Abstract
This educational review will highlight the historical and contemporary references that establish a basic understanding of measurements of kidney function in the neonate and its relevance for the life of an individual. Importantly, the differential renal function of preterm infants relative to term infants has become paramount with the increased viability of preterm infants and the realization that kidney function is associated with gestational age. Moreover, neonatal kidney function is primarily associated with absolute renal mass and hemodynamic stability. Neonatal kidney function and its early developmental progression predict lifelong cardiovascular and renal disease risks. Validation of estimation equations of kidney function in this population has provided important reference data for other investigations and a clinical basis for prospective and longitudinal follow-up. Future research should be directed towards a better understanding of surrogate markers of kidney function from infancy through adulthood. Pediatric nephrologists should be aware of the developmental aspects of kidney function including the importance of the congenital nephron endowment and the preservation of kidney function throughout a lifetime.
Key summary points
• Nephrogenesis occurs in utero in concert with other organ systems by branching morphogenesis, including the lungs, pancreas, and vascular tree, with over 60 % of nephrons being formed during the last trimester.
• Infants born preterm before 36 weeks’ gestation are in active nephrogenesis and are at increased risk of having a decreased nephron endowment from prenatal and postnatal genetic and epigenetic hazards that will impact the patient for a lifetime.
• Post-natal adaptation of kidney function is directly proportional to the number of perfused nephrons, estimated by total kidney volume (TKV), mean arterial pressure (MAP), and gestational age.
• Accurate measurement of glomerular filtration rate (GFR) in infants is problematic due to the unavailability of the gold standard inulin. The traditional use of creatinine to estimate GFR is unreliable in preterm infants due to its tubular reabsorption by immature kidneys and its dependence on muscle mass as an endogenous marker. Alternative endogenous markers to estimate GFR are cystatin C and beta trace protein (BTP).
• Long-term follow-up of renal function in those born preterm should be life long and should include assessment of GFR, total kidney volume (TKV) relative to body surface area (BSA), and cardiovascular risks including hypertension and vascular stiffness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite many decades of careful scientific investigation, the accurate assessment of kidney function in the preterm infant remains elusive [1, 2]. Nephrogenesis is intended to be complete by 36 weeks’ gestation in utero [3, 4]. Preterm infants are born during active nephrogenesis, making them particularly vulnerable to alterations imposed by the extra-uterine environment [5, 6]. The individual adaptive kidney function will depend on the effective nephron mass (or surface area), which is proportional to the number of perfused and fully formed glomeruli [7]. It is inversely proportional to the area of the “nephrogenic zone” composed of incompletely formed glomeruli in various stages of development immediately below the renal capsule [7, 8]. Among preterm infants, the number of nephrons formed during the first 4–6 weeks of post-natal life will determine that individual’s nephron endowment for a lifetime [9]. Taking advantage of this “window of opportunity” to support active extra-uterine nephrogenesis through judicial clinical management may be the most important determinant of the preterm infant’s final nephron endowment [10]. For example, management of the preterm infant commonly includes the use of nephrotoxic medications and correcting hemodynamic instability related to environmental stress [11–13]. Epigenetic exposures including environmental pollutants can also contribute to kidney injury in the developing fetus and child [14]. In addition, genetic predisposition plays a role in determining risk for chronic kidney disease (CKD). For example, African Americans have a higher rate of preterm birth and new evidence suggests that carrying the APOL1 risk allele, which is associated with focal segmental glomerulosclerosis (FSGS), may be an additive risk for CKD progression in these infants [15]. To that end, it is important to assess the preterm infant’s renal size and function from the outset [16–18]. Understanding the limitations and applications of endogenous markers of kidney function relative to gestational age and post-natal development should allow early recognition of acute kidney injury (AKI) [19–21]. This, in turn, should lead to more systematic longitudinal surveillance of renal size and function in infants after their perinatal course [13, 17–21]. For many neonatal intensive care units, this will require a cultural change and we make an appeal for broader adoption of the clinical assessment tools described herein.
In this review, we will emphasize the strong association of structure and function in neonatal kidneys especially regarding the transition from the intrauterine to a potentially hostile extra-uterine environment. We will also consider kidney size and function in the perinatal period and their implications for the life course of individuals. The maturation of renal function from birth to early childhood will be examined according to post-natal physiologic and structural adaptations. Methodologies for the “true” measurement of kidney function in infants will be analyzed from a historical perspective. The limited data available validating current estimation equations of glomerular filtration rate (eGFR) applicable to the neonatal population will also be summarized [22–24]. This review is an ambitious endeavor with a goal of guiding continued translational research in the field of human renal-functional maturation. Most importantly, we provide an important educational update on current directions in neonatal renal functional assessment.
