Abstract
Keymessage
Under non-extreme drought conditions, reduced stem growth is not associated with reserve or hydraulic impairment in beech suffering from long-term decline.
Abstract
Global change is expected to increase the frequency and the intensity of drought events in temperate ecosystems. In some regions, this might be associated with an increase in tree decline. Of the ecophysiological mechanisms that have been proposed to explain tree decline, an impairment of the vascular transport system and/or carbon function are two of the most discussed. In a context of long-term decline caused by droughts, we investigated the functional differences between the carbon, nitrogen, and hydraulic functions of healthy and declining mature beech (Fagus sylvatica L.) trees. The study was carried out over two consecutive years with contrasting water availabilities. The radial growth of declining trees was clearly less than that of healthy trees, due to a lower growth rate, associated during the wet year with a shorter growth period. Leaf functional characteristics and hydraulic parameters (native embolism and cavitation vulnerability curves) were similar in healthy and declining trees. However, at the end of a growing season characterized by a dry spring, carbon reserves concentrations in young branches of declining trees were lower than those in healthy trees, though they recovered during the following non-constraining growing season. Our results did not indicate carbon starvation, nitrogen deficiency, or hydraulic failure. However, there seems to be some compensation mechanism related to reserve dynamics in the remaining living tissue of the declining trees. This study shows that the climate conditions of successive years are probably crucial for these functional adjustments to be operational.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is resulting in more frequent and more intense droughts, and this tendency is expected to continue in the coming decades, particularly in temperate climates (Intergovernmental Panel on Climate Change 2014). At the same time, the frequency of drought-induced forest declines is increasing (Lorenz and Becher 2012). As a consequence, there has been renewed interest in studying forest decline in the scientific community (McDowell et al. 2008; Adams et al. 2009; Breshears et al. 2009; Leuzinger et al. 2009; Sala 2009; McDowell and Sevanto 2010). However, an accurate prediction of the amplitude of this increased tree mortality in coming decades is not possible (Allen et al. 2010), due to a lack of understanding of the functional mechanisms involved in the responses of trees to climate change. The functional mechanisms underlying tree decline therefore need to be investigated thoroughly (Bréda and Badeau 2008).
The potential roles of carbon (C) and/or water balances in tree decline or mortality during stressful events have long been suggested (Bossel 1986; MartÍnez-Vilalta et al. 2002). In a review based on field observations, Waring (1987) suggested that the C balance of trees is key to understand their reactions to diverse stressors. More recently, research efforts have focused on drought-induced tree mortality (McDowell et al. 2008; Anderegg et al. 2012b; Adams et al. 2013; Hartmann et al. 2013a, b). Two functional mechanisms underlying drought-induced tree mortality have been hypothesized: a failure of the hydraulic system and carbon starvation. The latter is defined as either an exhaustion of C reserves (referred to as NSC—non-structural carbohydrates) of the tree, or a failure to remobilize them (McDowell et al. 2008; Sala et al. 2010). The exclusivity of these two hypotheses has since been reconsidered (Sala et al. 2010; Sevanto et al. 2014). Moreover, it is now known that insects and/or pathogens can play a major role in tree decline (Durand-Gillmann et al. 2012).
Recent studies have investigated the roles of C and/or water balances during drought-related tree declines and mortality (Brodribb and Cochard 2009; Galiano et al. 2011; Galvez et al. 2011; Amoroso et al. 2012; Anderegg et al. 2012a). Several studies of the role of C starvation have yielded contradictory results (Piper 2011; Hartmann et al. 2013b; Zhao et al. 2013). C starvation has been difficult to demonstrate empirically for three reasons: firstly, because this hypothesis relies on the assumption that C reserves accumulate mainly passively (Sala et al. 2010), which has recently been challenged (Wiley et al. 2013; Dietze et al. 2014); secondly, because the NSC pools are never fully depleted (Sala et al. 2010), suggesting that there is a threshold below which the NSC pools are too low to sustain the functioning of the tree in the absence of C assimilation (McDowell et al. 2011); and thirdly, because even if this threshold could be defined for each species, the tree might not be able to remobilize and/or transport the stored NSC (Sala et al. 2010).
Non-structural nitrogen (N) compounds supply N for spring growth and winter maintenance (Wildhagen et al. 2010; Bazot et al. 2013). However, the tree internal N cycle has been largely overlooked in studies of drought-related tree decline, and to our knowledge, the N reserves of trees during decline have seldom, if ever, been assessed. N reserves are essential for long-term tree survival and health status, as N is often a limiting element in forest ecosystems (Waring 1987; LeBauer and Treseder 2008). As a consequence, we hypothesized that N reserves (here referred to as NSNC—non-structural nitrogen compounds) would be lower in declining trees than in healthy trees.
Studies of hydraulic failure, on the other hand, have shown that loss of hydraulic conductance via cavitation may be linked to tree mortality. Indeed, Urli et al. (2013) showed that in temperate angiosperms, a high degree of embolism (around 90 %) was associated with tree mortality, and that drought resistance and cavitation resistance were strongly linked. Moreover, the link between the hydraulic limits of trees and drought-induced tree mortality has been well documented, at both local and global scales (Brodribb and Cochard 2009; Poot and Veneklaas 2012; Choat et al. 2012; Nardini et al. 2013; Barigah et al. 2013). Several studies have also shown that hydraulic failure was the final mechanism causing death of drought-affected trees (Anderegg et al. 2012b; Adams et al. 2013; Hartmann et al. 2013a). However, as pointed out by Plaut et al. (2012), hydraulic failure and C starvation might often interact when drought-related tree mortality occurs.
Despite several reports of multi-year periods of mortality following drought events reported in the literature (Allen et al. 2010; Anderegg et al. 2012a), the ecophysiological differences between healthy and declining trees during long-term declines have seldom been studied. During these events, affected trees may survive for years, or even decades with severely thinned crowns. As described above, short-term responses of C and N reserve concentrations and hydraulic conductance to drought remain unclear. Understanding long-term tree functional responses to repeated droughts is particularly critical for forest survival and predictions of the response of forest productivity to future climate scenarios (Bréda et al. 2006). Since reserve depletion may already be important after a severe drought (Bréda et al. 2006), it can be hypothesized that recurrent droughts would induce drastic decreases in reserves, which could lead to a progressive loss of tree resilience, by reducing the ability to regenerate (López et al. 2009). Similarly, after being damaged by a drought event, the plant hydraulic system could continue to further deteriorate due to the accumulation of permanent damage (Anderegg et al. 2013; Savi et al. 2015) or slower growth of the xylem (Brodribb et al. 2010).
This paper investigates the long-term ecophysiological responses of mature beech trees (Fagus sylvatica L.) to recurrent droughts (especially in 1959, 1976, 1990, 1992, 1996, and 2003). The study plot is an even-aged beech stand (mean tree age of 95 years) growing in the Fontainebleau state forest (France). Low soil water availability has been shown to be a predisposing factor of decline for beech in this forest, and a dendrochronological approach revealed the triggering role of dry years (Silva 2010). In the plot, two categories of trees can be distinguished in terms of their crown conditions: healthy trees with dense crowns and declining trees with highly thinned crowns. Silva (2010) showed that until 1959, the growth of currently declining and healthy trees was similar and that after the dry 1959 growing season, radial growth of currently declining trees had been reduced. Since the 1996 drought, the currently declining trees displayed less interannual growth variation than the currently healthy trees. Furthermore, crown loss in the declining trees also started at the end of the 1990s (Silva 2010), and no biotic attack has been recorded since the regeneration of this stand in the 1910s (National Forest Office, personal communication). Microsite soil water availability is not linked to the health status of the trees (Silva 2010).
