Abstract
Background
The sensitivity and specificity of the leukocyte esterase test are relatively low for a screening test for urinary tract infection (UTI). More accurate tests could reduce both overtreatment and missed cases. This study aimed to determine whether neutrophil gelatinase-associated lipocalin (NGAL) can replace leukocyte esterase in the diagnosis of UTI and/or whether NGAL accurately identifies children with acute pyelonephritis.
Methods
Data sources—MEDLINE and EMBASE. We only considered published studies that evaluated the results of an index test (NGAL) against the results of urine culture (for UTI) or against the results of dimercaptosuccinic acid (for acute pyelonephritis) in children aged 0 to 18 years. Two authors independently applied the selection criteria to all citations and independently extracted the data.
Results
A total of 12 studies met our inclusion criteria. Four studies (920 children) included data on NGAL for UTI; eight studies (580 children) included data on NGAL for pyelonephritis. We did not pool accuracy values because the included studies used different cutoff values. For the diagnosis of UTI, urinary NGAL appeared to have better accuracy than the leukocyte esterase test in all included studies. For the diagnosis of pyelonephritis, neither plasma NGAL nor urinary NGAL had high sensitivity and/or specificity. The number of studies was the main limitation of this systematic review.
Conclusions
Urinary NGAL appears promising for the diagnosis of UTI; however, larger studies are needed to validate this marker as a replacement for leukocyte esterase. The use of NGAL for diagnosing acute pyelonephritis requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Current bedside tests for UTI are not sufficiently accurate. The best available point-of-care test for UTI, the leukocyte esterase test, has a 79% sensitivity (i.e., it misses 21% of children with a true UTI) and 87% specificity (i.e., 13% of those negative for UTI will be incorrectly diagnosed with UTI and receive antimicrobial treatment) [1]. Accordingly, there has been interest in identifying tests that can more accurately differentiate children with and without UTI. More accurate tests could reduce both overtreatment and missed cases.
Current point-of-care tests for the diagnosis of acute pyelonephritis (APN) are also suboptimal; among these, the best markers are procalcitonin (PCT) and C-reactive protein (CRP), which are only useful for ruling in and ruling out APN, respectively [2].
NGAL is a small protein released by activated neutrophils in response to infection. Thus, several investigators have compared the accuracy of NGAL to the leukocyte esterase test as a screening test for UTI. Furthermore, because renal tubular cells release NGAL in response to injury or infection [3], others have investigated its potential as a marker of APN.
Although the accuracy of NGAL for acute kidney injury has been reviewed, its utility in the diagnosis of UTI and APN has not. This systematic review will examine whether plasma or urine NGAL (pNGAL and uNGAL, respectively) can be used to accurately diagnose UTI or APN.
Methods
Types of studies
The protocol for this review was submitted to PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) on November 13, 2018 (CRD42020137166). We considered published studies that evaluated the results of an index test (NGAL) against the results of urine culture (for UTI) or dimercaptosuccinic acid (DMSA) for acute pyelonephritis (APN). Cross-sectional and cohort studies were acceptable; case-control designs were included only if controls were representative of children being tested for UTI or APN (case-control studies with extreme cases or controls tend to result in biased estimates) [4]. We excluded studies in which another inflammatory marker (e.g., C-reactive protein, erythrocyte sedimentation rate) was used to select those getting DMSA. We included studies of symptomatic children from birth to 18 years of age with suspected UTI. Studies limited to children with neurogenic bladder or children with major genitourinary abnormalities were excluded. Studies in which another test was initially used to screen patients (e.g., WBC count to screen for UTI) were excluded.
Index tests
Studies that examined the accuracy of NGAL were considered, regardless of how NGAL was measured. Some studies reported creatinine normalized uNGAL values (uNGAL/creatinine), while others just reported uNGAL.
Target conditions and reference standards
UTI and APN were the two target conditions examined. The reference standard for diagnosis of UTI was the urine culture. We defined UTI as growth of one or two organism(s) at ≥ 10,000 colony-forming unit/ml (CFU/ml) from a catheterized specimen, or ≥ 100,000 CFU/ml clean catch, midstream, or bag specimen, or any growth from a suprapubic specimen [5]. The reference standard for assessing the presence and extent of pyelonephritis is a DMSA scan obtained within 2 weeks of diagnosis of a UTI [6].
Search strategy
We searched MEDLINE and EMBASE. Searches were carried out on December 7, 2019. Search strategies are presented in the electronic supplement (online resource 1). The reference lists of all included articles and relevant systematic reviews were reviewed to identify additional studies not found through the electronic review. Two authors independently applied the selection criteria to all citations (titles and abstracts). The full text of all articles identified by either author was retrieved and reviewed. We limited the review to articles written in English, Spanish, French, German, Portuguese, or Italian.
