jena, Germany).
Ultrastructural analyses
For ultrastructural analyses, stem apex samples undergoing elongation were fixed in Karnovsky’s solution (Karnovsky 1965) for 24 h, post-fixed with 1% osmium tetroxide (0.1 M phosphate buffer, pH 7.2), dehydrated in acetone series, and embedded in Araldite resin (Leica Microsystems, Heidelberg, Germany). Ultrathin Sects. (50 nm thick) were obtained using a UC6 ultramicrotome (Leica Microsystems, Heidelberg, Germany), contrasted in a saturated solution of uranyl acetate and lead citrate (Robards 1978; Roland 1978), and examined using a CM 100 transmission electron microscope (Philips/FEI Corporation, Eindhoven, Netherlands) at 80 kV.
Results
Laticifer distribution and structural type
Laticifers differentiate early from the ground meristem in the stem primary structure by incorporating cells and by partial lysis of their adjacent walls—forming an anastomosed branched system (Fig. 2a, b). The vascular system is syphonostelic, the vascular bundles are bicollateral, and the vascular cambium is located exclusively between the primary external phloem and xylem. Laticifers are located in the inner cortex and outer pith of the stem, close to the vascular cylinder (Fig. 2c, d). The laticifers develop early in the secondary phloem (Fig. 3a), and the anastomosis processes in the precursor cells occur by dissolving the transverse and longitudinal walls of the fusiform derivatives (Fig. 3b–d).
Distributions of anastomosed laticifers in the stem apex of Hancornia speciosa Gomes (Apocynaceae). a, b Longitudinal sections of the apical meristem. Laticifers differentiate early from the ground meristem by incorporating cells and by partial lysis of adjacent walls, forming an anastomosed branched duct (arrow). The boxes refer to the regions shown in Fig. 4. c, d Cross sections: Siphonostelic vascular system, with bicollateral bundles and vascular cambium exclusively between the xylem and the external primary phloem (arrowhead). Laticifers in the cortex and pith near the vascular cylinder (arrow). ca vascular cambium, co cortex, ep epidermis, gm ground meristem, op outer phloem, pc procambium, pi pith, px primary xylem, sx secondary xylem
Distribution of anastomosed laticifers in the secondary phloem of stem apices of Hancornia speciosa Gomes (Apocynaceae). a, b Cross sections. c, d Longitudinal sections. a Phloem development. b–d Laticifer formation from phloem fusiform derivatives (arrowhead). co cortex, cz cambial zone, px primary xylem, ra ray, si sieve plate, sp secondary phloem, sx secondary xylem
Laticifer ontogenesis and latex chemical composition
The differentiation of laticifers and the synthesis of latex in the apical meristem occurs early and involves cells of the promeristem and ground meristem. Lipophilic droplets and bulky vacuoles fuse in the cytoplasm to form an emulsion (Fig. 4a, b). The anastomosed structural type is confirmed by the lysis of adjacent precursor cell walls (Fig. 4c, d).
Ultrastructural aspects of laticifers in the stem apical meristem of Hancornia speciosa Gomes (Apocynaceae). Analyzes in the regions marked in Fig. 2a. a, b Latex synthesis and early differentiation of laticifers from promeristem and ground meristem cells. Lipophilic droplets in the cytoplasm fused with bulky vacuoles to form an emulsion. c, d Anastomosis process by lysis of adjacent precursor cell walls (arrow). dv dictyosome vesicles, em emulsion, la laticifer, nu nucleus, od oil droplets, pl plastids, va vacuole
Laticifers begin to differentiate immediately after the division of the fusiform initials of the vascular cambium in the secondary phloem (Fig. 5a). A thin layer of dense parietal cytoplasm persists on the periphery of the cell, and bulky vacuoles are observed that arise from the fusion of vesicles containing fibrillar material released from the dictyosomes (Fig. 5b). The droplets of lipophilic material synthesized in the plastids (Fig. 5c) are engulfed by vacuoles containing fibrillar material, forming an emulsion (Fig. 5d). The white latex contained in the vacuole was identified through histochemical tests as a mixture of hydrophilic compounds [mucilage (Fig. 6a), proteins (Fig. 6b, c), alkaloids (Fig. 6d)], and lipophilic material [terpenes (Fig. 6e–h)].