In this study, we intended to determine whether the altered growth of declining trees was associated with reduced internal carbon and/or nitrogen concentrations, and/or with hydraulic dysfunction. More precisely, we asked the following questions:
-
1.
To what extent and how is the stem radial growth reduced in declining trees at the seasonal time scale? Does it depend on climatic conditions?
-
2.
Is the growth reduction associated with altered foliar functioning?
-
3.
Is the altered seasonal growth in declining trees related to a dysfunction in reserve mobilization and/or storage?
-
4.
Is water transport damaged during the growing season in declining trees?
To answer these questions, tree radial growth was monitored weekly over two growing seasons with contrasting climatic conditions: one with a spring drought (2011) and one without water constraint (2012). Together with stem radial growth, the seasonal changes of C and N reserve concentrations were assessed over 2 years in stems and young branches. In 2012, foliar functioning was characterized by the percentage of N in the total organic matter, carbon isotope composition (δ13C), and stomatal density measurements on mature leaves. Hydraulic functioning was studied at the branch level by measurements of water potential, vulnerability to cavitation, and native embolism.
Materials and methods
Site and stand description
The study was carried out in the Fontainebleau state forest, France (48°22′N, 02°36′E, mean elevation 120 m a.s.l.) during the 2011 and 2012 growing seasons. This forest extends over 17,000 ha, 60 km southeast of Paris. The climate is temperate, with a mean annual temperature of 10.6 °C and mean annual precipitation of 749 mm, well distributed throughout the year (for the 1960–2010 period). During the 1960–2010 growing seasons (from March to September), mean temperature was 14.1 °C, mean precipitation was 426 mm, and there were on average 91 days with precipitation. The 2011 growing season was remarkable for being unusually dry and warm, particularly in spring: the mean temperature was 15.1 °C, precipitation was 307 mm, and the number of days with precipitation was 69. In contrast, the 2012 growing season had average climatic conditions, with a mean temperature of 14.4 °C, precipitation of 364 mm, and 95 days with precipitation.
The study site is a mature, monospecific beech (F. sylvatica L.) stand which has shown strong signs of decline since the late 1990s (observation by the forester in charge of the plot), where mean tree age in 2011 was 95 years and dominant height was 27 m. During the decline, the thinning of the crown has been observed to be episodic. The forester in charge of the plot has observed that crown loss episodes usually coincided with low precipitation periods. The crown condition typically does not improve during favorable years (with abundant and well temporally distributed precipitation). Mortality of some declining trees was observed by the forester in charge of the plot ca. 10 years after the onset of the visual symptoms of decline. During the 2011 growing season, death of upper branches was observed in the crowns of declining trees, resulting in further thinning of their crowns. Despite more favorable climatic conditions in 2012, the crown health of declining trees did not improve.
Trees were growing on a rather shallow podzoluvisol soil (mean profile depth 0.6 m), with a C:N ratio of 18.7:1, and an extractable soil water content of 101 mm. The soil texture is dominated by Stampian sand mixed with loam and clay at different depths.
Sampling design
Within the plot, ten trees from the dominant storey were chosen according to their health status, so that they formed two homogeneous groups: five “healthy” trees with intact crowns and five “declining” trees with altered crowns. The crown loss was evaluated using the DEPEFEU (DEPErissement des FEUillus) protocol (Nageleisen and Goudet 2011), which quantifies crown thinning on a scale from 0 (healthy tree) to 4 (dead tree). The selected healthy trees had large, dense crowns and abundant fine ramification (average DEPEFEU score 0.9), while the declining trees had reduced crown areas, very transparent crowns, only one or two main branches remaining and very limited fine ramification (average DEPEFEU score 3.6). On average, declining trees had 70 % less leaf area than healthy trees, as estimated with the DEPEFEU protocol. The mean diameters at breast height (45.9 ± 5 cm) and the heights of the two groups (26.5 ± 4 m) were similar. Radial stem growth was followed weekly with manual band dendrometers (DB20, EMS Brno, Brno, Czech Republic) placed at breast height. The spatial distribution of trees was not taken into account. An assessment of the competitive status of the chosen trees carried out as described in Takahashi (1996) after their selection showed that healthy and declining trees were subjected to similar levels of competition. The key phenological stages (budburst, evolution of the leaf mass per area—LMA and yellowing) were also monitored.
Sampling for C and N measurements
Trees were sampled twice in both 2011 and 2012: at budburst (50 % of the tree buds broken) and at yellowing (senescence of 50 % of tree leaves). The time of budburst was determined as described in Vitasse et al. (2009): a bud was considered broken when its leaves started unfolding. In beech, budburst has been shown to correspond to minimum reserve concentrations, while at yellowing the reserve concentrations are at a maximum (Barbaroux and Bréda 2002; Hoch et al. 2003).
On each sampling date, we collected light-exposed branches formed in 2010 using a shotgun; two trunk cores taken at breast height (one for C reserve assessment, one for N reserve assessment) containing phloem and xylem; and two coarse root cores (diameter ≥5 mm, see Le Goff and Ottorini 2001, taken approximately 50 cm from the trunk). Sampling was always performed between 10:00 and 12:00.
Samples were transported in a cooler to the laboratory, where their fresh weight was determined. The samples were then immediately placed in a freezer at −80 °C. The samples destined for carbohydrate analysis were lyophilized for 7 days, and dry weight determined immediately after freeze drying to estimate the humidity content of each organ. The samples destined for soluble protein assessment were kept frozen until analysis.
Light-exposed leaves were collected during the 2012 growing season, lyophilized, and ground to a fine powder. 1 mg subsamples were then placed in tin capsules in order to determine the δ13C and percentage of N with a CN analyzer and a mass spectrometer (Sercon Ltd., Cheshire, UK).
Hydraulic parameters
Water potentials
Pre-dawn potentials were measured twice in each tree during the 2011 and 2012 growing season (on 2011/06/29, 2011/08/23, 2012/05/25, and 2012/08/20) in three light-exposed branches, between 10 and 20 cm long. Midday water potentials were measured only in 2012 growing season. Measurements were immediately made in the field using a pressure chamber (Model 600, PMS Instruments, USA), after recutting 2 cm away from the last visible lead shot injury.
Native embolism
Light-exposed branches in the upper third part of the crown of each tree were sampled three times during the 2012 growing season (on 2012/04/25, 2012/05/29, and 2012/09/07) with a shotgun. The whole branch was immediately wrapped in moist paper towels, placed in a black plastic bag (to prevent any photosynthesis and transpiration), and brought back to the laboratory in a cooler. They were then preserved in a cold room at 7 °C until analysis. Native embolism (% loss of hydraulic conductivity, PLC) on ca. 3 cm branch segments using a XYL’EM® embolism meter (Bronkhorst, Montigny-les-Cormeilles, France). The technique requires hydraulic conductivity measurements before and after water refilling (for further details, see Cochard 2002). The branch segments were allowed to relax for ca. 60 min before measurement to prevent any measurement artifact (Wheeler et al. 2013), then perfused with a degassed 100 mmol l−1 KCl − 10 mmol l−1 CaCl2 solution, filtered at 0.2 µm. Samples were flushed three times for 3 s at 0.2 MPa to remove embolism. Native embolism was computed as described in Sperry et al. (1988).