Nephrogenesis and congenital nephron endowment
The human kidney forms from three primordial “attempts” beginning with the primitive, but obligatory anlage, called the “pronephros” at about 3 weeks’ gestation and lasting for only 3–5 days [3, 4, 25–27]. The pronephros never functions but provides the pattern of ciliated epithelial cells that are reminiscent of the proximal tubular epithelial cells that will compose a major part of the human nephron [4, 25–27]. With the regression of the pronephros, the mesonephros emerges as a much more complex structure with nephrotomes and filtration bundles developing around the incursion of the Wolffian duct into the mesonephric mesenchyme. Subsequently, the mesonephric nephrons begin their involution and the Wolffian duct gives rise to the ureteric bud, which starts its incursion into the metanephric mesenchyme, giving rise to the metanephros, the third anlage and true human kidney. From the 5th gestational week, the ureteric bud has begun sequential branching into the metanephric mesenchyme propagated by a complex system of signaling molecules emanating from both tissues [25–27]. By the 20th gestational week, the fetal urine comprises 80 % of the amniotic fluid at a rate of approximately 800 ml per day (300 ml/kg fetal weight/day) [28, 29]. The fetal urine flow rate is 2–5 ml/h at 20–25 weeks’ gestation; 5–10 ml/h by 26–30 weeks’ gestation, and 15–30 ml/h by 31 to 40 weeks’ gestation [28, 29].
The kidneys form in parallel with other organ systems by a process of “branching morphogenesis” [25–27]. Of profound interest and scientific relevance, these various organ systems share genetic and physiologic functional determinants within the “fetal origins of adult disease paradigm” [30–32]. Among the organ systems that develop by “branching morphogenesis” are the lungs, pancreas, vascular tree, and kidneys [30–32]. As a consequence, parallel burdens of post-natal maturation, and growth may impact insulin resistance, vascular stiffness, chronic lung disease, as well as early renal senescence [28–32]. Over 80 % of the nephrons form during the last trimester, with a rapid succession of “nephron generations” from the cortico-medullary junction extending up to the “nephrogenic blue zone” under the capsule [3–5]. From 22 to 36 weeks’ gestation, 10–12 generations of nephrons form, similar to the rings of a tree [3–5]. With cessation of glomerulogenesis, the nephrogenic zone disappears [25, 26]. Thereafter, only tubular and vascular growth and interstitial tissue expansion occurs [25, 26]. The glomerular epithelial cells are cuboidal in shape and the capillary loops are poorly perfused, receiving less than 2 % of the fetal cardiac output [28, 29]. It appears that the epithelial cells decrease in density as the nephrons expand with the inner glomeruli becoming more “mature”, while those of the outer cortex are the last to expand, making them the more vulnerable to adverse circulation ex-utero [27]. If birth occurs prematurely during active nephrogenesis, there may be potential for ongoing or even accelerated nephrogenesis [5, 6, 33]. However, our knowledge of ongoing nephrogenesis in preterm humans is limited to a few postmortem studies [5, 6, 33]. Rodriguez et al. showed that extra-uterine nephrogenesis was active until 40 post-natal days in extremely preterm infants. Among those infants who survived >40 days, a full complement of nephrons was never achieved compared to term newborns [5]. Moreover, those infants who had AKI, defined as a serum creatinine >1.5 mg/dl (133 mM/l), had fewer nephron generations compared to those without AKI [5]. Sutherland et al. [33] conducted an autopsy study of preterm infants ranging from 24 to 35 weeks’ gestation and showed active or even accelerated nephrogenesis with a high percentage of morphologically abnormal glomeruli, including “atubular” and cystic glomeruli, which would not be able to function [33]. The same group has demonstrated similar morphology in a non-human primate model of prematurity [34]. In the final analysis, preterm birth is an independent risk factor for a decrease in one’s nephron endowment [5, 6, 33, 34].