Ultrastructural aspects of laticifers in the secondary stem phloem of Hancornia speciosa Gomes (Apocynaceae). a Laticifers during early differentiation, after the cambium fusiform derivatives division. b Thin layer of dense parietal cytoplasm persisting at the cell periphery. Bulky vacuoles from the fusion of vesicles released from the dictyosomes containing fibrillar material. c, d Droplets of lipophilic material synthesized in plastids and engulfed by vacuoles containing fibrillar material, forming an emulsion. di dictyosomes, dv dictyosome vesicles, la laticifer, mi mitochondria, od oil droplets, pc peripheral cytoplasm, pi pits, pl plastids, st starch, va vacuole
Histochemical characterization of the latex produced by the secondary phloem of the stem apices of Hancornia speciosa Gomes (Apocynaceae). a–f, h Cross sections g Longitudinal sections. a Mucilage (black, tannic acid). b Protein (blue, bromophenol). c Protein (red, Xilydine Poinceau). d Alkaloid (brown, Dragendorff). e, f Neutral lipids (rose, Nile blue). g General lipids (red, Sudan). h Terpenes (purple, α-naphthol dimethyl paraphenylene diamine). Arrow—latex in the laticifer, arrowhead—exuded latex
Climatic seasonality
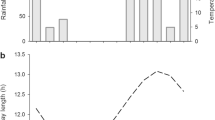
The resumption of meristematic activity of H. speciosa, with the elongation and thickening of the branches, occurs at the end of the dry austral winter with increasing day length (Jul–Sep) (Fig. 7a–e). The numbers of internodes, the lengths and the thickness of the branches, and the thickness of the vascular cambium reach their maximum values in the months with the highest temperatures and rainfall rates (Oct–Feb) (Fig. 7a–c). The increase in thickness of the vascular cambium does not correspond to an increase in the thickness of the bases of the branches (Fig. 7c). That vascular cambium activity begins with the exclusive development of secondary xylem (Jul–Jan) (Fig. 8a–d) and ends with the exclusive development of secondary phloem (in Feb) (Fig. 8e, f).
Climatic seasonality effects on branches of Hancornia speciosa Gomes (Apocynaceae). Tukey test 5% probability. Resumption of meristematic activity at the end of the dry winter, with increasing day length (Jul–Sep). a–c Maximum values for the length of the branches, the number of internodes, the thickness of the vascular cambium, and the thickness of the bases of the branches in the months with the highest temperature and rainfall (Oct–Feb). The increase in thickness of the vascular cambium does not correspond to an increase in the thickness of the bases of the branches. d Day length. e temperature and rainfall
Cambial activity in the stem apex of Hancornia speciosa Gomes (Apocynaceae). Cross sections. a–d Beginning of vascular cambium activity at the end of the dry period, and exclusive development of secondary xylem during the rainy period (Jul–Jan). Note laticifers in the cortex (white arrowhead) and anastomosis between vessel elements (asterisk). e–f Termination of vascular cambium activity (arrow) at the end of the rainy season, and exclusive development of secondary phloem (Feb). Note laticifers in the secondary phloem (black arrowhead). co cortex, ip inner primary phloem, op outer primary phloem, pi pith, px primary xylem, sp secondary phloem, sx secondary xylem
Discussion
Laticifer distribution in stem primary and secondary structures
The branched laticifers in the primary stem structure of H. speciosa are distributed in the cortex and pith and differentiate early from the ground meristem. That distribution pattern is common in Apocynaceae (Lopes et al. 2009; Krentkowski and Duarte 2012; Canaveze and Machado 2016; Gama et al. 2017; Pirolla-Souza et al. 2019; Naidoo et al. 2020) and families such as Euphorbiaceae (Kumar et al. 2012; Rajeswari et al. 2013), Malpighiaceae (Pace et al. 2019), and Cannabaceae (Leme et al. 2020).