Data extraction and management
For each study meeting the inclusion criteria, we extracted the following information: age of participants, fever required (yes, no), used perineal bags to collect urine specimen (yes, no), and case control (yes, no). Two by two tables were constructed independently by two authors from the data in the publication. Only studies for which two by two data were available (or could be reconstructed) were included. Two authors independently used the QUADAS II questionnaire to assess the risk of bias in all studies meeting inclusion criteria [7]. Disagreements were resolved by discussion.
Statistical analysis and data synthesis
The primary analysis was to compare the NGAL test against the reference standard. We had planned to pool accuracy values (using a random-effects bivariate model using SAS Proc NLMIXED in SAS; see registered protocol for more detail) and statistically investigate heterogeneity only if 5 or more studies reported accuracy values for the NGAL test using the same cutoff. Otherwise, if different studies use different cutoff values when reporting accuracy, instead of reporting pooled accuracy (i.e., sensitivity and specificity) values, we had planned to examine how accuracy varied with threshold on a summary receiver operating characteristic curve (i.e., curve used to summarize performance of a diagnostic test based on data from a meta-analysis).
Results
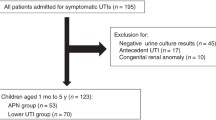
The results of the search strategy are shown in Fig. 1. Of the 1892 records identified, 60 records were retrieved and reviewed. A total of 12 studies met our inclusion criteria; one author was successfully contacted for clarification. The characteristics of key excluded studies are detailed in the electronic supplement (online resource 2).
uNGAL or uNGAL/creatinine for UTI
Four studies (920 patients) provided data for these analyses [8,9,10,11]. Two [9, 11] presented data for uNGAL over creatinine and three [8, 10, 11] presented data for NGAL not normalized to urine creatinine. Of the four studies, two used bags for urine collection, three included only febrile populations, and two were case control in design (Table 1). In all studies (Table 2, also see online resource 3 for graphical representation), NGAL had markedly higher accuracy than previously reported pooled sensitivity and specificity values [95% confidence interval] for the leukocyte esterase test (0.79 [0.77, 0.81] and 0.87 [0.86, 0.88], respectively) [1]. Direct comparison between uNGAL and existing tests for the diagnosis of UTI (leukocyte esterase test or WBC count) was limited due to the small number of studies, but data from studies that provided this is reported in Table 3; in all but one study, the NGAL test (or NGAL/creatinine) was more sensitive than the currently available test and had comparable or better specificity; in one small study [9], uNGAL was more sensitive but less specific than the leukocyte esterase test. An important methodological limitation was that in two of the four included studies, patient selection could have introduced bias (online resource 4).
uNGAL for APN
Three studies (162 patients) provided data for this analysis [9, 12, 13]. Of the three studies, one used bags for urine collection, all three included only febrile children, and two were case-control studies (Table 1); two studies reported uNGAL, and two reported uNGAL/creatinine. An important methodological limitation was that in one of the three included studies, patient selection could have introduced bias (online resource 4). The accuracy of uNGAL was low in all three studies (Table 2 and online resource 3 for a graphical representation).
pNGAL for APN
Six studies (474 patients) provided data for this analysis [12, 14,15,16,17,18]. Of the 6 studies, none used bags for urine collection, four included only febrile children, and none of the studies were case control in design (Table 1). Both sensitivity and specificity were above 80% in two of the six studies [14, 16] (Table 2 and online resource 3 for a graphical representation).
Discussion
In this review we examined the accuracy of urinary NGAL for the diagnosis of UTI and the accuracy of urinary and plasma NGAL for the diagnosis of pyelonephritis. With regard to UTI diagnosis, we found that urinary NGAL consistently outperformed the leukocyte esterase test in both sensitivity and specificity in the majority of included studies. The non-uniformity of cutoffs used across the different studies precluded the calculation of an overall sensitivity and specificity. Nevertheless, in most studies examined, the accuracy of NGAL significantly exceeded the accuracy of the leukocyte esterase test.
