Abstract
Objectives
This study was designed to compare the diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin (NGAL) with procalcitonin (PCT), C-reactive protein (CRP), and white blood cells (WBCs) for predicting acute pyelonephritis (APN) in children with febrile urinary tract infections (UTIs).
Materials and methods
In total, 138 children with febrile UTIs (APN 59, lower UTI 79) were reviewed retrospectively. Levels of NGAL, PCT, CRP, and WBCs in blood were measured on admission. The diagnostic accuracy of the biomarkers was investigated. Independent predictors of APN were identified by multivariate logistic regression analysis.
Results
Receiver operating curve (ROC) analyses showed good diagnostic profiles of NGAL, PCT, CRP, and WBCs for identifying APN [area under the curve (AUC) 0.893, 0.855, 0.879, and 0.654, respectively]. However, multivariate analysis revealed only plasma NGAL level was an independent predictor of APN (P = 0.006). At the best cutoff values of all examined biomarkers for identifying APN, sensitivity (86 %), specificity (85 %), positive predictive value (81 %), and negative predictive value (89 %) of plasma NGAL levels were the highest. The optimal NGAL cutoff value was 117 ng/ml. The positive likelihood ratio [odds ratio (OR) 5.69, 95 % confidence interval (CI) 3.56–8.78], and negative likelihood ratio (OR 0.16, 95 % CI 0.08-0.29) of plasma NGAL for APN diagnosis also showed it seemed to be more accurate than serum PCT, CRP, and WBCs.
Conclusion
Plasma NGAL can be more useful than serum PCT, CRP, and WBC levels for identifying APN in children with febrile UTIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile urinary tract infection (UTI) is a common problem among infants and children [1]. Subsequent renal scarring related to acute pyelonephritis (APN) has been considered an important cause of long-term morbidity, such as chronic renal failure [2, 3]. Since symptoms of APN in children are nonspecific, establishing an APN diagnosis often requires imaging studies to detect renal parenchymal involvement. Among these, ultrasonography (US) is a noninvasive technique that can reveal various anatomical abnormalities. However, this imaging technique is not reliable in detecting pyelonephritis or vesicoureteral reflux (VUR). Although 99mTc-dimercaptosuccinic acid (DMSA) renal scintigraphy has been accepted as the gold standard for diagnosing APN in children [1, 4], there seems no consensus regarding its indication during the acute phase of UTI [5–7]. Thus, a more practical tool that could help clinicians determine the presence of renal parenchymal damage in an acute phase of febrile UTI would be of great clinical value.
The noninvasive measurement of suitable biomarkers to aid the recognition of APN in children has attracted interest. As traditional biomarkers for infection, serum C-reactive protein (CRP) level and peripheral white blood cell (WBC) count are used to diagnose invasive bacterial infections and determine UTI level [8, 9]. However, several studies do not support the significance of these markers in discriminating upper from lower UTIs [10, 11]. Procalcitonin (PCT), a 116‐amino acid propeptide of calcitonin, has been identified as an early, sensitive, and specific marker of bacterial infection [12, 13]. It is almost undetectable in healthy individuals or during viral infections, but its level increases in response to bacterial endotoxins, with the extent of increase being proportional to infection severity [14]. Several studies suggest that PCT levels are correlated with APN, and a meta-analysis [15, 16] showed that a serum PCT level > 0.5 ng/ml predicts early renal parenchymal involvement reasonably well. PCT ≥ 0.5 ng/ml yielded an adjusted odds ratio (OR) of 7.9, with 71 % sensitivity and 72 % specificity for APN. PCT was a better predictor than CRP or WBC count for identifying children with APN during the early stages of UTI [16]. Neutrophil gelatinase-associated lipocalin (NGAL), a member of the lipocalin superfamily, is a 25-kDa protein originally purified from human neutrophils [17]. NGAL has been reported to be a useful predictor for distinguishing acute bacterial infections from viral infections in all populations, from newborns to adults [18–20]. Besides neutrophils, NGAL expression is also markedly induced in injured epithelial cells, including the kidney [21]. This appears to be mediated via nuclear factor kappa B (NF-kB), which is known to be promptly activated in epithelial cells after acute injuries [22]. For many years, NGAL has been identified as one of the most reliable biomarkers of acute kidney injury (AKI) [23].