In the secondary stem structure of H. speciosa, the latex that exudes from laticifers present in the bark region had differentiated from cambium fusiform derivatives of the phloem. There are records of laticifers in the secondary phloem of Apocynaceae (Gimenez 2004; Canaveze and Machado 2016), Rubiacee (Lara and Marcati 2016), Moraceae (Alonso et al. 2013), Cannabaceae (Leme et al. 2020) and, in particular, Hevea brasiliensis (Euphorbiaceae) due to its economic importance for rubber production (Tan et al. 2017). Laticifers do not occur in the rays of H. speciosa as they do in Ficus maxima (Moraceae) (Alonso et al. 2013) and Carica papaya (Caricaceae) (Rao et al. 2013), nor do they occur in the secondary xylem, as seen in lianas of the Malpighiaceae family (Pace et al. 2019). Laticifers were recorded in the intraxylemic phloem in Apocynaceae, having developed from unusual vascular cambium activity between the primary xylem and the internal primary phloem in the bicollateral bundles of Vallaris solanacea (Gondaliya and Rajput et al. 2016b) and Leptadenia pyrotechnica (Gondaliya and Rajput et al. 2016a). Laticifers are largely distributed close to the phloem in both primary and secondary structures, and that phloem contributes nutrients to the latex secretion process to supply its high energy demands. Laticifer distribution is of significant economic importance, and its structural type will be decisive for high latex extraction yields such as the network of anastomosed laticifers distributed in concentric layers in Hevea brasiliensis (Sando et al. 2009).
Laticifer structural types
The anastomosis process described in this work, which is the fusion of adjacent cells during the ontogenesis of the laticiferous ducts in H. speciosa, results in laticifer elongation and increased caliber, favoring the accumulation and release of latex. The laticifers are formed by the dissolution of the transverse walls of ground meristem cells, and those in the secondary phloem are formed by the dissolution of the transverse and longitudinal walls of cambium fusiform derivatives of the phloem. That anastomosis process can be observed in the discontinuity of the precursor cell walls (Gama et al. 2017) and the continuity of the protoplasts (Gonçalves et al. 2018). During anastomosis, the nuclei assume parietal positions (Lopes et al. 2009; Pirolla-Souza et al. 2019) and eventually collapse (Gonçalves et al. 2018). The cell walls become thick and hydrated, and the plasmodesmata become clogged thus preventing the transit of compounds out of the laticifer—which keeps the latex under pressure (Gonçalves et al. 2018).
Anastomosis differs from laticifer formation by intrusive growth, as it involves successive mitosis and cell polarization, with the nucleus positioned close to the cell end to guide elongation (Gama et al. 2017). The cell extremity is acuminated and its penetration into intercellular spaces is facilitated by pectinase secretions that dissolve the middle lamella (Canaveze et al. 2019). That intrusive growth leads to shorter and smaller caliber ducts, as in the embryos of E. lathyris (Euphorbiaceae) (Castelblanque et al. 2016) in contrast to the development by anastomosis, which gives rise to long and large caliber laticifers, as in the rubber tree H. brasiliensis (Euphorbiaceae) (Sando et al. 2009).
Laticifer ontogenesis and latex secretory process
In H. speciosa, the hydrophilic mucilage and proteins of the latex originate from dictyosome and ribosome activities. The lipophilic terpenes are synthesized in the cytosol of the ground meristem cells and in plastids in the cambium fusiform derivatives of secondary phloem. Secretory structures in plants are good models for studying the dynamics of the synthesis and compartmentalization of chemical compounds relating the compounds to their location of synthesis and accumulation (Mercadante-Simões and Paiva 2013, 2015). The intensity of the secretory process is evidenced by the large volumes of the nucleus and nucleoli (which collapse in later phases) (Lopes et al. 2009), and the cytoplasm is dense due to the abundance of organelles related to the synthesis of the chemical components of the latex. That population of organelles is associated with numerous mitochondria and amyloplasts, which are responsible for providing the energy necessary for intensive synthetic processes (Cai et al. 2009; Kumar et al. 2012; Gama et al. 2017). The hydrophilic and lipophilic compounds produced are progressively engulfed by a bulky central vacuole, which stores that emulsion and occupies almost the entire cell volume (Lopes et al. 2009). Mucilage and proteins have electrontranslucid and fibrillar appearances under transmission electron microscopy, which contrasts with the electron-dense aspect and osmiophilic nature of the terpenes (Krentkowski and Duarte 2012, Gonçalves et al. 2018). At the end of the secretory process, the vacuoles demonstrate autophagic activities and degrade the organelles that gave rise to the latex. The organelle fragments will be integrated into the latex and the cytoplasm reduced to a thin layer pressed against the wall of the mature laticifer (Gonçalves et al. 2018). Secondary compounds present in latex, such as terpenes, alkaloids, and phenolic compounds, favor plant survival in its surrounding biotic and abiotic environment, and serve as deterrents against herbivores and phytopathogens (Freitas et al. 2010; Ramos et al. 2019; Tan et al. 2017).