Cavitation vulnerability curves
To establish vulnerability curves, two south-oriented light-exposed branches from each tree were sampled at the end of May 2012. From each branch, segments ca. 20 cm long were selected. The average age of these segments was 20 years. Vulnerability curves were established using the Cavitron technique (Cochard 2002), as described in Wortemann et al. (2011). Beech vessel length (90 % of beech vessels are shorter than 12 cm, see Hacke and Sauter 1995) is ideal to establish reliable vulnerability curves, as discussed in Cochard et al. (2010). The slope (% MPa−1), xylem pressure inducing losses of conductance of 12 % (P 12), 50 % (P 50), and 88 % (P 88) were computed as described in Wortemann et al. (2011). P 12 is considered as the xylem pressure at which embolism begins to appear in the xylem conduits (Sparks and Black 1999), and P 88 the xylem pressure at which the embolism level in the xylem conduits becomes critical (Domec and Gartner 2001).
Foliar functioning characterization
During the 2012 growing season, light-exposed leaves were sampled at the same phenological stages as trees were sampled for C and N measurements. The total area of 10 leaves randomly selected from each tree was measured using an area meter (Delta-T Area-meter, Delta-T Devices, UK). These 10 leaves were then dried at 60 °C for 4 days, and their LMA computed.
In mid-August 2012, 10 sun-exposed leaves were sampled in the upper third of the crown. On each of these leaves, a 1 cm2 area was covered with a thin layer of nail polish, and left to dry for approximately 20 min. This was then carefully removed with a piece of clear adhesive tape, and mounted on a glass slide. With a magnification of 200 times under an optical microscope (Leitz Wetzlar, Germany), stomata were counted in three separate fields from each leaf. The field width was 930 µm, as calculated with a graduated slide. The stomatal densities of the three fields were averaged to obtain the mean stomatal density of each leaf (number of stomata mm−2). The average stomatal densities of each of the 10 leaves of a given tree were then averaged to obtain the tree’s average stomatal density.
Quantification of N and C compounds
Total non-structural N (TNSN)
Soluble proteins were assessed in the 2010 growth units, the phloem, the outermost 1.5 cm of xylem, and the coarse roots, using an adaptation of the protocol described by Bahrman et al. (1997). 400 mg of fresh matter were ground in liquid N. The proteins were then precipitated with 10 ml of precipitation solution (acetone, 10 % TCA, and 0.07 % β-mercapto-ethanol). After a night at −20 °C, the extracts were centrifuged (15 min, −4 °C, 12,000g) and the supernatants eliminated. The remaining pellets were rinsed with 10 ml 0.07 % β-mercapto-ethanol in acetone, incubated for 1 h at −20 °C, centrifuged (30 min, 4 °C, 12,000g), and the supernatants were again eliminated. The pellets were then dried in a centrifugal vacuum concentrator (Centrivap, Labconco, USA) and 10–20 µl mg−1 of pellet of a solubilization solution (7 M urea, 2 M thiourea, 100 mM DTT, 4 % CHAPS, 0.4 % Triton X-100 in water) were added. After sonication during 30 min, the tubes were centrifuged (30 min, 15 °C, 12,000g), and the supernatants were transferred to other tubes.
Proteins were assessed in these supernatants by the Bradford method (Bradford 1976). Bradford reagent (BioRad Protein Assay, BioRad, USA) was diluted four times, and 3.5 ml of the diluted dye reagent were added to 10 µl of the protein extract mixed with 10 µl of HCl 0.1 mol l−1 and 80 µl of distilled water. After 15 min in the dark, absorbance was read at 595 nm with a spectrophotometer (UVIKON 938, NorthStar, UK). Protein concentration was calculated using solutions of increasing ovalbumine concentrations as standards. The protein concentrations were expressed in grams of reserve compound per 100 g of dry matter (g 100 g DW−1).
Non-structural carbohydrates
Soluble sugars and starch were extracted from 10 mg (±0.5 mg) of finely ground dry matter from the 2010 growth units, phloem, outermost 0.5 cm of xylem, and coarse roots. Samples were ground using a ball mill (MM 301, Retsch, Germany) with 5 mm balls shaken at 30 Hz three times for 2 min. Soluble sugars were extracted twice with 1 ml of boiling 80 % ethanol. These 2 ml were pooled and centrifuged. The supernatants and the pellets containing starch were dried in a centrifugal vacuum concentrator to remove ethanol (Centrivap, Labconco, USA). Soluble sugars and the pellets containing starch were then rehydrated with 0.02 mol l−1 NaOH. Glucose, fructose, and sucrose were assessed enzymatically by the method described in Barbaroux et al. (2003) and Brummer and Cui (2005). Their concentrations were then added to determine the concentration of total soluble sugars. Starch was quantified as the glucose equivalent, using the enzymatic method described for soluble sugars. The concentrations of total non-structural carbohydrates (TNSC), starch, and total soluble sugars were expressed in grams of reserve compound per 100 g of dry matter (g 100 g DW−1).
Statistical analyses
The growth data of each tree for each year were fitted with a sigmoid curve using Matlab software (MATLAB 2008a, MathWorks, Natick, MA, USA):
in which CG is the cumulative stem diameter growth (mm), a is the upper asymptote (mm), S is the growth rate (mm day−1), D is the date (day of year), and b is the inflexion point of the curve.
The dates of start and end of growth were determined by deriving the function six times for the start date and five times for the end date. The dates obtained with the fitted curves were then matched with the closest of our measurement dates. Additionally, annual basal area increment (BAI) was computed from the radial growth data for 2011 and 2012.
All the variables studied (growth-related variable, functional leaf parameters, C and N reserve concentrations, and hydraulic-related parameters) were checked for normality using Shapiro–Wilk normality test. The effects of health status on the different variables were determined with Student’s t tests performed separately for each sampling date. The differences between sampling dates were tested using pairwise Student’s t tests. Concerning C and N reserve concentrations, a potential difference of reserve variations between the 2 years of measurement was tested by creating an annual synthetic variable: the difference between concentrations at budburst and at yellowing for each compound in each organ. On this synthetic variable, we applied paired Student’s t tests to test for a year effect. The same technique was used to test for a possible interaction between health status and phenological stage that could result in differences in reserve dynamics between healthy and declining trees. The relationship between growth and reserve concentrations and dynamics were tested with Pearson correlation tests. Data were analyzed with the R software package (http://www.r-project.org/). In the text, values are presented as averages ± SE.
Results
Stem radial growth
Declining trees had lower diameter increments (P < 0.05, Fig. 1a) and lower BAI (basal area increment, data not shown) than healthy trees during both years. In 2011, the diameter increment was on average 2.4 mm in healthy trees and 1.3 mm in declining trees. The 2012 diameter increments were more than twice those in 2011: 5.7 mm in healthy trees and 3.0 mm in declining trees. Lower growths in declining trees were related to lower growth rates (P < 0.05) and a growth duration difference in 2012 (Fig. 1a): declining trees stopped growing 19 days earlier than healthy ones that year (P < 0.05). The dates of the onset of growth were not significantly different between declining and healthy trees in both years. However, trees stopped growing earlier in 2011 (P < 0.01), resulting in a shorter growing season than in 2012 (73 days in 2011 for both healthy and declining trees, and 121 and 102 days in 2012 for healthy and declining trees, respectively, P < 0.01, Fig. 1a).