To the best of our knowledge, there have been no comparison analyses between the diagnostic accuracy of plasma NGAL levels and PCT concentrations in children with APN. We previously reported that plasma and urinary NGAL levels may be instrumental in the diagnosis and therapeutic monitoring of APN in pediatric patients with UTIs [24–26]. Our study aimed to compare the accuracy of plasma NGAL levels with those of serum concentrations of PCT, CRP, and WBC to predict APN in children with febrile UTIs. The accuracy of candidate biomarkers to diagnose VUR and hydronephrosis was also determined based on the area under the receiver operating characteristic (ROC) curve.
Materials and methods
Patient characteristics and inclusion criteria
We retrospectively reviewed the electronic medical records of UTI patients admitted to our Department of Pediatrics between 1 October 2014 and 30 September 2015. In order to make sure the retrospective search identified all eligible individuals, we searched our hospital registry for pediatric patients hospitalized with a diagnosis of UTI. Children who were aged 1 month to 13 years and had a first episode of a febrile UTI were included. A febrile UTI was defined as the presence of fever (body temperature ≥ 38 °C), pyuria (≥5 WBCs/high-power field), and a positive urine culture (pure bacterial growth of >105 colony forming units/ml), primarily based on the UTI guideline from the American Academy of Pediatrics [6]. Urine samples for culture were obtained by catheterization or suprapubic aspiration for non-toilet-trained children and from a clean voided midstream for toilet-trained patients. Patients with a history of UTI, AKI based on pediatric Risk, Injury, Failure, Loss, and End-stage Renal Disease Criteria [27], chronic kidney disease, other infectious diseases (comorbid bacterial, viral or congenital infections), and known abnormalities or malformations of the urinary tract except VUR, were excluded from the study. This retrospective chart review was approved by our institutional review board before its initiation and the study performed in accordance with the Declaration of Helsinki.
Laboratory and radiological assessment
According to our department protocol, NGAL, PCT, CRP, and WBC counts were measured at the time of admission. Plasma NGAL level was measured in ethylenediaminetetraacetic acid (EDTA), anticoagulated, whole-blood specimens using the Triage NGAL assay (Alere, San Diego, CA, USA), which has a detection range of 15–1300 ng/ml. Inter- and intra-assay coefficients of variation of the assay were 13.5 % and 11 %, respectively. Renal US and DMSA scintigraphy were performed within the first 3 days after admission, and voiding cystourethrogram was performed before discharge (within 7–10 days after admission) to detect VUR. Hydronephrosis was diagnosed according to the Society for Fetal Urology classification [28], and APN was defined as focal, multifocal, or diffuse areas of decreased cortical uptake on DMSA renal scan. Patients were divided into two groups, APN and lower UTI, according to DMSA scan results. Laboratory, clinical, and imaging results were compared between groups.
Statistical analysis
Statistical comparisons were performed with the SPSS program for Windows (version 18.0, SPSS, Chicago, IL, USA) and the MedCalc program for Windows (version 15.4, MedCalc Software, Belgium). For continuous variables, normality tests were performed. Continuous variables showing a normal distribution were compared between groups using Student’s t test, and nonnormally distributed variables were compared using the Mann–Whitney U test. Categorical variables were compared using the chi-square test. To evaluate clinical outcomes, all associated risk factors of APN were tested with univariate logistic regression analysis. Predictors significantly associated with APN from univariate analysis (P ≤ 0.05) were included in multivariate logistic regression analysis, and backward selection techniques were used to determine baseline risk factors. Collinearity among variables was also assessed by examining the variance inflation factor. Diagnostic accuracy of APN biomarkers was investigated using ROC analysis, which was used to calculate the best cutoff values. The area under the ROC curve (AUC), including 95 % confidence intervals (CIs), were calculated and compared using a pairwise comparison method [29]. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated for each biomarker. Diagnostic accuracy of biomarkers for VUR and hydronephrosis was assessed by calculating the AUCs. Data are presented as median [IQR (interquartile range)] for continuous variables and proportions for categorical variables. Statistical significance was set at P < 0.05.