Climatic seasonality and laticifer development
The initiation of laticifer development in H. speciosa is related to increasing day length at the end of the dry austral winter (in the period still without rainfall) with the resumption of meristematic activity in the stem apex and vascular cambium. The greatest intensity of laticifer formation occurs, however, in the hot and rainy period, and is associated with the highest rate of cambium activity. Some studies have shown that increasing day length is the main inducer of the resumption of vascular cambium activity in several neotropical shrubby and woody species (Lara and Marcati 2016; Marcati et al. 2016). High temperatures and precipitation, on the other hand, are associated with increased vascular cambium activity and phloem differentiation, the sites where laticifers preferentially develop in tropical species (Krepkowski et al. 2011; Volland-Voigt et al. 2011; Aref et al. 2014). Fluctuations in light availability, temperature, and humidity can affect photosynthesis, nutrients availability and plant metabolism, and thus latex production (Souza et al. 2016). The latex aids in controlling herbivores and pathogens (whose populations are also affected by climate components) (Freitas et al. 2010; Ramos et al. 2019; Tan et al. 2017). Understanding the relationship between vascular cambium activity, laticifer formation in the secondary phloem, and climatic factors could help in management planning for efficient latex extraction. Latex collection at the time of the development of laticifers can minimize plant injuries.
Conclusion
Laticifers are found in the cortex and pith of the primary structure, and in the midst of the axial cells of the phloem in the plant secondary structure. The laticifers in the stem apex are branched, and their ontogenesis occurs by anastomosis of the transverse and lateral walls of the ground meristem cells. Laticifer ontogenesis in the secondary phloem is principally by anastomosis of the lateral walls of the cambium fusiform derivatives of the secondary phloem. Latex is an emulsion composed of lipophilic terpenes synthesized in the cytoplasm of the apical meristem cells and in plastids of the fusiform derivatives of the secondary phloem, and includes mucilage, proteins, and hydrophilic alkaloids. The development of laticifers in the stem apex and vascular cambium of H. speciosa is triggered by increasing day lengths during the breaking of apical dormancy and the resumption of cambial activity, with the highest rate of laticifer development occurring in the warmer rainy season of the year. In-depth studies on the formation of laticifers in the secondary phloem in Apocynaceae have been lacking. This work brings advances in our knowledge of the location of laticifers between the phloem cells, the cellular compartmentalization of terpene synthesis and the period of development of the laticifers during cambial activity.
Author's contribution statement
MOMS conceived and designed research, and performed the ultra-structural analyses. LMR analyzed data. AIRCS, KRC, and MPG carried out the phytochemical, anatomical, and histochemical analyses. All of the authors collaborated to the bibliographical revision and writing the text.
References
Almeida LM, Floriano JF, Ribeiro TP, Magno LN, Mota LSLS, Peixoto N, Mrué F, Melo-Reis P, Junior RSL, Graeff CFO (2014) Hancornia speciosa latex for biomedical applications: physical and chemical properties, biocompatibility assessment and angiogenic activity. J Mater Sci Mater Med 25:2153–2162. https://doi.org/10.1007/s10856-014-5255-8
Almeida AL, de Freitas PF, Ferreira CP, Ventrella MC (2020) Syncytial development of annatto (Bixa orellana L.) pigment gland: a curious type of anastomosed articulated laticifer. Flora 274:151727. https://doi.org/10.1016/j.flora.2020.151727