Importantly, a low nephron endowment has been linked to the development of hypertension and cardiorenal disease in adult life [35]. As pertains to renal organogenesis, there is a wide normal distribution in the “congenital nephron endowment” [36, 37]. This has been confirmed in numerous other studies, with a tenfold variance in nephron numbers (Nglom) ranging from approximately 200,000 to over 2,000,000 per kidney [37–44]. Epigenetic and environmental influences play a prominent role in the nephron endowment, which may be affected by simple genetic variants or polymorphisms within a given population accounting for the wide normal distribution that has been described [37, 45]. Nyengaard also described the positive correlation between the human nephron endowment and kidney size by gram weight across all age ranges [37]. Of note, renal size and nephron numbers are 10–15 % less in females, beginning at an early age [37].
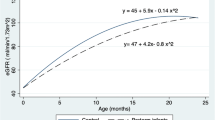
Since the number of nephrons in each kidney is determined before term birth, and the size of the kidney must accommodate the size of the infant, the nephron density (Dglom) or Nglom per gram kidney weight is at least 10 × greater during infancy and gradually declines until maturation when the final mature kidney mass is obtained. At each age, there is a wide confidence interval following the normal distribution that is found [37–45]. In Fig. 1a, we have plotted the regression equation of a composite of data points extracted from references that provided kidney size with nephron numbers per kidney over an age range from <3 months of age to >60 years of age in postmortem kidneys from subjects with preserved renal function [37–45]. Similarly, the glomerular density relative to kidney size is plotted in Fig. 1b. Both relationships are significant and the graphic depiction of glomerular number (Nglom) and density (Dglom) relative to advancing age and kidney weight provides a clear representation of the renal structural changes that occur from birth to maturation. After 40 years of age, there is a gradual loss in Nglom through senescence manifest as glomerular sclerosis and GFR declines by 10 ml/min/1.73 m2 each decade [46]. Its relevance for preterm infants is paramount, since there is evidence that low nephron endowment and preterm birth lead to early senescence with a higher set-point for the onset of progressive CKD [24, 46, 47].
a Linear regression of the Natural Log (LN)10 of glomerular number (Nglom) per kidney ranging from kidney weights of 20 g to >200 g at autopsy from individuals with normal kidney function ranging in age from <3 months to >60 years. Data were extracted from references [35–42]. The red line indicates the average Nglom = 813,000 with an average mature kidney weight of 150 g. b Non-linear regression of the glomerular density (Dglom) as the number of glomeruli (N/glom) per gram kidney weight. The data were extracted from references [35–42] and included kidneys at autopsy from individuals with normal kidney function ranging in age from <3 months to >60 years. The red line indicates the average Dglom = 5500 or Nglom = 825,000 in an average kidney weighing 150 g
A recent educational review on the determinants of nephron number in early life covers the subject well and is recommended for more comprehensive reading [45]. However, the methodologies devoted to the actual counting of nephrons deserve closer attention since there is an important research effort directed towards being able to assess the nephron endowment in the living individual for prediction of cardio-renal longevity as well as to determine the suitability for kidney donation [48, 49]. Hinchcliffe et al. first devised the “disector” method for estimating glomerular number and size, and determined from autopsied infants that those with intrauterine growth restriction had fewer nephrons [50]. Shortly thereafter, Hinchliffe described the “medullary ray glomerular counting” method; now called the “radial glomerular counts” of nephron generations, which provides not only an estimate of nephron numbers but allows an assessment of the stages of active nephrogenesis in preterm infants [51]. The “gold standard” has become the “dissector/ fractionator” method, which is laborious, expensive, and requires the destruction of the tissue [48, 49, 52]. More recently, the injection of cationic ferritin followed by ex vivo MRI scanning in animals appears to be a promising technique directed towards developing methodologies for in vivo studies [53]. Until then, we must rely on surrogate and easily accessible modalities for assessing renal mass, such as renal ultrasound, while we continue to refine other non-invasive methods of clinical assessment of nephron mass in infants and small children currently limited to experimental use [54–56].