Results
Patient characteristics
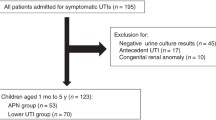
Among 186 patients with UTIs, 48 were excluded and 138 were enrolled based on the aforementioned criteria (Fig. 1). Fifty-nine patients were included in the APN group and 79 in the lower UTI group. Although patient ages ranged from 1 month to 13 years, 97 patients (70.3 %) were younger than 1 year, and there was no difference in the median age between groups. Among the enrolled 138 patients (65 girls and 73 boys), a higher distribution of girls was seen in the APN group (P < 0.05). The levels of NGAL, PCT, CRP, and WBC counts were all significantly higher in the APN group than in the lower-UTI group (all P < 0.05). Although no differences were found in the presence of hydronephrosis between groups, the incidence of VUR was higher in the APN group than in the lower UTI group (P < 0.05) (Table 1).
Univariate and multivariate logistic regression analyses
Univariate analysis led to the identification of several clinical variables associated with the presence of APN, as shown in Table 2. The analysis showed that female sex, a high plasma level of NGAL, elevated serum concentrations of PCT, CRP, and WBC, and the presence of VUR were associated with APN (all P < 0.05). However, multivariate analysis revealed that only plasma NGAL level was an independent predictor of APN (OR 1.01, 95 % CI 1.00-1.02, P = 0.005). No collinearity was detected among variables, because none of the variance inflation factors was >10.
ROC analyses and ORs
ROC analyses were performed to define diagnostic profiles of the parameters and to compare plasma NGAL with serum PCT and CRP levels and WBC counts in predicting APN in patients with UTIs. NGAL had the highest AUC value, of 0.893 (95 % CI 0.835-0.950, P < 0.001), followed by CRP of 0.879 (95 % CI 0.807-0.928, P < 0.001), PCT of 0.855 (95 % CI 0.790-0.920, P < 0.001), and WBC of 0.654 (95 % CI 0.560-0.748, P = 0.001) (Fig. 2). Differences in AUCs were not statistically significant, except between plasma NGAL level and WBC counts (P < 0.001). According to ROC analyses, the best cutoff levels for predicting APN with NGAL, PCT, CRP, and WBC were 117 ng/ml, 0.17 ng/ml, 2.78 mg/dl, and 15,870/mm3, respectively. Sensitivity (86 %) and specificity (85 %) measured as part of the ROC analysis were the highest for plasma NGAL. Plasma NGAL showed the greatest PPV (81 %) and NPV (89 %) for predicting APN among all examined biomarkers. PLR and NLR for detecting APN at each optimal biomarker cutoff value also showed that plasma NGAL seemed to be more accurate than the other examined biomarkers. Plasma NGAL had a higher PLR (5.69, 95 % CI 3.56-8.78) and lower NLR (0.16, 95 % CI 0.08-0.29) than the other examined biomarkers. To compare our results with findings from other studies [14, 28], we performed a further ROC analysis for serum PCT using a cutoff of > 0.5 ng/ml. At this cutoff point, the AUC to predict APN was 0.750 (95 % CI, 0.669-0.820, P = 0.044), with a sensitivity of 63 % and a specificity of 87 %. This AUC value for PCT was significantly lower than AUCs for NGAL, CRP, and PCT with our optimal cutoff level of 0.17 ng/ml (P < 0.05) (Table 3).
In ROC analyses for evaluating the presence of VUR and hydronephrosis (Fig. 3), plasma NGAL levels and serum PCT and CRP levels also showed good diagnostic performances for the presence of VUR. The AUC was 0.798 (95 % CI 0.704-0.872) for NGAL, 0.756 (95 % CI 0.658-0.837) for PCT, and 0.845 (95 % CI 0.757-0.910) for CRP (all P < 0.001). Cutoff levels of NGAL, PCT, and CRP for diagnosing VUR were 118 ng/ml (sensitivity 96 %, specificity 61 %), 0.92 ng/ml (sensitivity 70 %, specificity 76 %), and 4.14 mg/dl (sensitivity 87 %, specificity 72 %), respectively. AUC for serum CRP level was significantly higher than those for the PCT level and WBC counts (P = 0.04 and P < 0.001, respectively). There were no differences in AUCs between serum CRP and plasma NGAL level. In contrast to APN and VUR, parameters analyzed in this study did not correlate with the presence of hydronephrosis (Table 4).