Alonso AAA, Mendonça MS, Reis RS, Biondo PLTA, Alonso RRP (2013) Laticifers distribution in secondary phloem of the Amazon wood species. Comunicata Scientiae 4:212–215
APG (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Lin Soc 181:1–20. https://doi.org/10.1111/boj.12385
Aref IM, Khan PR, Al-Mefarrej H, Al-Shahrani T, Ismail A, Iqbal M (2014) Cambial periodicity and wood production in Acacia ehrenbergiana Hayne growing on dry sites of Saudi Arabia. J Environ Biol 35:301–310
BFG (2015) Growing knowledge: an overview of Seed Plant diversity in Brazil. Rodriguésia 66:1085–1113. https://doi.org/10.1590/2175-7860201566411
Cai X, Li W, Yin L (2009) Ultrastructure and cytochemical localization of acid phosphatase of laticifers in Euphorbia kansui Liou. Protoplasma 238:3–10. https://doi.org/10.1007/s00709-009-0065-4
Cain AJ (1947) The use of Nile Blue in the examination of lipoids. J Cell Sci 3:383–392
Calle Z, Schlumpberger BO, Piedrahita L, Leftin A, Hammer SA, Tye A, Borchert R (2010) Seasonal variation in daily insolation induces synchronous bud break and flowering in the tropics. Trees 24:865–877. https://doi.org/10.1007/s00468-010-0456-3
Canaveze Y, Machado SR (2016) The occurrence of intrusive growth associated with articuled laticifers in Tabernaemontana catharinensis A.DC. A new record for Apocynaceae. Int J Plant Sci 177:1–23. https://doi.org/10.1086/685446
Canaveze Y, Mastroberti AA, Mariath JEA, Machado SR (2019) Cytological differentiation and cell wall involvement in the growth mechanisms of articulated laticifers in Tabernaemontana catharinensis A.DC. (Apocynaceae). Protoplasma 256:131–146. https://doi.org/10.1007/s00709-018-1284-3
Castelblanque L, Balaguer B, Marti C, Rodríguez JJ, Orozco M, Vera P (2016) Novel insights into the organization of laticifer cells: a cell comprising a unified whole system. Plant Physiol 172:1032–1044. https://doi.org/10.1104/pp.16.00954
David R, Carde JP (1964) Coloration différentielle dês inclusions lipidique et terpéniques des pseudophylles du pine maritime au moyen du réactif Nadi. CR Acad Sci Paris, Série D 258:1338–1340
Fahn A (1979) Secretory tissues in plants. Academic Press, San Francisco
Freitas CDT, Souza DP, Araújo ES, Cavalheiro MG, Oliveira LS, Ramos MV (2010) Anti-oxidative and proteolytic activities and protein profile of laticifer cells of Cryptostegia grandiflora, Plumeria rubra and Euphorbia tirucalli. Braz J Plant Physiol 22:11–22. https://doi.org/10.1590/S1677-04202010000100002
Gama TSS, Rubiano VS, Demarco D (2017) Laticifer development and its growth mode in Allamanda blanchetii ADC (Apocynaceae). J Torrey Bot Soc 144:303–312. https://doi.org/10.3159/Torrey-D-16-00050
Gimenez AM (2004) Anatomía de leño y corteza de Tabernaemontana catharinensis A. DC (Apocynaceae). Quebracho 11:22–32
Gonçalves MP, Mercadante-Simões MO, Ribeiro LM (2018) Ontogeny of anastomosed laticifers in the stem apex of Hancornia speciosa (Apocynaceae): a topographic approach. Protoplasma 255:1713–1724. https://doi.org/10.1007/s00709-018-1262-9
Gondaliya AD, Rajput KS (2016a) Stem anatomy and development of intraxylary phloem in Vallaris solanacea (Roth) Kuntze (Apocynaceae). J Indian bot Soc 95:202–215
Gondaliya AD, Rajput KS (2016b) Stem anatomy and development of inter- and intraxylary phloem in Leptadenia pyrotechnica (Forssk.) Decne. (Asclepiadaceae). Plant Biosystems 151:855–865. https://doi.org/10.1080/11263504.2016.1218968
Hagel JM, Yeung EC, Facchini PJ (2008) Got milk? The secret life of laticifers. Trends Plant Sci 13:631–639. https://doi.org/10.1016/j.tplants.2008.09.005
Jensen WA (1962) Botanical histochemistry. WH Freeman, San Francisco
Karnovisky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Bio 27:137–138
Krentkowski FL, Duarte MR (2012) Morpho-anatomical analysis of Aspidosperma olivaceum and A. polyneuron. Apocynaceae Braz J Pharmacog 22:937–945