For differentiating pyelonephritis from cystitis, neither uNGAL nor pNGAL demonstrated sufficient accuracy to replace the DMSA scan for definitive diagnosis. Furthermore, the number of studies in each of the three analyses (uNGAL for APN, uNGAL/creatinine for APN, pNGAL for APN) was small. This lack of data was compounded by methodological shortcomings in a large proportion of studies (use of bag specimen, non-representative patient populations, inclusion of children without fever). The pre-test probability of pyelonephritis in young febrile children with a UTI is approximately 60% [20]. A positive DMSA scan increases the probability of UTI to 93% and a negative DMSA scan decreases the probability of pyelonephritis to 18% (assuming the DMSA has a sensitivity and specificity of 86% and 91%, respectively). To rule in pyelonephritis, a useful test would need to increase post-test probability of pyelonephritis to at least 85%, with 85% selected as a clinically reasonable threshold above which many clinicians would feel reasonably confident in their diagnosis of pyelonephritis and which is almost a high as a DMSA test. The post-test probability of pyelonephritis exceed 85% in only two [14, 16] of the six studies of pNGAL and one [9] of three studies of uNGAL. To rule out pyelonephritis, a useful test would need to reduce the post-test probability of pyelonephritis to less than 20%, with 20% representing the cutoff below which most clinicians would feel reasonably confident in their diagnosis of cystitis and which is close to the DMSA test. The post-test probability of pyelonephritis was less than 20% in only two [16, 17] of the six studies of pNGAL and one [13] of three studies of uNGAL. In summary, the bulk of the data, albeit sparse, suggest that the utility of both pNGAL and uNGAL for the diagnosis of pyelonephritis is limited.
The small number of studies with relevant data was a major limitation on this analysis. This was especially patent in the diagnosis of pyelonephritis, where cutoffs and estimates of test accuracy varied widely between studies without explanation. Another potentially large, but less obvious, limitation of studies examining the accuracy of NGAL as a diagnostic marker of pyelonephritis was the failure to differentiate between the monomeric and dimeric forms of the protein. Previous studies have demonstrated that while the renal epithelium produces NGAL predominantly in the monomeric form, activated neutrophils in the urine (and in the infected kidney) secrete NGAL primarily in its dimeric form [21]. Since assays that could differentiate the two forms were not readily available until recently, none of the studies included attempted to differentiate the two forms and thus did not consider the effects that differentiating the two forms of the protein might have had on diagnostic accuracy. This may explain why uNGAL was more accurate for diagnosis of UTI compared to diagnosing APN. This suggests that future studies of the diagnostic accuracy of NGAL should pay particular attention to the form of NGAL being measured. Inclusion of children without fever was another limitation. However, except for two studies, all children in the included studies were febrile. Thus, only a small number of children without fever (n = 31 across all studies) were included. This is unlikely to have significantly affected our results. Furthermore, only one of the 5 analyses conducted (plasma NGAL for APN) included studies with afebrile children. Differences in the definition of UTI across studies were another limitation. Of the studies looking at the accuracy of NGAL for UTI, two studies allowed for the presence of a second organism on culture as long as the child met the criteria for UTI with the first pathogen. Neither of these studies used perineal bags to collect samples. We frequently encounter cases in which children with likely UTI have growth of a second organism, usually with counts < 10,000 CFU/ml, and thus feel that inclusion of these studies is reasonable; similar criteria have been used in large recent studies [22,23,24].
In summary, urinary NGAL offers a promising alternative to the current leukocyte esterase test for diagnosing UTI. Although we used 96-well plates and ELISA kits to measure NGAL, individual testing using existing analyzers in many hospital laboratories is becoming increasingly available. Larger studies are needed to validate the superiority of this marker for the diagnosis of UTI in children. For the differentiation of cystitis from pyelonephritis, we found that neither plasma nor urine NGAL levels appeared to be sufficiently accurate; however, further study examining different molecular forms of NGAL (monomeric vs. dimeric) for the diagnosis of APN are warranted.
