Abstract
Background
Children with chronic kidney disease (CKD) are at risk for abnormalities in pubertal development. We aimed to describe the timing of pubertal onset by luteinizing hormone (LH) levels and the association between hormonal onset of puberty with changes in GFR.
Methods
Data from the Chronic Kidney Disease in Children (CKiD) study were collected prospectively. GFR was estimated at annual visits and measured by iohexol clearance every other year. LH was measured from stored repository serum samples in a nested sample of 124 participants. Hormonal onset of puberty was defined as LH level greater than or equal to 0.3 IU/L. A mixed effects model with random intercepts and slopes was used to compare the slope of decline of GFR before and after hormonal onset of puberty. The model was adjusted for age, glomerular disease diagnosis, baseline proteinuria on the log scale, and BMI.
Results
Median age at hormonal onset of puberty was 9.9 years (IQR 8.1, 11.9) in girls and 10.2 years (IQR 9.2, 11.0) in boys. The mixed effects model showed faster decline in both estimated GFR and measured GFR in boys after hormonal onset of puberty (p < 0.001), and a similar but attenuated accelerated estimated GFR decline was observed for girls with no difference for measured GFR.
Conclusions
LH levels in the post-pubertal range were observed prior to clinical manifestations of puberty in children with CKD. Hormonal onset of puberty was associated with faster decline in GFR, particularly among boys with CKD.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Puberty is a period of marked change including development of secondary sexual characteristics, acceleration of linear growth, and development of reproductive capability [1,2,3,4,5]. Pubertal delay is a recognized complication in children with chronic kidney disease (CKD) [6]. In addition, puberty itself has been shown to be a risk factor for progression of CKD [7, 8].
Puberty is primarily controlled through the hypothalamic-pituitary–gonadal (HPG) axis. The hypothalamus initially secretes gonadotropin-releasing hormone (GnRH) in a pulsatile manner. In response to GnRH, the pituitary releases the gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH). LH acts on the theca cells of the ovary to form androgenic precursors of estradiol or the Leydig cells of the testes to secrete testosterone. FSH acts on the granulosa cells of the ovary or Sertoli cells of the testes to stimulate gametogenesis and gonadal growth, and in the ovary, FSH stimulates aromatase to form estradiol from thecal androgens [9]. The HPG axis remains quiescent throughout childhood due to suppression by the central nervous system. Puberty ultimately results from an increase in HPG activity with increased pulsatile secretion of GnRH as CNS inhibition declines [9]. Multiple factors likely contribute to the timing of puberty including genetics, environmental factors, and nutrition. Increase in LH is used to signal hormonal onset of puberty [10]. From a clinical standpoint, pubertal onset can be defined by thelarche in girls (Tanner breast stage 2) and testicular enlargement in boys (Tanner genital stage 2).
Previous studies among children with CKD have demonstrated that pubertal onset may be associated with more rapid progression of kidney disease [7, 8]. We previously demonstrated that there was a significant decline in glomerular filtration rate (GFR) after pubertal onset when defined by clinical features including Tanner stage, peak growth velocity, and menarche for girls [7]. However, increase in LH is a more objective marker of pubertal onset, and activation of the HPG-axis via pulsatile increase in various pubertal hormones occurs before certain physical manifestations of puberty such as growth spurt. In addition, children with CKD may have alterations in the HPG axis and impaired kidney metabolism of LH. Therefore, we aimed to (1) describe LH levels in a cohort of children with CKD progressing from pre-puberty to puberty, and (2) evaluate the association between hormonal onset of puberty and GFR decline among children with CKD.
Materials and methods
Study population
We used prospective data from the Chronic Kidney Disease in Children (CKiD) study. The CKiD study’s design and methods have been published previously [11]. A nested sample of participants age 6 through 16 years with at least three consecutive study visits was used in order to follow serial measurements of LH with the goal of detecting change in LH associated with pubertal onset. Baseline clinical and demographic data were obtained at study entry including body mass index (BMI), glomerular versus non-glomerular diagnosis, and urine protein-to-creatinine ratio.
Exposure and definition of pubertal onset
LH levels were measured at study entry and at yearly visits using the ultra-sensitive LH ELISA kit. Limit of detection for LH was 0.009 mIU/mL. The inter-assay coefficient of variance for controls averaged 3.84%. Across the samples, the inter-assay coefficient of variance for the replicates averaged 5.38%. Hormonal onset of puberty was defined as achieving an LH level greater than or equal to 0.3 IU/L based on definitions of puberty in the general population [12, 13].