Krepkowski J, Bräuning A, Gebrekirstos A, Strobl S (2011) Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees 25:59–70. https://doi.org/10.1007/s00468-010-0460-7
Kumar SP, Rajeswari B, Rao AP, Reddy LV, Khan PSSV (2012) Anatomical and ultrastructural peculiarities in the laticifers of Euphorbia antiquorum L.: a potential source for the biofuel production. Curr Bot 4:12–17
Lara NOT, Marcati CR (2016) Cambial dormancy lasts 9 months in a tropical evergreen species. Trees 30:1331–1339. https://doi.org/10.1007/s00468-016-1369-6
Leme FM, Borella PH, Marinho CR, Teixeira SP (2020) Expanding the laticifer knowledge in Cannabaceae: distribution, morphology, origin, and latex composition. Protoplasma 257:1183–1199. https://doi.org/10.1007/s00709-020-01500-5
Lopes KLB, Thadeo M, Azevedo AA, Soares AA, Meira RMSA (2009) Articulated laticifers in the vegetative organs of Mandevilla atroviolacea(Apocynaceae, Apocynoideae). Can J Bot 87:202–209. https://doi.org/10.1139/b08-126
Marcati CR, Machado SR, Podadera DS, Podadera DS, Lara NOT, Bosio F, Wiedenhoeft AC (2016) Cambial activity in dry and rainy season on branches from woody species growing in Brazilian Cerrado. Flora 223:1–10. https://doi.org/10.1016/j.flora.2016.04.008
Marinho CR, Teixeira SP (2019) Cellulases and pectinases act together on the development of articulated laticifers in Ficus montana and Maclura tinctoria (Moraceae). Protoplasma 256:1093–1107. https://doi.org/10.1007/s00709-019-01367-1
Mazia D, Brewer PA, Alfert M (1953) The cytochemical staining and measurement of protein with mercuric bromophenol blue. Biol Bull 104:57–67
Medeiros ES, Galiani PD, Moreno RMB, Mattoso L, Malmonge J (2010) A comparative study of the non-isothermal degradation of natural rubber from Mangabeira (Hancornia speciosa Gomes) and Seringueira (Hevea brasiliensis). J Therm Anal Calorim 100:1045–1050. https://doi.org/10.1007/s10973-009-0477-6
Mercadante-Simões MO, Paiva EAS (2013) Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): Ecological, morphologicaland structural aspects. C R Biol 336:400–406. https://doi.org/10.1016/j.crvi.2013.06.007
Mercadante-Simões MO, Paiva EAS (2015) Anatomy and ultrastructure of the floral nectary of Tontelea micrantha (Celastraceae: Salacioideae): Floral Nectary of Tontelea micrantha. Plant Species Biol 31:117–124. https://doi.org/10.1111/1442-1984.12093
Milanez FR (1961) Contribuição ao conhecimento anatômico de Cryptostegia grandiflora-II. Sobre os laticíferos da estrutura primária (Asclepiaceae). Rodriguésia 35/36:99–128
Milanez FR (1966) Contribuição ao conhecimento anatômico de Cryptostegia grandiflora – III. Nota sobre a estrutura secundária. Rodriguésia 25:335–350
Naidoo C, Naidoo Y, Dewir YH (2020) The secretory apparatus of Tabernaemontana ventricosa Hochst ex A.DC. (Apocynaceae): laticifer identification characterization and distribution. Plants 9:686. https://doi.org/10.3390/plants9060686
O’Brien TP, Feder N, Mccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Oliveira JM, Santarosa E, Pillar VD, Roig FA (2009) Seasonal cambium activity in the subtropical rain forest tree Araucaria angustifolia. Trees 23:107–115. https://doi.org/10.1007/s00468-008-0259-y
Pace MR, Cunha Neto IL, Santos-Silva LNN, Melo-de-Pinna FA, Acevedo-Rodríguez P, Almeida RF, Amorim M, Angyalossy V (2019) First report of laticifers in lianas of Malpighiaceae and their phylogenetic implications. Am J Bot 106:1156–1172. https://doi.org/10.1002/ajb2.1350
Pearse AGE (1980) Histhochemistry theoretical and applied: preparative and optical technology. Churchill Livingston, Edinburgh
Pereira AC, Pereira ABD, Moreira CCL, Botion LM, Lemos VS, Braga FC, Cortes SF (2015) Hancornia speciosa Gomes (Apocynaceae) as a potential anti-diabetic drug. J Ethnopharmacol 161:30–35. https://doi.org/10.1016/j.jep.2014.11.050