Abbreviations
- UTI:
-
Urinary tract infection
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- pNGAL:
-
Plasma neutrophil gelatinase-associated lipocalin
- uNGAL:
-
Urine neutrophil gelatinase-associated lipocalin
- APN:
-
Acute pyelonephritis
- PCT:
-
Procalcitonin
- CRP:
-
C-reactive protein
- DMSA:
-
Dimercaptosuccinic acid
- WBC:
-
White blood cell
- CFU:
-
Colony-forming unit
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
References
Williams GJ, Macaskill P, Chan SF, Turner RM, Hodson E, Craig JC (2010) Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta-analysis. Lancet Infect Dis 10:240–250
Shaikh N, Borrell JL, Evron J, Leeflang MM (2015) Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev 1:CD009185
Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D'Agati V, Lin CS, Qiu A, Al-Awqati Q, Ratner AJ, Barasch J (2014) α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest 124:2963–2976
Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM (2006) Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469–476
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595–610
Stokland E, Hellstrom M, Jacobsson B, Jodal U, Lundgren P, Sixt R (1996) Early 99mTc dimercaptosuccinic acid (DMSA) scintigraphy in symptomatic first-time urinary tract infection. Acta Paediatr 85:430–436
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, JAC S, Bossuyt PMM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Jung N, Byun HJ, Park JH, Kim JS, Kim HW, Ha JY (2018) Diagnostic accuracy of urinary biomarkers in infants younger than 3 months with urinary tract infection. Korean J Pediatr 61:24–29
Lee HE, Kim DK, Kang HK, Park K (2015) The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr Nephrol 30:123–130
Lubell TR, Barasch JM, Xu K, Ieni M, Cabrera KI, Dayan PS (2017) Urinary neutrophil gelatinase-associated lipocalin for the diagnosis of urinary tract infections. Pediatrics 140:e20171090
Valdimarsson S, Jodal U, Barregard L, Hansson S (2017) Urine neutrophil gelatinase-associated lipocalin and other biomarkers in infants with urinary tract infection and in febrile controls. Pediatr Nephrol 32:2079–2087
Shaikh N, Martin JM, Hoberman A, Skae M, Milkovich L, Nowalk A, McElheny C, Hickey RW, Kearney D, Majd M, Shalaby-Rana E, Tseng G, Alcorn JF, Kolls J, Kurs-Lasky M, Huo Z, Horne W, Lockhart G, Pohl H, Shope TR (2019) Host and bacterial markers that differ in children with cystitis and pyelonephritis. J Pediatr 209:146–153 e1
Yim HE, Yim H, Bae ES, Woo SU, Yoo KH (2014) Predictive value of urinary and serum biomarkers in young children with febrile urinary tract infections. Pediatr Nephrol 29:2181–2189
Krzemien G, Panczyk-Tomaszewska M, Kotula I, Demkow U, Szmigielska A (2019) Serum neutrophil gelatinase-associated lipocalin for predicting acute pyelonephritis in infants with urinary tract infection. Cent Eur J Immunol 44:45–50
Yun BA, Yang EM, Kim CJ (2018) Plasma neutrophil gelatinase-associated lipocalin as a predictor of renal parenchymal involvement in infants with febrile urinary tract infection: a preliminary study. Ann Lab Med 38:425–430
Kim BK, Yim HE, Yoo KH (2017) Plasma neutrophil gelatinase-associated lipocalin: a marker of acute pyelonephritis in children. Pediatr Nephrol 32:477–484
Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH (2015) Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatr Res 78:48–55
Seo WH, Nam SW, Lee EH, Je BK, Yim HE, Choi BM (2014) A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. Eur J Pediatr 173:229–232
Shaikh N, Hoberman A, Hum SW, Alberty A, Muniz G, Kurs-Lasky M, Landsittel D, Shope T (2018) Development and validation of a calculator for estimating the probability of urinary tract infection in young febrile children. JAMA Pediatr 172:550–556
Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126:1084–1091
Martensson J, Bellomo R (2014) The rise and fall of NGAL in acute kidney injury. Blood Purif 37:304–310
Hoberman A, Chesney RW, Trial Investigators RIVUR (2014) Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med 371:1072–1073
Keren R, Carpenter MA, Hoberman A, Shaikh N, Matoo TK, Chesney RW, Matthews R, Gerson AC, Greenfield SP, Fivush B, McLurie GA, Rushton HG, Canning D, Nelson CP, Greenbaum L, Bukowski T, Primack W, Sutherland R, Hosking J, Stewart D, Elder J, Moxey-Mims M, Nyberg L (2008) Rationale and design issues of the randomized intervention for children with vesicoureteral reflux (RIVUR) study. Pediatrics 122(Suppl 5):S240–S250
Keren R, Shaikh N, Pohl H, Gravens-Mueller L, Ivanova A, Zaoutis L, Patel M, deBerardinis R, Parker A, Bhatnagar S, Haralam MA, Pope M, Kearney D, Sprague B, Barrera R, Viteri B, Egigueron M, Shah N, Hoberman A (2015) Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics 136:e13–e21
Author information
Authors and Affiliations
Contributions
Dr. Nader Shaikh is responsible for the study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, and statistical analysis. He had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Kai Shaikh, Victor Osio, and Vinod Rajakumar are responsible for the acquisition of data, analysis and interpretation of data, and drafting of the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaikh, K., Rajakumar, V., Osio, V.A. et al. Neutrophil gelatinase-associated lipocalin for urinary tract infection and pyelonephritis: a systematic review. Pediatr Nephrol 36, 1481–1487 (2021). https://doi.org/10.1007/s00467-020-04854-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04854-3