Discussion
This study aimed to determine the accuracy of NGAL levels in predicting APN and comparing it with accuracies of serum PCT and CRP levels and WBC counts in children with febrile UTIs. The results demonstrated that plasma NGAL level could improve prediction of APN beyond serum PCT, CRP, or WBC levels. Using univariate and multivariate analyses, we showed that only plasma NGAL level was an independent predictor of APN. Sensitivity and specificity was superior to that of serum PCT, CRP, and WBC measurement. PPV, NPV, PLR, and NLR of each biomarker demonstrated that plasma NGAL was more accurate than the other examined biomarkers. These findings suggest that plasma NGAL could be one of the most promising biomarkers for detecting APN in patients with febrile UTI and without AKI.
Since serum PCT has been proven to be an early, sensitive, and specific marker of bacterial infection [12, 13], several studies showed that serum PCT could also be a good diagnostic biomarker for APN [30, 31]. In a recent study published in 2013, serum PCT level was a more sensitive predictor than CRP level or WBC counts for selective identification of children with APN during the early stages of UTI [16]. As the traditional biomarker for infection, serum CRP level has been measured diagnosing UTIs and assessment of treatment effects. According to a review published in 2015 that analyzed CRP data from 13 studies (1638 children), overall sensitivity and specificity were 94 % and 39 % at a cutoff value of 2 mg/dl [9]. However, serum CRP level does not appear to be useful in ruling out APN because of the low specificity and inconsistencies among the published studies [8, 10, 11].
NGAL is expressed at low levels in normal organs and at increased levels in injured epithelia, including the kidney [32, 33]. NGAL released from developing nephrons also participates in the conversion of metanephric tissue into glomeruli and proximal renal tubules [34]. It has been proven by several clinical studies that NGAL is an excellent biomarker for monitoring and determining the prognosis of AKI [35, 36]. Similar to an AKI, APN is characterized by acute, mostly tubulointerstitial, kidney damage. Accordingly, NGAL is thought to be up-regulated with renal parenchymal injury in the setting of UTI [37] and, indeed, in our study, plasma NGAL level was substantially higher in children with APN than those with lower UTIs, which is consistent with our previous findings [24, 25]. Compared with PCT, CRP, and WBC, NGAL appeared to be more highly associated with a positive prediction for APN in children with febrile UTIs.
In this study, female gender, an increased plasma level of NGAL, high serum concentrations of PCT, CRP, and WBC, and the presence of VUR were related with APN. However, multivariate logistic regression analysis showed that only plasma NGAL level independently predicted the presence of APN. NGAL had the highest AUC value, followed by CRP, PCT, and WBC, as determined by ROC curves. AUCs showed no statistical differences, except between plasma NGAL level and WBC counts. Although a widely used test to compare the difference between two AUCs depends on the method by DeLong et al. [29], recent reports suggest P values for the change in AUC are not necessary and should be avoided [38, 39]. Because AUC is mainly a measure of the quality of discrimination, its usefulness lies in the magnitude of the increase in model performance [39]. Accordingly, PCT and CRP may also be sufficiently accurate to be helpful in differentiating children with APN from children with lower UTIs. Nevertheless, diagnostic accuracy of plasma NGAL for predicting APN appears to be higher and have greater sensitivity (86 %), specificity (85 %), PPV (81 %), NPV (89 %), and PLR (5.69), as well as the smaller NLR (0.16).
The best cutoff level of 0.17 ng/ml for PCT to differentiate patients with or without APN is lower than that of > 0.5 ng/ml for diagnosing APN in previous studies [15, 16]. Since a serum PCT > 0.5 ng/ml has been known to predict early renal parenchymal involvement reasonably well [15, 16], we performed a further ROC analysis for serum PCT using a cutoff of > 0.5 ng/ml. AUC value of 0.75 for PCT with this cutoff point (sensitivity 63 %, specificity 87 %) was lower than the AUCs for NGAL (0.893), CRP (0.879), and PCT (0.855), with the optimal cutoff of 0.17 ng/ml (sensitivity 85 %, specificity 79 %). This AUC value also seemed lower than that (0.82) reported in the study of Leroy et al. (sensitivity 71 %, specificity 72 %) [16]. For CRP, our study showed findings consistent with the previous publication of Sim et al. [24], in which the AUC of serum CRP for an APN diagnosis was 0.842, with the best cutoff being 3.86 mg/dl (sensitivity 87 %, specificity 73 %). In our study, serum CRP showed a higher AUC of 0.879 with a lower cutoff of 2.78 mg/dl (sensitivity 83 %, specificity 81 %). In both studies, AUC difference between NGAL and CRP was not significant. Univariate analysis identifying serum CRP was associated with the presence of APN. However, in a multivariate logistic regression analysis, serum CRP was not retained as a predictor of APN. Plasma NGAL was the only independent predictor of APN in multivariate analysis.