Post-natal physiologic and structural adaptations
Birth is a hemodynamically intense event that has been characterized as one of the most dangerous moments of a lifetime. The fetus has been completely protected and nurtured within the amniotic fluid and perfused through a blood circuit of low oxygen tension [28, 29]. The physiologic, structural, and functional alterations of the kidneys in transition from the fetal to the extra-uterine environment include an abrupt increase in renal perfusion pressure mediated by an increase in the percentage of cardiac output received by the kidneys from 3 to 25 %. There is also a gradual decrease in filtration fraction (FF) from as high as 50 % to a mature level of 20 % after the glomerular capillary resistance decreases and tubular growth and expansion occurs. Filtration fraction (FF) is the ratio of GFR over renal plasma flow (RPF); FF = GFR/RPF. At birth, both GFR and RPF are low due to the low perfusion pressure, high blood viscosity, and increased efferent arteriolar tone [12, 13]. Ultimately, the GFR will equal 120 ml/min/1.73 m2 and the RPF will be over 600 ml/min/1.73 m2 giving a FF of 20 % at full maturation. This FF is higher and takes longer to mature in preterm infants. This may also partially explain the “back diffusion” of creatinine described in preterm infants who have persistently elevated serum creatinine and low urinary creatinine excretions with appropriate renal function in the first weeks of life [57, 58].
Glomerular perfusion is dependent on local and systemic mediators of afferent and efferent arteriolar tone, with the primary humoral mediators being prostaglandins for afferent relaxation and the renin–angiotensin–aldosterone system maintaining efferent arteriolar constriction [28, 29]. The extremely sensitive glomerular-tubular feedback at the juxtaglomerular apparatus (JGA) may insert a potentially damaging reflex that can acutely constrict the afferent arteriole through the generation of adenosine in response to anoxia or hypotension [28].
Measurement of glomerular filtration in infants by exogenous markers
Historically, inulin and its analog sinistrin have been considered the “gold standard” for measurement of GFR in infants and, to date, have not been replaced [59]. It is a naturally occurring polysaccharide that is a derivative of the Jerusalem artichoke and other legumes such as chicory. It meets the essential criteria for accurate measurement of GFR, including being freely filtered through the glomerulus and neither being secreted nor re-absorbed in its transit down the nephron [16, 60]. Moreover, it must not be metabolized or protein bound after injection. Importantly, it must be safe and innocuous for clinical investigations in infants and children. During the early years of investigation into the renal function of infants, there were a number of studies that reported inulin clearances relative to gestational and post-natal age [61–71]. These studies were considered the foundation for the assessment of neonatal kidney function and development. Unfortunately, the product is no longer readily available for clinical use and, as will be discussed below, has been supplanted by estimation equations [16, 60]. Other direct measures of GFR include non-radioactive iothalamate [72]. Although it is partially protein bound and there is possible tubular secretion, it is inexpensive and, in the neonate, closely approximates inulin clearance [72]. Iohexol is a non-radiopaque contrast agent used in the measurement of GFR in children with CKD, but has not been validated in infants [23, 73].
Estimation of GFR by endogenous markers
Despite the imperative for having a reliable assessment of GFR in the neonate, the performance of tedious clearance studies, especially in sick infants, is impractical. As a consequence, endogenous markers of GFR have been sought with the construction of estimating equations [22, 23, 73, 74]. To serve as an accurate marker of GFR, the plasma solute must be freely filtered across the glomerular membrane and neither secreted nor reabsorbed along the tubular network. It must be solely excreted via the kidney and the endogenous production rate must be constant. The endogenous markers that have been applied in the assessment of neonatal kidney function across gestational age groups are creatinine, cystatin C, and beta-trace protein (BTP) [17, 74–81]. Using a composite analysis of historic clearance studies and contemporary estimating equations applicable to infants, Table 1 and Fig. 2 have been provided as a resource [62–68].
Plot and comparison of the estimated glomerular filtration rates (eGFR) derived from three endogenous markers: creatinine (cr), cystatin C (CysC), and beta trace protein (BTP) in colors against the measured inulin clearance from preterm birth through the first 36 months of life. At birth, especially in the preterm infant, eGFRcr markedly underestimates GFR-inulin; whereas GFR CysC and GFR BTP closely approximate measured or “true” GFR. After 6 months, GFRcr and GFR BTP overestimate and GFR CysC underestimate “true” GFR. It is not until 3 years of age that all the eGFR seem to accurately assess GFR. Data are derived from references used to develop Table 1 [17, 74–82]
Schwartz and others, using inulin clearances in their early clinical investigations, provided reliable data that allowed the derivation of estimating equations from serum creatinine values [23, 73, 74]. More recently, the Schwartz equation has been modified using iohexol clearances in a large cohort of children with CKD (CKiD study) [74]. The need for the adjustment of the Schwartz constant “k” by 25 % from 0.55 to 0.413 was due to the adoption of the enzymatic assay for serum creatinine over the historical Jaffe method, which decreased the results by 20–25 % [74]. Pottel et al., in a meta-analysis of creatinine-based estimating equations, have provided the Flanders equation, which adjusts the k by age so that those subjects <3 years of age can have more accurate assessment of GFR [81]. Similarly, Zappitelli et al. have provided iothalamate clearances in infants and children and applied estimating equations using cystatin C and creatinine [76]. More recently, Filler et al. have extensively reviewed the application and potential advantages of using BTP in estimating GFR in neonates [79].