Outcome
The primary outcome of interest was CKD progression as measured by GFR. GFR was estimated at annual study visits using the CKiD U25 (eGFR) equation based on serum creatinine and cystatin C. We also described changes in GFR measured by iohexol clearance (mGFR) which was conducted every other year. Direct measurement of iohexol is not dependent on serum creatinine which can be affected by muscle mass and pubertal development.
Statistical methods
A linear mixed-effects model with random intercepts and slopes with log-transformed GFR as the outcome was used to compare the average eGFR or mGFR percent change per year before and after hormonal onset of puberty. Models were adjusted for glomerular diagnosis, baseline proteinuria (log-transformed), and BMI category as a time-varying covariate. We fit additional models stratified by glomerular disease status. Analyses were conducted using Stata, version 16.1 [14]. This study was approved by the Institutional Review Board of Penn State College of Medicine.
Results
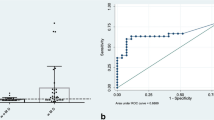
The cohort included 124 children (48 girls and 76 boys) (Table 1) contributing 585 observations. The median duration of follow-up for girls was 4 (IQR 3, 6) years and for boys was 5 (IQR 3, 6) years. Eight children entered following hormonal onset of puberty (i.e., prevalent puberty). There were 89 children with incident hormonal onset of puberty during study follow-up (31 girls and 58 boys) (Table 2). The median age at pubertal LH level for girls was 9.9 (8.8, 11.9) years and for boys was 10.2 (9.2, 11.0) years. Figure 1 displays the individual trajectories of LH; for those with incident hormonal onset of puberty, the enhanced boxplot depicts the distribution of ages at which onset was first observed. As expected, there is a general upward trend in absolute LH level over time after pubertal onset. The majority of children had a normal BMI and a non-glomerular disease diagnosis. The median eGFR and mGFR for those with incident hormonal onset of puberty was 47 ml/min|1.73 m2 and 43 ml/min|1.73 m2, respectively, at the time of pubertal onset.
Luteinizing hormone levels for each subject with incident hormonal onset of puberty and enhanced boxplots describing age at hormonal onset of puberty (girls, n = 31—contributing 162 samples; boys, n = 58—contributing 314 samples). Percentile box plots depict the distributions of age at first luteinizing hormone ≥ 0.3 IU/L
Table 3 presents results from mixed effects linear models. Faster decline in eGFR and mGFR after onset of hormonal puberty was observed for boys, and a similar but attenuated accelerated eGFR decline was observed for girls and no difference for mGFR. For boys, the percent change in eGFR was − 1.1% prior to hormonal pubertal onset and − 5.7% following hormonal pubertal onset (p < 0.001). Similarly, the percent change in mGFR was − 0.9% prior to hormonal pubertal onset and − 6.6% following onset (p < 0.001). For girls, the percent change in eGFR was − 3.5% prior to hormonal pubertal onset and − 5.0% following onset (p = 0.092). Using mGFR, the percent change in GFR was − 4.8% prior to hormonal pubertal onset and − 4.4% following hormonal pubertal onset (p = 0.84). Figure 2 displays the model-estimated change in eGFR for a child with non-glomerular disease, urine protein-creatinine ratio of 0.5 and normal BMI. In a secondary analysis, results stratified by glomerular disease status showed consistent results as the main analysis for the non-glomerular group. The glomerular diagnosis group did not have enough data for reliable inference (Table 4).
Example of change in estimated glomerular filtration rate (eGFR) before and after hormonal onset of puberty from a mixed effects model for a hypothetical profile experiencing LH ≥ 0.3 IU/L at 9.9 years for girls and 10.2 year for boys with a normal BMI, non-glomerular disease diagnosis, and urine protein-to-creatinine ratio of 0.5
Discussion
In this longitudinal cohort study of children with CKD, we found that pubertal levels of LH occurred at a median age of 9.9 years among girls and 10.2 years among boys. Although we did not directly study children with CKD compared to healthy controls, the age at hormonal onset of puberty was generally similar as compared to previously reported literature from the general population at approximately 10 years of age for both boys and girls [10, 13, 14]. We found that hormonal onset of puberty was associated with a faster decline in GFR particularly among boys with CKD.