In this study, an NGAL cutoff level for predicting APN of 117 ng/ml (sensitivity 86 %, specificity 85 %) was suggested, which is somewhat higher than our previous findings. The best cutoff levels for predicting APN were 102.5 ng/ml (sensitivity 89 %, specificity 71 %) [24] and 61.0 ng/ml (sensitivity 75 %, specificity 78 %) [25] in those previous studies. Regarding this, there was a difference in the plasma NGAL measurement method between studies. In the study by Seo et al. [25], NGAL levels were measured at the bedside, directly after adding several drops of whole blood to a disposable device without using EDTA-anticoagulated tubes. In our study and previous work by Sim et al. [24], plasma NGAL levels were measured in EDTA-anticoagulated whole blood specimens using the Triage NGAL assay. It is possible that a source of variation in NGAL levels arose in the period from sampling to measurement. Nevertheless, the specificity determined based on the best cutoff value of 117 ng/ml for ruling out APN is higher than in our previous studies.
VUR is a common renal problem in children with febrile UTIs. Renal scarring can develop in children with reflux-related UTI, which may lead to chronic renal failure [3]. To date, no effective biomarkers have been identified that can predict the occurrence of VUR. Previous studies have reported conflicting results as to whether the correlation between tested biomarkers and VUR is significant. Serum PCT level was shown to be elevated in children with VUR [40]. However, other studies have found no difference in PCT levels in children with or without VUR [41]. With regard to the role of NGAL for predicting VUR, NGAL level might also be increased in children with VUR. NGAL is released in the course of regeneration of renal tubular cells after kidney injury, and a high NGAL level may be a consequence of NGAL release from the renal tubular cells damaged by VUR and/or renal scarring [42–44]. According to previous studies, urinary NGAL levels were significantly increased in children with VUR and without evidence of UTI, and they correlated with the presence of renal scarring regardless of the reflux grade [44]. The authors suggested urinary NGAL might become a useful diagnostic indicator for renal scarring when monitoring patients with VUR. On the other hand, our previous studies shown no relationship between plasma or urinary NGAL levels and the presence of VUR and/or renal scarring [24–26]. To the best of our knowledge, there have been no reports regarding the role of plasma NGAL for detecting VUR in the absence of UTI.
In this study, AUCs for plasma NGAL and serum PCT and CRP were high enough to predict the presence of VUR. AUC for serum CRP was significantly higher than that for serum PCT and WBC counts, while the difference in AUCs between plasma NGAL and serum CRP was not significant. However, it is debatable whether plasma NGAL and serum CRP levels are more reliable than other biomarkers. First, as mentioned above, there are conflicting results from several studies. Second, in this study, the prevalence of VUR in patients with APN was much higher than in patients with lower UTI (39 % versus 8 %). Since VUR is one of the risk factors for APN, especially in children with high-grade reflux [3], the infectious condition of APN might have contributed to these findings. To accurately evaluate the correlation between biomarkers and the presence of VUR, measurements of these parameters should be performed in patients without UTIs and/or other confounders, such as AKI and renal scarring.
Hydronephrosis is also frequently diagnosed during the radiologic workup for UTI. Several studies have attempted to identify an effective biomarker for predicting hydronephrosis, but results were controversial [45, 46]. Recently, Noyan et al. demonstrated that the ratio of urinary NGAL level to creatinine was significantly higher in a group of patients with severe hydronephrosis with dysfunction than in the group with mild hydronephrosis without dysfunction [47]. However, in the study reported here, no differences were observed in the levels of biomarkers, including NGAL, between patients with and without hydronephrosis. In this regard, most hydronephrosis cases were mild (Society for Fetal Urology grade 1 or 2), and there were no cases complicated by renal or ureteral dysfunction. There could also be transient hydronephrosis, because renal sonography was performed during the acute phase of UTI. Therefore, our findings may provide only limited information on biomarker variations associated with hydronephrosis.