Leaf characteristics
At each studied phenological stage (budburst, leaf maturity, yellowing), all the functional leaf characteristics measured (δ13C of total leaf organic matter, %C, %N, mean leaf surface and LMA) were similar in healthy and declining trees (Table 1). The patterns of seasonal variation of these variables were similar for healthy and declining trees (Table 1). LMA increased between budburst and leaf maturity (P < 0.05), and decreased slightly at yellowing (P < 0.05, Table 1). The mean leaf area increased between budburst and leaf maturity (P < 0.05), and did not vary significantly thereafter. %N and δ13C of total organic matter decreased throughout the growing season (P < 0.05).
C and N reserves
In 2011, no effect of health status on TNSC concentrations was detected at budburst in the 2010 growth units. However, at yellowing, TNSC concentrations were lower in declining trees than in healthy ones (P < 0.05, Fig. 2a). No effect of health status on TNSC concentrations in the trunk phloem and xylem (Fig. 2b, c), and in coarse roots (data not shown), was noticed at any date. Starch concentrations in the 2010 growth units exhibited patterns similar to TNSC. No effect of health status on starch concentrations in the trunk phloem and xylem (Fig. 2b, c), or in coarse roots (data not shown), was observed at any date. Health status had no significant effect on soluble sugar concentrations in the 2010 growth units at any phenological stage. No effect of health status on soluble sugars in the trunk phloem was detected at budburst. However, at yellowing, soluble sugars were lower in the trunk phloem of declining trees than in that of healthy ones (P < 0.05, Fig. 2b). Declining trees had higher soluble sugar concentrations in the xylem than healthy trees at budburst (P < 0.05), a difference which had disappeared by yellowing (Fig. 2c).
Concentrations of carbon (soluble sugar and starch, a–c) and nitrogen reserves (soluble proteins, d–f) in healthy (H) and declining (D) Fagus sylvatica trees in Fontainebleau state forest (France) during the 2011 growth season: 2010 growth units (a, d); phloem (b, e); and xylem (c, f). The error bars represent SE. The letters correspond to the results of pairwise Wilcoxon–Mann–Whitney (WMW) sum rank tests. Different letters indicate significant differences in average values over all studied trees between dates (P < 0.05); in (a), (b), and (c) for TNSC concentrations (upper line), for soluble sugar concentrations (middle line), and for starch concentrations (lower line), in (d), (e), and (f) for protein concentrations. At each date, significant differences (WMW tests) between healthy and declining trees are noted by asterisk (P < 0.05) for TNSC concentrations (upper line), for soluble sugar concentrations (middle line), and starch concentrations (lower line)
In 2012, declining trees had lower TNSC concentrations in the 2010 growth units than healthy trees at budburst (P < 0.05, Fig. 2a), but no such difference was observed at yellowing. Health status had no significant effect on TNSC concentrations in the trunk phloem and xylem (Fig. 2b, c) and in the coarse roots (data not shown) whatever the date. No effect of health status on starch and soluble sugar concentrations was observed in any organ at any date (Fig. 2a–c).
Nitrogen reserve concentrations were similar for healthy and declining trees, except at yellowing in 2011 in the xylem, where protein concentrations were lower in healthy than in declining trees (P < 0.05, Fig. 2f).
Interannual variations of growth and reserves
Globally, TNSC concentrations were higher in 2012 than in 2011 in the 2010 growth units, at both budburst and yellowing (P < 0.05, Fig. 2a). TNSC concentrations in the phloem were also higher in 2012 than in 2011 at budburst (P < 0.01, Fig. 2b), but were similar at yellowing in both years. No difference was noted in TNSC concentrations in the xylem between 2011 and 2012 at budburst. However, TNSC concentrations at yellowing were higher in 2012 compared to 2011 (P < 0.05, Fig. 2c). TNSC concentrations in coarse roots did not differ between 2011 and 2012 (data not shown). The interannual variations of starch concentrations were similar to those of TNSC (Fig. 2a–c). Soluble sugar concentrations in the 2010 growth units were higher at budburst in 2012 than in 2011 (P < 0.01), but as it was the opposite at yellowing: the soluble sugar concentrations were higher in 2011 than in 2012 (P < 0.01, Fig. 2a). In the phloem, the soluble sugar concentrations were higher in 2012 than in 2011 at budburst (P < 0.001), but they were not different between years at yellowing (Fig. 2b). In the xylem, at budburst, the soluble sugar concentrations were higher in 2011 than in 2012 (P < 0.05), but this difference had disappeared at yellowing (Fig. 2c). Soluble sugar concentrations in the coarse roots did not significantly differ between 2011 and 2012 (data not shown).
Soluble protein concentrations in the 2010 growth units did not significantly differ between 2011 and 2012 at budburst, whereas they were higher at yellowing in 2011 than in 2012 (P < 0.05, Fig. 2d). Soluble protein concentrations in the trunk phloem were higher in 2011 than in 2012 at both budburst and yellowing (P < 0.05, Fig. 2e). Protein concentrations in the xylem were higher in 2012 than in 2011 at budburst (P < 0.05), but at yellowing the opposite held true (P < 0.01, Fig. 2f). In the coarse roots, soluble protein concentrations were higher in 2012 than in 2011 (P < 0.01, data not shown).
Considering all the trees, the relationship between stem radial growth and seasonal variations in C reserves differed between years: the replenishment of starch reserves in the 2010 growth units was positively correlated with stem radial growth (R 2 = 0.50, P < 0.05) in 2011, but not in 2012 (Fig. 3). A similar correlation was found for replenishment of TNSC in the stem xylem and stem radial growth (R 2 = 0.39, P < 0.05). The seasonal variations of the C reserves were not significantly correlated between years, with the exception of starch in the xylem (P < 0.01, R 2 = 0.66). Stem radial growth was significantly correlated between years (P < 0.01, R 2 = 0.70).
Relationship between basal area increment (BAI, mm2 year−1) and seasonal variations in starch concentration in the 2010 growth units (% dry weight, calculated as the difference between the starch concentrations at yellowing and budburst), in 2011 (gray dots) and 2012 (black dots). A simple regression line for 2011 is shown
Hydraulic characteristics
Tree water status
Health status had no significant effect on pre-dawn water potential whatever the sampling year and date. The average midday water potential was significantly lower in August 2012 (−2.1 MPa) than in May (−1.4 MPa, P < 0.0001, Table 2).
Branch xylem embolism
Healthy and declining trees had similar PLCs at all sampling dates (P > 0.05, Fig. 4). Between April 25th and May 29th, the PLC in both groups decreased significantly, from 35.5 to 20.1 % (Fig. 4), but it did not show any significant variation in September. Regarding vulnerability to water-stress induced cavitation, the parameters describing the curves did not differ significantly between healthy and declining trees (P > 0.05). Average values, considering all trees, were P 12 −2.3, P 50 −3.0, and P 88 −3.6 MPa, and the average slope of the linear part of the curve was 87.3 PLC MPa−1 (Fig. 5).
Average percentage loss of conductivity (±SE) in young branches (1–2 years old) of healthy (H, black) and declining (D, gray) Fagus sylvatica trees in Fontainebleau state forest (France) during the 2012 growing season. Letters correspond to the results of Wilcoxon–Mann–Whitney (WMW) sum rank tests. Different letters indicate significant differences between dates (P < 0.05)
Average cavitation vulnerability curves of healthy and declining Fagus sylvatica trees in 2012. The symbols represent the measured values of percentage loss of conductivity (± SE, n = 5), and the dashed lines represent the model fitted as described in the “Materials and methods” section
Discussion
How does health status affect stem radial growth and its response to climatic conditions, at the seasonal and interannual scales?