Pirolla-Souza AO, ArrudaPaceFarinaccio RCMRMA (2019) Leaf anatomical characters of Rhabdadenia (Rhabdadenieae, Apocynaceae), their taxonomic implications, and notes on the presence of articulated laticifers in the genus. Plant Syst Evol 305:797–810. https://doi.org/10.1007/s00606-019-01608-z
Pizzolato TD, Lillie RD (1973) Mayer’s tannic acid-ferric chloride stain for mucins. J Histochem Cytochem 21:56–64
Rajeswari B, Kumar SP, Rao AP, Khan PSSV (2013) A distribution and ultrastructure of laticifers in the phylloclade of Euphorbia caducifolia Haines, a potential hydrocarbon yielding CAM plant. Am J Plant Sci 5:70–79. https://doi.org/10.4236/ajps.2014.51011
Ramos MV, Demarco D, Souza ICC, Freitas CDT (2019) Laticifers, latex, and their role in plant defense. Trends Plant Sci 24:553–567. https://doi.org/10.1016/j.tplants.2019.03.006
Rao KS, Rajput KS, Kim YS (2013) Secondary growth and occurrence of laticifers in the root of papaya (Carica papaya L). Acta Bot Gallica 160:253–258. https://doi.org/10.1080/12538078.2013.830072
Robards AW (1978) An introduction to techniques for scanning electron microscopy of plant cells. In: Hall JL (ed) Electron microscopy and cytochemistry of plant cells. Elsevier, New York, pp 343–403
Roland AM (1978) General preparations and staining of thin sections. In: Hall JL (ed) Electron microscopy and cytochemistry of plant cells. Elsevier, New York, pp 1–62
Sando T, Hayashi T, Takeda T, Akiyama Y, Nakazawa Y, Fukusaki E, Kobayashi A (2009) Histochemical study of detailed laticifer structure and rubber biosynthesis-related protein localization in Hevea brasiliensis using spectral confocal laser scanning microscopy. Planta 230:215–225. https://doi.org/10.1007/s00425-009-0936-0
Souza TR, Pereira IR, Oliveira AHC, Gonçalves PJ, Almeida LM (2016) Caracterização química do látex de Hancornia speciosa e produtividade associada às características fenotípicas. Rev Agrot 7:61–66. https://doi.org/10.12971/2179-5959
Svendsen AB, Verpoorte R (1983) Chromatography of alkaloids. Elsevier, New York
Tan D, Hu X, Fu L, Kumpeangkeaw A, Ding Z, Sun X, Zhang J (2017) Comparative morphology and transcriptome analysis reveals distinct functions of the primary and secondary laticifer cells in the rubber tree. Sci Rep 7:3126. https://doi.org/10.1038/s41598-017-03083-3
Teixeira SP, Marinho CR, Leme FM (2020) Structural diversity and distribution of laticifers In: Advances in Botanical Research, Elsevier, p 27–54. https://doi.org/10.1016/bs.abr.2019.09.003
Vidal BC (1970) Dichroism in collagen bundles stained with Xylidine Ponceau 2R. Ann Histochim 15:289–296
Volland-Voigt F, Bräuning A, Ganzhi O, Peters T, Maza H (2011) Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 25:39–48. https://doi.org/10.1007/s00468-010-0461-6
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico for funding (423340/2018-2) and granting Scientific Initiation Scholarships (PRP02/2018 PIBIC-CNPq/UNIMONTES, PRP03/2018PIBIC-AF-CNPq/UNIMONTES, PRP02/2019 PIBIC-CNPq/UNIMONTES, PRP 03/2019PIBIC-AF-CNPq/UNIMONTES) and Research Productivity Grants to MOMS (423340/2018-2) and LMR (302216/2018-9); the Centro de Microscopia Eletrônica da Universidade Federal de Minas Gerais—CM/UFMG for the ultrastructural analyses; and Waldimar Ferreira Ruas and Cleidimar Pereira Farias Cardoso for their support during fieldwork and laboratory tests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by B. Fernandez-Marin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, A.I.R.d., Cordeiro, K.R., Gonçalves, M.P. et al. The development of anastomosed laticifers in the stem apical meristem and vascular cambium of Hancornia speciosa (Apocynaceae) is related to climatic seasonality. Trees 35, 1317–1328 (2021). https://doi.org/10.1007/s00468-021-02118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-021-02118-7