There were some limitations to this study. First, it was retrospective, there was a relatively small population and only from a single center, and the age group was also limited. Second, the small sample size and the presence of acute infection in VUR or hydronephrosis may have interfered with precise evaluation of biomarkers for predicting VUR or hydronephrosis. Third, we chose to use the results of the univariate analysis as predictors in multivariate analysis rather than choosing physiologically plausible predictors. While this is a valid method, it does limit the generalizability of the results. Finally, studies of the association between biomarkers and renal scarring were not performed because of the short study period and the lack of follow-up. Results need to be validated in a larger, multicenter, prospective study of various children from age groups over a longer period.
Conclusion
Data from this study indicate that in children with their first febrile UTI, plasma NGAL level can be more helpful than serum PCT and CRP levels and WBC counts for predicting APN. NGAL, PCT, and CRP may serve as laboratory indices for APN diagnosis, but the diagnostic accuracy of NGAL can be higher than PCT and CRP. Determination of plasma NGAL levels might be a useful, noninvasive tool for identifying renal parenchymal involvement. However, larger and longer studies are needed to better assess the role of plasma NGAL level as a predictor of APN, renal scarring, and VUR in children with febrile UTIs.
References
Saadeh SA, Mattoo TK (2011) Managing urinary tract infections. Pediatr Nephrol 26:1967–1976
Vachvanichsanong P (2007) Urinary tract infection: one lingering effect of childhood kidney diseases-review of the literature. J Nephrol 20:21–28
Montini G, Tullus K, Hewitt I (2011) Febrile urinary tract infections in children. New Engl J Med 365:239–250
Jaksic E, Bogdanovic R, Artiko V, Saranovic DS, Petrasinovic Z, Petrovic M, Bojic L, Pavlovic S (2011) Diagnostic role of initial renal cortical scintigraphy in children with the first episode of acute pyelonephritis. Ann Nucl Med 25:37–43
Baumer J, Jones R (2007) Urinary tract infection in children, National Institute for Health and Clinical Excellence. Arch Dis Child Educ Pract Ed 92:189–192
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595–610
Stein R, Dogan HS, Hoebeke P, Kočvara R, Nijman RJ, Radmayr C, Tekgül S, European Association of Urology; European Society for Pediatric Urology (2015) Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol 67:546–558
Biggi A, Dardanelli L, Pomero G, Cussino P, Noello C, Sernia O, Spada A, Camuzzini G (2001) Acute renal cortical scintigraphy in children with a first urinary tract infection. Pediatr Nephrol 16:733–738
Shaikh N, Borrell JL, Evron J, Leeflang MM (2015) Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev. doi:10.1002/14651858.CD009185
Ayazi P, Mahyar A, Daneshi MM, Jahani Hashemi H, Pirouzi M, Esmailzadehha N (2013) Diagnostic accuracy of the quantitative C-reactive protein, erythrocyte sedimentation rate and white blood cell count in urinary tract infections among infants and children. Malays J Med Sci 20:40–46
Garin EH, Olavarria F, Araya C, Broussain M, Barrera C, Young L (2007) Diagnostic significance of clinical and laboratory findings to localize site of urinary infection. Pediatr Nephrol 22:1002–1006
Assicot M, Bohuon C, Gendrel D, Raymond J, Carsin H, Guilbaud J (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518
Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39:206–217
Zaffanello M, Brugnara M, Franchini M, Fanos V (2008) Is serum procalcitonin able to predict long-term kidney morbidity from urinary tract infections in children? Clin Chem Lab Med 46:1358–1363
Mantadakis E, Plessa E, Vouloumanou EK, Karageorgopoulos DE, Chatzimichael A, Falagas ME (2009) Serum procalcitonin for prediction of renal parenchymal involvement in children with urinary tract infections: a meta-analysis of prospective clinical studies. J Pediatr 155:875–881
Leroy S, Fernandez-Lopez A, Nikfar R, Romanello C, Bouissou F, Gervaix A, Gurgoze MK, Bressan S, Smolkin V, Tuerlinckx D (2013) Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics 131:870–879
Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N (1993) Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Bio Chem 268:10425–10432
Nasioudis D, Witkin SS (2015) Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol 204:471–479
Björkqvist M, Källman J, Fjaertoft G, Xu S, Venge P, Schollin J (2004) Human neutrophil lipocalin: normal levels and use as a marker for invasive infection in the newborn. Acta Paediatr 93:534–539