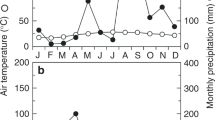
Greater growth rates and a longer growing season in 2012 led to increased growth compared to 2011, in healthy and declining trees. The stem radial growth differences can be explained by climatic conditions: in 2012, precipitation events were frequent and evenly distributed (Fig. 1b), leading to high water availability throughout the growing season, whereas 2011 was marked by a dry spring (Fig. 1a) which probably imposed a hydric limitation on growth. Indeed, it has long been demonstrated that low water availability can impose growth limitations via a decrease in turgor pressure: Hsiao (1973), reviewing the response of plants to water stress, already described this effect more than 40 years ago. More recently, Zweifel et al. (2006), investigating the intra-annual growth patterns of different tree species (notably beech) growing on dry sites in the Alps, showed the importance of the drought sensitivity of turgor-related cell expansion in intra-annual stem radial growth patterns.
As expected, we observed significantly less growth in declining trees than in healthy ones. Numerous studies have presented similar observations, sometimes long before the appearance of crown-level symptoms: studying silver fir dieback in Slovenia, Torelli et al. (1986) noted that declining trees exhibited narrower rings at least 10 years before the occurrence of the first crown-level symptoms of decline. Jenkins and Pallardy (1995), Pedersen (1998), and Demchik and Sharpe (2000) made similar observations of North American oak species experiencing high mortality following recurrent droughts: growth decreased up to 20 years before mortality. More recently, Bréda and Badeau (2008) showed in a meta-analysis that following severe droughts, the stem radial growth of some trees was permanently decreased. Even when the decline or mortality event cannot be related exclusively to dry conditions, affected trees can show decreased growth, as shown by Amoroso et al. (2012) in a study of declining Austrocedrus chilensis. The decreased annual growth of declining trees we observed is consistent with data already obtained from other trees at the same site (Silva 2010), which showed that currently declining trees had exhibited a decreased stem radial increment since 1959.
The average growth difference between healthy and declining trees was greater in 2012 than in 2011. Declining trees thus seem to have a weaker response to interannual climatic variations than healthy trees. Silva (2010) also observed that declining trees displayed reduced interannual variations since the dry year of 1996, when crown-level symptoms of decline appeared. Pedersen (1998) observed a similar reduction of interannual growth variations during the years preceding tree death in North American oaks. However, the long-term studies of Amoroso et al. (2012) and Klein et al. (2014) showed the opposite trend: declining trees showed a stronger response to interannual climatic variation than healthy trees.
Is the growth reduction associated with altered foliar functioning?
All the leaf functional variables (LMA, mean leaf surface, percentage of N, δ13C of total organic matter, and stomatal densities) studied were within the range reported for healthy beech trees in the literature (Aussenac and Ducrey 1977; Balsberg Påhlsson 1989; Keitel et al. 2006; Wang et al. 2008, 2011; Closa et al. 2010; Bresson et al. 2011). All these parameters were similar for healthy and declining trees. Similarly, the observed phenological variations of the measured parameters were consistent with those reported in the literature (Wang et al. 2008; Fotelli et al. 2009; Maunoury-Danger et al. 2010).
Taken together, these parameters give us a partial image of the foliar functioning of the two groups. The δ13C of the total leaf organic matter is known to be related to the tree’s water use efficiency (Farquhar et al. 1989). Combined with the stomatal densities, N content, and the pre-dawn and midday water potentials, it gives us a proxy for stomatal conductance, which is probably similar in healthy and declining trees. This result is not in agreement with observations made at the canopy level in Populus tremuloides by Anderegg et al. (2014): aspen canopies in healthy plots had higher stomatal conductance than the canopies in declining plots. However, this apparent contradiction could stem from differences in spatial scales: while Anderegg et al. (2014) studied aspen decline at the plot level, we focused on individual trees growing in the same plot. It would therefore be highly interesting to apply Anderegg et al.’s (2014) approach to healthy and declining beech plots to enable a proper comparison. Concerning the photosynthetic apparatus, the LMA and N contents of leaf organic matter are known to be related to the biochemical leaf photosynthetic capacities (Evans 1989; Poorter et al. 2009). As a consequence, the C-assimilation capacity at leaf level is likely to be similar in healthy and declining trees. Thus, the observed growth reduction in declining trees is probably not associated with altered leaf functioning but rather with a reduction of carbon uptake due to a reduced leaf area per tree (indirectly quantified here by the crown loss index), as demonstrated in poplars by Anderegg et al. (2014).
Is altered seasonal growth in declining trees related to a dysfunction in reserve mobilization and/or storage?
There is a lack of information concerning the N relations of declining trees. The only studies relevant to this subject in the literature deal with soil N absorption and partitioning by trees during experimental droughts (Fotelli et al. 2002, 2004; Geßler et al. 2004; Peuke and Rennenberg 2004). In our study, health status had little effect on N reserve concentrations and dynamics. The measured protein concentrations were within the ranges reported by Bazot et al. (2013) and Gilson et al. (2014) for temperate oaks. Similarly, the percentage of N in sun-exposed leaves was similar in healthy and declining trees, and within the range reported in the literature (Påhlsson 1992; Bussotti et al. 2005). N limitation is therefore unlikely at our study site, and is probably not significantly implicated in the beech decline reported. The results from literature are rather contradictory, and are not directly comparable to those presented in this paper. Even during the moderate drought year 2011, leaf N and protein contents were similar in declining and healthy trees, an observation consistent with previous results. Indeed, Fotelli et al. (2002) found no effect of drought on the protein N content of beech seedlings. Moreover, Fotelli et al. (2004) and Peuke and Rennenberg (2004) observed no effect of drought on N assimilation in beech seedlings. However, Fotelli et al. (2002) and Geßler et al. (2004) showed that under dry conditions, N absorption by beech trees was reduced.
Young branches of declining trees had less C reserves than healthy ones following the constraining 2011 growing season. This reserve deficit was caused by lower starch concentrations in the declining trees. A reduction of C starch concentrations following a drought was documented in the stems of beech trees that had suffered defoliation during the 2003 heat wave and drought (Bréda et al. 2006). Similar observations were made in Mediterranean forest trees: a clear association between drought-induced defoliation and lower C reserve concentrations in Quercus ilex was observed (Galiano et al. 2012; Rosas et al. 2013). In our study, this difference in C reserve concentrations was still present at the following budburst. However, after the moist 2012 growing season, C reserve concentrations in young branches of healthy and declining trees were similar (Fig. 2a). It therefore seems that in 2012, declining trees made a functional adjustment at the tissue level by storing more C reserves in young branches than healthy trees. The reduced stem radial growth of declining trees was therefore associated with differences in reserve concentrations between budburst and yellowing. This relationship between growth and C reserves can be examined more precisely by correlating stem radial growth and reserve replenishment of individual trees during the two contrasted growing seasons. In 2011, stem radial growth was positively correlated with the replenishing of C reserves in young branches (Fig. 3): the more a tree grew, the greater the increase of C reserve concentrations during the growing season. This correlation was however not significant in 2012. Declining trees in fact increased their stem radial growth between 2011 and 2012 to a lesser extent than healthy trees, while increasing the replenishment of their C reserves in stems and young branches between the 2 years. In beech, C reserve storage and growth were concomitant for both healthy and declining trees in 2011 and 2012 (data not shown). Barbaroux and Bréda (2002) and Scartazza et al. (2013) similarly showed that in beech, growth and C reserve formation were not disconnected. Nevertheless, it was noticeable that in 2012, the growth of declining trees stopped almost 2 weeks before that of healthy trees (Fig. 1a; beginning of August in declining trees and mid-August in healthy trees). Net C assimilation at the tree level has been shown to occur from May to September in beech (Lebaube et al. 2000; Pilegaard et al. 2001). Thus, the assimilated C could be stored after the cessation of growth, explaining the higher increase of C reserve concentrations in branches of declining trees. The concomitance of growth and C storage at the beginning of the growing season confirmed the active nature of C reserve formation, as described by Chapin et al. (1990), whereas C storage is likely to become a passive process after the cessation of growth. However, if the stem radial growth of declining trees was actively down regulated, C reserve formation at the end of the 2012 growing season could be considered semi-active, as described by Dietze et al. (2014). It would therefore mean that in declining trees, during the 2012 growing season, the stem radial growth was compromised to ensure the replenishing of C reserves. Carbon reserve concentrations are known to correlate with the ability of trees to recover from stressful events (Bréda et al. 2006; Galiano et al. 2011) and with tree survival (Canham et al. 1999; Gleason and Ares 2004; Myers and Kitajima 2007). Our results point towards a potential strategy of C storage at the expense of short-term growth to ensure long-term survival, as proposed in Sala et al. (2012).