We previously demonstrated that GFR declined faster after clinical onset of puberty [7]. There are several proposed mechanisms to explain the accelerated GFR decline associated with puberty. One proposed mechanism for the loss of kidney function around the time of puberty includes hyperfiltration of remaining glomeruli as a result of acceleration in growth, body mass, and blood pressure. In the ItalKid study of children with CKD, a faster decline in eGFR was demonstrated after pubertal growth spurt. However, the authors concluded that increase in body mass was likely not the sole explanation for decline in GFR after pubertal onset given that eGFR continued to rapidly decline despite slowing or cessation of growth [8]. We previously showed that the decline in GFR after clinical onset of puberty remained significant after adjustment for BMI, and in this study, we show a faster decline in GFR after hormonal onset of puberty in boys which occurs prior to the pubertal growth spurt. For reference, peak growth velocity was shown to occur at age 11.9 years in boys and 11.1 years in girls which is later than hormonal onset of puberty (Fig. 2) [7]. Interestingly, the difference before and after hormonal onset of puberty in girls is attenuated as compared to our previous reports of more subjective clinical markers (Tanner stage, peak growth velocity, and menarche). This could be a reflection of fewer girls included in the study, but there is also literature that suggests there are sex differences in progression of CKD with males having been described as having faster disease progression [15, 16]. It is still possible the physical development that occurs after initial hormonal onset plays a larger role in disease progression rather than upregulation of LH levels directly or that other hormones upregulated secondary to LH and FSH such as testosterone and estradiol to higher levels later in puberty play a stronger role than LH itself.
Numerous animal models have shown a negative effect of androgens on kidney disease progression. Androgens have been implicated in the development of proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis among males [17,18,19,20,21]. Estrogen may also contribute to CKD progression among females. In a study of over 4000 women, pre-menopausal women on oral contraceptives and post-menopausal women on hormone replacement therapy had an increased risk of microalbuminuria [22]. In a study of over 5000 post-menopausal women, hormone replacement therapy was associated with a significant decrease in eGFR. A higher cumulative dose of estrogen was associated with a greater decline in eGFR [23]. The association of sex hormones and kidney disease progression has not been studied in the pediatric CKD population. We hypothesize that sex hormones could contribute to the acceleration in GFR decline after pubertal onset, but do not minimize the role that physical growth itself may play. This needs to be evaluated further in future studies. In addition, changes in medication compliance and self-care during adolescence may contribute to the decline in kidney function with puberty [24].
This study has several limitations. Although we utilized data from the large CKiD study, our sample size was small as we restricted the cohort to children who were likely to be in the pubertal period and had three consecutive visits. The models indicated accelerated GFR decline after puberty, but it is not known whether the acceleration persists or the GFR decline stabilizes after completion of puberty. Further work could assess long-term GFR changes, but would need to address the competing risk problem of dialysis or transplant. Second, LH is released in a pulsatile fashion, and the level can vary throughout the day. As our samples were obtained from stored serum that were obtained at various times of day there may be variability in LH levels, and these results may not be peak levels. It is also possible that the LH level used to define hormonal onset of puberty in children with CKD may need to be different than the definition used for the general population. LH is thought to be primarily cleared by the kidney where it is endocytosed by tubular cells and catabolized. Therefore, patients with CKD are thought to be at increased risk for elevated LH levels as a result of reduced clearance by the kidney. When synthetic LH-releasing hormone was administered to adult patients on hemodialysis, prolonged plasma elevations of both LH and FSH were seen, and children with CKD and on dialysis have been shown to have longer half-life of several isoforms of LH [25,26,27,28]. Further study is needed to optimize the definition of LH level indicative of hormonal onset of puberty in CKD. Finally, the absence of testosterone and estradiol levels limits our ability to more definitively comment on the impact of sex hormones on kidney disease progression.
In conclusion, hormonal onset of puberty was associated with faster decline in GFR, particularly among boys with CKD. Clinicians should be aware that puberty is a high-risk period for kidney disease progression. Future studies should explore the role of sex hormones on CKD progression in children and interventions to mitigate the risk of progressive disease.
Data availability
Data from the Chronic Kidney Disease in Children Cohort Study [(V7 https://doi.org/10.58020/dzq8-ct80] reported here are available for request at the NIDDK Central Repository (NIDDK-CR) website, Resources for Research (R4R), https://repository.niddk.nih.gov/.