Fjaertoft G, Foucard T, Xu S, Venge P (2005) Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr 94:661–666
Cowland JB, Borregaard N (1997) Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase associated lipocalin from humans. Genomics 45:17–23
Devarajan P (2010) Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med 4:265–280
Martensson J, Bellomo R (2014) The rise and fall of NGAL in acute kidney injury. Blood Purif 37:304–310
Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH (2015) Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatr Res 78:48–55
Seo WH, Nam SW, Lee EH, Je B-K, Yim HE, Choi BM (2014) A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. Eur J Pediatr 173:229–232
Yim HE, Ym H, Bae ES, Woo SU, Yoo KH (2014) Predictive value of urinary and serum biomarkers in young children with febrile urinary tract infections. Pediatr Nephrol 29:2181–2189
Akcan-Arikan A, Zappitelli M, Loftis L, Washburn K, Jefferson L, Goldstein S (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Fernbach S, Maizels M, Conway J (1993) Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol 23:478–480
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Pecile P, Miorin E, Romanello C, Falleti E, Valent F, Giacomuzzi F, Tenore A (2004) Procalcitonin: a marker of severity of acute pyelonephritis among children. Pediatrics 114:e249–e254
Bressan S, Andreola B, Zucchetta P, Montini G, Burei M, Perilongo G, Da Dalt L (2009) Procalcitonin as a predictor of renal scarring in infants and young children. Pediatr Nephrol 24:1199–1204
Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P (2003) Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63:1714–1724
Xu S, Venge P (2000) Lipocalins as biochemical markers of disease. Biochim Biophys Acta 1482:298–307
Mori K, Nakao K (2007) Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 71:967–970
Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR (2008) Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 36:1297–1303
Aghel A, Shrestha K, Mullens W, Borowski A, Tang WW (2010) Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 16:49–54
Ichino M, Kuroyanagi Y, Kusaka M, Mori T, Ishikawa K, Shiroki R, Kurahashi H, Hoshinaga K (2009) Increased urinary neutrophil gelatinase associated lipocalin levels in a rat model of upper urinary tract infection. J Urol 181:2326–2331
Pepe MS, Kerr KF, Longton G, Wang Z (2013) Testing for improvement in prediction model performance. Stat Med 32:1467–1482
Demler OV, Pencina MJ, D’Agostino RB Sr (2012) Misuse of DeLong test to compare AUCs for nested models. Stat Med 31:2577–2587
Ipek IO, Sezer RG, Senkal E, Bozaykut A (2012) Relationship between procalcitonin levels and presence of vesicoureteral reflux during first febrile urinary tract infection in children. Urology 79:883–887
Sheu JN, Chang HM, Chen SM, Hung TW, Lue KH (2011) The role of procalcitonin for acute pyelonephritis and subsequent renal scarring in infants and young children. J Urol 186:2002–2008
Gobet R, Cisek LJ, Chang B, Barnewolt CE, Retik AB, Peters CA (1999) Experimental fetal vesicoureteral reflux induces renal tubular and glomerular damage, and is associated with persistent bladder instability. J Urol 162:1090–1095
Peters C, Rushton HG (2010) Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol 184:265–273
Ichino M, Kusaka M, Kuroyanagi Y, Mori T, Morooka M, Sasaki H, Shiroki R, Shishido S, Kurahashi H, Hoshinaga K (2010) Urinary neutrophil-gelatinase associated lipocalin is a potential noninvasive marker for renal scarring in patients with vesicoureteral reflux. J Urol 183:2001–2007
Papachristou F, Pavlaki A, Printza N (2014) Urinary and serum biomarkers in ureteropelvic junction obstruction: a systematic review. Biomarkers 19:531–540
Karakus S, Oktar T, Kucukgergin C, Kalelioglu I, Seckin S, Atar A, Ander H, Ziylan O (2016) Urinary IP-10, MCP-1, NGAL, Cystatin-C, and KIM-1 levels in prenatally diagnosed unilateral hydronephrosis: the search for an ideal biomarker. Urology 87:185–192
Noyan A, Parmaksiz G, Dursun H, Ezer SS, Anarat R, Cengiz N (2015) Urinary NGAL, KIM-1 and L-FABP concentrations in antenatal hydronephrosis. J Pediatr Urol 11:249
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding
No funding was secured for this study.
Ethical approval
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Kim, B.K., Yim, H.E. & Yoo, K.H. Plasma neutrophil gelatinase-associated lipocalin: a marker of acute pyelonephritis in children. Pediatr Nephrol 32, 477–484 (2017). https://doi.org/10.1007/s00467-016-3518-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3518-y