Is water transport altered by health status during the growing season?
Similar pre-dawn water potentials in healthy and declining trees in both 2011 and 2012 indicate that the trees probably have similar access to soil water resources (Ritchie and Hinckley 1975). This finding is consistent with that presented by Silva (2010): the local variability of soil depth around beech trees growing in the same plot (as in this paper) was not spatially correlated with the pattern of decline. Our results showed similar native embolism levels throughout the growing season in healthy and declining trees, well below levels lethal to beech (PLC around 90 % Barigah et al. 2013). Moreover, healthy and declining trees also had similar vulnerabilities to cavitation, well within the range reported for beech (Wortemann et al. 2011). As a consequence, our data suggest that hydraulic failure sensu stricto (without considering the potential interactions with C metabolism) is probably not a chronic functional mechanism involved in beech decline in Fontainebleau state forest. However, hydraulic failure during an extreme dry year such as 2003 leading to observed loss of young branches in declining trees cannot be excluded. In this species, the xylem function seems to fail only under very prolonged and extreme hydric stress (Barigah et al. 2013, study on saplings).
Potential interaction between C and hydraulics
Sala et al. (2012) exposed that the hydraulic and C systems of trees are likely to interact with each other. Since declining trees had less C reserves than healthy ones in young branches at the end of the constraining 2011 growing season, it is possible that, in the case of a more severe drought, or of recurrent droughts, declining trees could fail to maintain sufficient C reserve concentrations in other storage compartments in order to ensure the growth of new organs at the beginning of the following growing season, and proper osmoregulation of the xylem sap, and consequently suffer from C-related hydraulic failure. Indeed, the importance of sufficient C reserve concentrations in the maintenance of the xylem transport via osmoregulation processes has recently been demonstrated: Sevanto et al. (2014) showed that under drought conditions, hydraulic failure in Pinus edulis might be associated with a loss of C reserve content required for osmoregulation, in turn promoting hydraulic failure. A study of within-crown variability of both C reserves and hydraulic function could clarify the link between these two aspects of tree function, and improve our understanding of the increased fine branch mortality observed in declining trees.
In conclusion, this study documents tree functional responses after a long-term, drought-related decline in beech, at the individual level. Declining trees were characterized by growth clearly lower than that of healthy trees. This decrease is linked to reduced carbon assimilation, mainly due to a thinned canopy rather than an impairment of leaf function. During a favorable year following a constraining one, their growth recovery was limited especially by the length of the growing season. This apparent limitation could in fact be beneficial, by allowing a seasonal recovery of the reserve content in woody tissue. With the exception of growth, functioning of declining trees is globally still close to that of healthy trees for the surviving tissues (for C, N, and hydraulic functioning). This study concerned favorable or moderately stressful years, and should be completed by examining tree functioning during an extremely constraining year, when hydraulic and reserve dynamics are probably impacted to greater and different extents.
Author contribution statement
AD, SB, and CD conceived and designed experiments. AD conducted all laboratory analysis, carried out data analysis, wrote the main part of the manuscript and prepared the figures. CD and SB contributed to the redaction of the manuscript.
References
Adams HD, Guardiola-Claramonte M, Barron-Gafford GA et al (2009) Reply to Sala: temperature sensitivity in drought-induced tree mortality hastens the need to further resolve a physiological model of death. Proc Natl Acad Sci USA 106:E69. doi:10.1073/pnas.0905282106
Adams HD, Germino MJ, Breshears DD et al (2013) Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol 197:1142–1151. doi:10.1111/nph.12102
Allen CD, Macalady AK, Chenchouni H et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684. doi:10.1016/j.foreco.2009.09.001
Amoroso MM, Daniels LD, Larson BC (2012) Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: the use of tree-rings as an indicator of forest decline. For Ecol Manag 265:62–70
Anderegg WRL, Berry JA, Field CB (2012a) Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends Plant Sci 17:693–700
Anderegg WRL, Berry JA, Smith DD et al (2012b) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA 109:233–237
Anderegg WRL, Plavcová L, Anderegg LDL et al (2013) Drought’s legacy: multiyear hydraulic deterioration underlies widespread aspen forest die-off and portends increased future risk. Glob Chang Biol. doi:10.1111/gcb.12100
Anderegg WRL, Anderegg LDL, Berry JA, Field CB (2014) Loss of whole-tree hydraulic conductance during severe drought and multi-year forest die-off. Oecologia 175:11–23. doi:10.1007/s00442-013-2875-5
Aussenac G, Ducrey M (1977) Etude bioclimatique d’une futaie feuillue (Fagus silvatica L. et Quercus sessiliflora Salisb.) de l’Est de la France. I—Analyse des profils microclimatiques et des caractéristiques anatomiques et morphologiques de l’appareil foliaire. Ann des Sci For 34:265–284
Bahrman N, Plomion C, Petit RJ, Kremer A (1997) Contribution of two-dimensional electrophoresis of proteins to maritime pine genetics. Ann For Sci 54:225–236
Balsberg Påhlsson AM (1989) Mineral nutrients, carbohydrates and phenolic compounds in leaves of beech (Fagus sylvatica L.) in southern Sweden as related to environmental factors. Tree Physiol 5:485–495
Barbaroux C, Bréda N (2002) Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 22:1201–1210
Barbaroux C, Bréda N, Dufrêne É (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615. doi:10.1046/j.1469-8137.2003.00681.x
Barigah TS, Charrier O, Douris M et al (2013) Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Ann Bot 112:1431–1437. doi:10.1093/aob/mct204
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees Struct Funct 27:1023–1034. doi:10.1007/s00468-013-0853-5
Bossel H (1986) Dynamics of forest dieback: systems analysis and simulation. Ecol Modell 34:259–288. doi:10.1016/0304-3800(86)90008-6
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bréda N, Badeau V (2008) Forest tree responses to extreme drought and some biotic events: towards a selection according to hazard tolerance? Comptes Rendus Geosci 340:651–662. doi:10.1016/j.crte.2008.08.003
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644. doi:10.1051/forest
Breshears DD, Myers OB, Meyer CW et al (2009) Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Front Ecol Environ 7:185–189. doi:10.1890/080016