References
Lane PH (2005) Puberty and chronic kidney disease. Adv Chronic Kidney Dis 12:372–377
McKeever MO (2000) Delayed puberty. Pediatr Rev 21:250–252
Schaefer F, Seidel C, Binding A, Gasser T, Largo RH, Prader A, Schärer K (1990) Pubertal growth in chronic renal failure. Pediatr Res 28:5–10
Haffner D, Zivicnjak M (2017) Pubertal development in children with chronic kidney disease. Pediatr Nephrol 32:949–964
Kim HS, Ng DK, Matheson MB, Atkinson MA, Warady BA, Furth SL, Ruebner RL (2020) Delayed menarche in girls with chronic kidney disease and the association with short stature. Pediatr Nephrol 35:1471–1475
Meuwese CL, Carrero JJ (2013) Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res 44:591–600
Kim HS, Ng DK, Matheson MB, Atkinson MA, Akhtar Y, Warady BA, Furth SL, Ruebner RL (2022) Association of puberty with changes in GFR in children with CKD. Am J Kidney Dis 79:131–134
Ardissino G, Testa S, Daccò V, Paglialonga F, Viganò S, Felice-Civitillo C, Battaglino F, Bettinelli A, Bordugo A, Cecchetti V, De Pascale S, La Manna A, Li Volti S, Maringhini S, Montini G, Pennesi M, Peratoner L (2012) Puberty is associated with increased deterioration of renal function in patients with CKD: data from the ItalKid Project. Arch Dis Child 97:885–888
Bordini B, Rosenfield RL (2011) Normal pubertal development: part II: clinical aspects of puberty. Pediatr Rev 32:281–292
Addo OY, Miller BS, Lee PA, Hediger ML, Himes JH (2014) Age at hormonal onset of puberty based on luteinizing hormone, inhibin B, and body composition in preadolescent US girls. Pediatr Res 76:564–570
Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1:1006–1015
Sims EK, Addo OY, Gollenberg AL, Himes JH, Hediger ML, Lee PA (2012) Inhibin B and luteinizing hormone levels in girls aged 6–11 years from NHANES III, 1988–1994. Clin Endocrinol (Oxf) 77:555–563
Krishna KB, Fuqua JS, Rogol AD, Klein KO et al (2019) Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr 91:357–372
StataCorp (2017) Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX
Gretz N, Zeier M, Geberth S, Strauch M, Ritz E (1989) Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. Am J Kidney Dis 14:178–183
Oh ES, You Z, Nowak KL, Jovanovich AJ (2023) Sex differences in cardiovascular and all-cause mortality in adults with and without CKD: NHANES 1999–2018. Kidney 360. https://doi.org/10.34067/KID.0000000000000239
Elema JD, Arends A (1975) Focal and segmental glomerular hyalinosis and sclerosis in the rat. Lab Invest 33:554–561
Baylis C (1994) Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 94:1823–1829
Lemos CC, Mandarim-de-Lacerda CA, Dorigo D, Coimbra TM, Bregman R (2005) Chronic renal failure in male and female rats. J Nephrol 18:368–373
Metcalfe PD, Leslie JA, Campbell MT, Meldrum DR, Hile KL, Meldrum KK (2008) Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab 294:E435–E443
Sakemi T, Baba N (1993) Castration attenuates proteinuria and glomerular injury in unilaterally nephrectomized male Sprague-Dawley rats. Lab Invest 69:51–57
Monster TB, Janssen WM, de Jong PE (2001) Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med 161:2000–2005
Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, MacRae JM, Zhang J, Hemmelgarn BR (2008) Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int 74:370–376
Elhadd TA, Khan F, Kirk G, McLaren M, Newton RW, Greene SA, Belch JJ (1998) Influence of puberty on endothelial dysfunction and oxidative stress in young patients with type 1 diabetes. Diabetes Care 21:1990–1996
Holley JL (2004) The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis 11:337–341
Pimstone B, Epstein S, Hamilton SM, LeRoith D, Hendricks S (1977) Metabolic clearance and plasma half disappearance time of exogenous gonadotropin releasing hormone in normal subjects and in patients with liver disease and chronic renal failure. J Clin Endocrinol Metab 44:356–360
Schalch DS, Gonzalez-Barcena D, Kastin AJ, Landa LU, Lee LA, Zamora MT, Schally AV (1975) Plasma gonadotropins after administration of LH-releasing hormone in patients with renal or hepatic failure. J Clin Endocrinol Metab 41:921–925
Schaefer F, Veldhuis JD, Robertson WR, Dunger D, Schärer K (1994) Immunoreactive and bioactive luteinizing hormone in pubertal patients with chronic renal failure. Cooperative study group on pubertal development in chronic renal failure. Kidney Int 45:1465–1476
Funding
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri—Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116). The CKiD Web site is located at https://statepi.jhsph.edu/ckid. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data and biospecimens from the CKiD study reported here were supplied by the NIDDK Central Repository. This manuscript does not necessarily reflect the opinions or views of the NIDDK Central Repository or the NIDDK. Research reported in this publication was funded by The Children’s Hospital of Philadelphia Pediatric Center of Excellence in Nephrology and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number P50DK114786. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
For the CKiD Study investigators please see the Supplementary Material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H.S., Ng, D.K., Matheson, M.B. et al. Pubertal luteinizing hormone levels in children with chronic kidney disease and association with change in glomerular filtration rate. Pediatr Nephrol 39, 1543–1549 (2024). https://doi.org/10.1007/s00467-023-06210-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06210-7