Bresson CC, Vitasse Y, Kremer A, Delzon S (2011) To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol 31:1164–1174. doi:10.1093/treephys/tpr084
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149:575–584. doi:10.1104/pp.108.129783
Brodribb TJ, Bowman DJMS, Nichols S et al (2010) Xylem function sand growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol 188:533–542. doi:10.1111/j.1469-8137.2010.03393.x
Brummer Y, Cui SW (2005) Understanding carbohydrate analysis. Food Carbohydr Chem Phys Prop Appl. Taylor and Francis group, pp 67–104
Bussotti F, Prancrazi M, Matteucci G, Gerosa G (2005) Leaf morphology and chemistry in Fagus sylvatica (beech) trees as affected by site factors and ozone: results from CONECOFOR permanent monitoring plots in Italy. Tree Physiol 25:211–219. doi:10.1093/treephys/25.2.211
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11. doi:10.1007/s004420050900
Chapin SF, Schultze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Choat B, Jansen S, Brodribb TJ et al (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755
Closa I, Irigoyen JJ, Goicoechea N (2010) Microclimatic conditions determined by stem density influence leaf anatomy and leaf physiology of beech (Fagus sylvatica L.) growing within stands that naturally regenerate from clear-cutting. Trees Struct Funct 24:1029–1043. doi:10.1007/s00468-010-0472-3
Cochard H (2002) A technique for measuring xylem hydraulic conductance under high negative pressures. Plant Cell Environ 25:815–819. doi:10.1046/j.1365-3040.2002.00863.x
Cochard H, Herbette S, Barigah TS et al (2010) Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant Cell Environ 33:1543–1552. doi:10.1111/j.1365-3040.2010.02163.x
Demchik MC, Sharpe WE (2000) The effect of soil nutrition, soil acidity and drought on northern red oak (Quercus rubra L.) growth and nutrition on Pennsylvania sites with high and low red oak mortality. For Ecol Manag 136:199–207. doi:10.1016/S0378-1127(99)00307-2
Dietze MC, Sala A, Carbone MS et al (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. doi:10.1146/annurev-arplant-050213-040054
Domec J-C, Gartner BL (2001) Cavitation and water storage capacity in bole xylem segments of mature and young Douglas-fir trees. Trees Struct Funct 15:204–214. doi:10.1007/s004680100095
Durand-Gillmann M, Cailleret M, Boivin T et al (2012) Individual vulnerability factors of Silver fir (Abies alba Mill.) to parasitism by two contrasting biotic agents: mistletoe (Viscum album L. ssp. abietis) and bark beetles (Coleoptera: Curculionidae: Scolytinae) during a decline process. Ann For Sci 69:1–15. doi:10.1007/s13595-012-0251-y
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19. doi:10.1007/BF00377192
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Fotelli MN, Rennenberg H, Geßler A (2002) Effects of drought on the competitive interference of an early successional species (Rubus fruticosus) on Fagus sylvatica L. Seedlings: 15 N uptake and partitioning, responses of amino acids and other N compounds. Plant Biol 4:311–320. doi:10.1055/s-2002-32334
Fotelli MN, Rienks M, Rennenberg H, Geßler A (2004) Climate and forest management affect 15N-uptake, N balance and biomass of European beech seedlings. Trees Struct Funct 18:157–166. doi:10.1007/s00468-003-0289-4
Fotelli MN, Nahm M, Radoglou K et al (2009) Seasonal and interannual ecophysiological responses of beech (Fagus sylvatica) at its south-eastern distribution limit in Europe. For Ecol Manag 257:1157–1164
Galiano L, MartÍnez-Vilalta J, Lloret F (2011) Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol 190:750–759. doi:10.1111/j.1469-8137.2010.03628.x
Galiano L, MartÍnez-Vilalta J, Sabaté S, Lloret F (2012) Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean holm oak forest. Tree Physiol 32:478–489. doi:10.1093/treephys/tps025
Galvez DA, Landhäusser SM, Tyree MT (2011) Root carbon reserve dynamics in aspen seedlings: does simulated drought induce reserve limitation? Tree Physiol 31:250–257. doi:10.1093/treephys/tpr012
Geßler A, Keitel C, Nahm M, Rennenberg H (2004) Water shortage affects the water and nitrogen balance in central European beech forests. Plant Biol 6:289–298
Gilson A, Barthes L, Delpierre N et al (2014) Seasonal changes in carbon and nitrogen compound concentrations in a Quercus petraea chronosequence. Tree Physiol 34:716–729. doi:10.1093/treephys/tpu060
Gleason SM, Ares A (2004) Photosynthesis, carbohydrate storage and survival of a native and an introduced tree species in relation to light and defoliation. Tree Physiol 24:1087–1097. doi:10.1093/treephys/24.10.1087
Hacke UG, Sauter JJ (1995) Vulnerability of xylem to embolism in relation to leaf water potential and stomatal conductance in Fagus sylvatica f. purpurea and Populus balsamifera. J Exp Bot 46:1177–1183. doi:10.1093/jxb/46.9.1177
Hartmann H, Ziegler W, Kolle O, Trumbore S (2013a) Thirst beats hunger—declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol 200:340–349. doi:10.1111/nph.12331
Hartmann H, Ziegler W, Trumbore S (2013b) Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct Ecol 27:413–427. doi:10.1111/1365-2435.12046
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570. doi:10.1146/annurev.pp.24.060173.002511
Intergovernmental Panel on Climate Change (2014) Climate change 2014: synthesis report
Jenkins MA, Pallardy SG (1995) The influence of drought on red oak group species growth and mortality in the Missouri Ozarks. Can J For Res 25:1119–1127. doi:10.1139/x95-124
Keitel C, Matzarakis A, Rennenberg H, Geßler A (2006) Carbon isotopic composition and oxygen isotopic enrichment in phloem and total leaf organic matter of European beech (Fagus sylvatica L.) along a climate gradient. Plant Cell Environ 29:1492–1507. doi:10.1111/j.1365-3040.2006.01520.x
Klein T, Hoch G, Yakir D, Körner C (2014) Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol 34:981–992. doi:10.1093/treephys/tpu071
Le Goff N, Ottorini J-M (2001) Root biomass and biomass increment in a beech (Fagus sylvatica L.) stand in North-East France. Ann For Sci 58:1–13
Lebaube S, Le Goff N, Ottorini J-M, Granier A (2000) Carbon balance and tree growth in a Fagus sylvatica stand. Ann For Sci 57:49–61
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. doi:10.1890/06-2057.1
Leuzinger S, Bigler C, Wolf A, Körner C (2009) Poor methodology for predicting large-scale tree die-off. Proc Natl Acad Sci USA 106:E106. doi:10.1073/pnas.0908053106
López BC, Gracia CA, Sabaté S, Keenan T (2009) Assessing the resilience of Mediterranean holm oaks to disturbances using selective thinning. Acta Oecologica 35:849–854. doi:10.1016/j.actao.2009.09.001
Lorenz M, Becher G (2012) Forest condition in Europe 2012, technical report of ICP forests, Hamburg
MartÍnez-Vilalta J, Piñol J, Beven K (2002) A hydraulic model to predict drought-induced mortality in woody plants: an application to climate change in the Mediterranean. Ecol Modell 155:127–147. doi:10.1016/S0304-3800(02)00025-X
Maunoury-Danger F, Fresneau C, Eglin T et al (2010) Impact of carbohydrate supply on stem growth, wood and respired CO2 delta13C: assessment by experimental girdling. Tree Physiol 30:818–830. doi:10.1093/treephys/tpq039
McDowell NG, Sevanto S (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytol 186:263–264
McDowell NG, Pockman WT, Allen CD et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. doi:10.1111/j.1469-8137.2008.02436.x
McDowell NG, Beerling DJ, Breshears DD et al (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26:523–532. doi:10.1016/j.tree.2011.06.003
Myers JA, Kitajima K (2007) Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J Ecol 95:383–395. doi:10.1111/j.1365-2745.2006.01207.x
Nageleisen LM, Goudet M (2011) Manuel de Notation des dommages forestiers (symptômes, causes, état des cimes), Paris
Nardini A, Battistuzzo M, Savi T (2013) Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol 200:322–329. doi:10.1111/nph.12288
Påhlsson AM (1992) Influence of nitrogen fertilization on minerals, carbohydrates, amino acids and phenolic compounds in beech (Fagus sylvatica L.) leaves. Tree Physiol 10:93–100
Pedersen BS (1998) The role of stress in the mortality of midwestern oaks as indicated by growth prior to death. Ecology 79:79–93
Peuke AD, Rennenberg H (2004) Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): phosphorus most affected by drought. Trees Struct Funct 18:639–648. doi:10.1007/s00468-004-0335-x
Pilegaard K, Hummelshøj P, Jensen NO, Chen Z (2001) Two years of continuous CO2 eddy-flux measurements over a Danish beech forest. Agric For Meteorol 107:29–41. doi:10.1016/S0168-1923(00)00227-6
Piper FI (2011) Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann For Sci 68:415–424. doi:10.1007/s13595-011-0030-1
Plaut JA, Yepez EA, Hill J et al (2012) Hydraulic limits preceding mortality in a piñon–juniper woodland under experimental drought. Plant Cell Environ 35:1601–1617
Poorter H, Niinemets Ü, Poorter L et al (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. doi:10.1111/j.1469-8137.2009.02830.x
Poot P, Veneklaas EJ (2012) Species distribution and crown decline are associated with contrasting water relations in four common sympatric eucalypt species in southwestern Australia. Plant Soil 364:409–423. doi:10.1007/s11104-012-1452-8
Ritchie GA, Hinckley TM (1975) The pressure chamber as an instrument for ecological research. Adv Ecol Res 9:165–254. doi:10.1016/S0065-2504(08)60290-1
Rosas T, Galiano L, Ogaya R et al (2013) Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought. Front Plant Sci 4:1–16. doi:10.3389/fpls.2013.00400
Sala A (2009) Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proc Natl Acad Sci USA 106:E68. doi:10.1073/pnas.0904580106
Sala A, Piper FI, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281. doi:10.1111/j.1469-8137.2009.03167.x
Sala A, Woodruff DR, Meinzer FC (2012) Carbon dynamics in trees: feast or famine? Tree Physiol 32:764–775. doi:10.1093/treephys/tpr143
Savi T, Bertuzzi S, Branca S et al (2015) Drought-induced xylem cavitation and hydraulic deterioration: risk factors for urban trees under climate change? New Phytol 205:1106–1116. doi:10.1111/nph.13112
Scartazza A, Moscatello S, Matteucci G et al (2013) Seasonal and inter-annual dynamics of growth, non-structural carbohydrates and C stable isotopes in a Mediterranean beech forest. Tree Physiol 33:730–742. doi:10.1093/treephys/tpt045
Sevanto S, McDowell NG, Dickman LT et al (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161. doi:10.1111/pce.12141
Silva DE (2010) Ecologie du hêtre (Fagus sylvatica L.) en marge sud-ouest de son aire de distribution. Université Henri Poincaré, Nancy
Sparks JP, Black RA (1999) Regulation of water loss in populations of Populus trichocarpa: the role of stomatal control in preventing xylem cavitation. Tree Physiol 19:453–459. doi:10.1093/treephys/19.7.453
Sperry JS, Donnelly JR, Tyree MT (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11:35–40. doi:10.1111/j.1365-3040.1988.tb01774.x
Takahashi K (1996) Plastic response of crown architecture to crowding in understorey trees of two co-dominating conifers. Ann Bot 77:159–164
Torelli N, Čufar K, Robic D (1986) Some wood anatomical, physiological and silvicultural aspects of silver fir dieback in Slovenia (NW Yugoslavia). Iawa Bull 7:343–350
Urli M, Porté AJ, Cochard H et al (2013) Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol 33:672–683. doi:10.1093/treephys/tpt030
Vitasse Y, Delzon S, Dufrêne É et al (2009) Leaf phenology sensitivity to temperature in European trees: do within-species populations exhibit similar responses? Agric For Meteorol 149:735–744. doi:10.1016/j.agrformet.2008.10.019
Wang Q, Iio A, Tenhunen J, Kakubari Y (2008) Annual and seasonal variations in photosynthetic capacity of Fagus crenata along an elevation gradient in the Naeba Mountains, Japan. Tree Physiol 28:277–285. doi:10.1093/treephys/28.2.277
Wang L, Xu Y, Schjoerring J (2011) Seasonal variation in ammonia compensation point and nitrogen pools in beech leaves (Fagus sylvatica). Plant Soil 343:51–66. doi:10.1007/s11104-010-0693-7
Waring RH (1987) Characteristics of trees predisposed to die. Bioscience 37:569–574
Wheeler JK, Huggett BA, Tofte AN et al (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36:1938–1949. doi:10.1111/pce.12139
Wildhagen H, Dürr J, Ehlting B, Rennenberg H (2010) Seasonal nitrogen cycling in the bark of field-grown Grey poplar is correlated with meteorological factors and gene expression of bark storage proteins. Tree Physiol 30:1096–1110. doi:10.1093/treephys/tpq018
Wiley E, Huepenbecker S, Casper BB, Helliker BR (2013) The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiol 33:1216–1228. doi:10.1093/treephys/tpt093
Wortemann R, Herbette S, Barigah TS et al (2011) Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol 31:1175–1182
Zhao J, Hartmann H, Trumbore S et al (2013) High temperature causes negative whole-plant carbon balance under mild drought. New Phytol 200:330–339. doi:10.1111/nph.12400
Zweifel R, Zimmermann L, Zeugin F, Newbery DM (2006) Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. J Exp Bot 57:1445–1459. doi:10.1093/jxb/erj125
Acknowledgments
The authors would like to acknowledge the students who contributed to data collection and field work: Alain Sévéré for his help with the branch sampling, and Michèle Viel and Patricia Le Thuaut for technical assistance. We are grateful to the French National Forest Office (ONF) for allowing us to carry out these experiments. The authors thank Stéphane Herbette (UMR PIAF INRA, Université Blaise Pascal), for help with the measurements of vulnerability curves.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AD’s doctoral grant was provided by the French Ministry of Higher Education and Scientific Research. Additional financial support was provided by a CYTRIX project (EC2CO) funded by CNRS and INSU and also by the ESE laboratory supported by the University Paris-Sud, CNRS and AgroParisTech.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Y. Sano.
Rights and permissions
About this article
Cite this article
Delaporte, A., Bazot, S. & Damesin, C. Reduced stem growth, but no reserve depletion or hydraulic impairment in beech suffering from long-term decline. Trees 30, 265–279 (2016). https://doi.org/10.1007/s00468-015-1299-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1299-8