In summarizing the benefits of applying estimating equations for the accurate assessment of GFR in infants, it is evident that creatinine-based formulas lack accuracy. However, creatinine assays are universally standardized, readily available, and inexpensive. Cystatin C-based equations have been validated in infants and children and are independent of body mass and age [74–77]. Cystatin C-based equations are consistent with inulin and iohexol clearances and do not require length measurements [74–77]. Although novel, BTP may be a potential alternative to cystatin C; but the most accurate BTP estimating equations still contain creatinine and height [79]. Both cystatin C and BTP assays are not routinely available in the hospital chemistry laboratory as standard assays and are from 10–15 times more expensive [79]. Combined equations offer greater accuracy and are included in Table 2; but the univariate equations offer the best bedside assessments. Therefore, current recommendations would be to use a univariate cystatin C equation followed by the modified Schwartz equation adjusted by the Flanders’ constant in infants <2 years of age [16, 23, 74, 81].
Towards normative charts for creatinine, cystatin C, and eGFR
In prior years, experts have called for the adoption of normative charts for infants and children to be able to interpret measures of renal assessment similar to growth curves with the calculation of z-scores or percentiles relative to the normal pooled data [82]. Traditionally, the dogma has been to dismiss elevated serum creatinine in preterm infants during the neonatal period as unreliable. However, there is growing concern among pediatric nephrologists and neonatologists that failure to recognize kidney injury during the neonatal period may result in a lack of surveillance for the development of progressive kidney disease [13, 17, 83].
Table 1 provides the mean ± standard deviation (SD) calculated for preterm and term infants at birth and at 3, 6, and 12 months post-conceptual age for serum creatinine, cystatin C, and eGFR [74–82]. Table 2 provides a summary of the estimating equations most applicable to infants during the first 2 years of life with the caveat that none have been adequately validated in infants [74–82].
Total kidney volume in the assessment of kidney function
In the early post-natal period, maintenance of GFR is dependent primarily on gestational age, total kidney volume (TKV), and mean arterial pressure (MAP) [15]. Within days, with hemodynamic stability, the healthy neonate has adjusted to the extra uterine environment and 15–20 % of cardiac output is distributed to the kidneys [7, 26, 27]. However, maturation of GFR to adult levels will take almost 2 years in the healthy infant and is directly related to maturation and growth of the kidney and increase in the surface area of those glomeruli that were present at birth (nephron endowment).
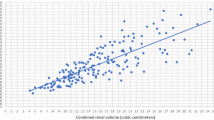
Preterm birth imposes immediate and potential long-term alterations in kidney size and function. Infants born before 36 weeks’ gestation during active nephrogenesis will have a decreased number of filtering nephrons and the GFR will be proportionate to the number of nephrons being perfused. The GFR will also be a function of the gestational age since renal size in utero follows gestational growth curves [17]. In a small autopsy study of term neonates (n = 15) glomerular number correlated to kidney weight [43]. This is consistent with adult autopsy studies in various populations, suggesting that nephron endowment can be estimated by following kidney size [35–42]. Even more compelling is that kidney size individualized to body surface area (BSA) correlates closely with kidney function across all age groups [15, 16, 83–85]. Individual kidney volume can be measured using the equation for an ellipsoid: volume = length × width × depth × 0.523 and then left and right kidney volumes can be summated for the TKV (ml) [54, 55, 85]. Ultra-sonographers can estimate these measurements from standard renal ultrasound images [54, 55, 85].
Moreover, there is a normal Gaussian distribution of TKV individualized to BSA (TKV/m2 = ml/m2) when measured by non-invasive renal ultrasound from birth to adulthood [85]. A cross-sectional study of renal ultrasounds in 624 healthy German children from birth to 18 years of age showed an average TKV/m2 to be 132 ml/m2 with the 10th and 90th percent confidence interval being 90 and 171 ml/m2, respectively [85]. In a separate Canadian cohort of 136 full-term healthy Caucasian infants <3 months of age, an identical curve was generated with the mean TKV/m2 = 132 ± 29 ml/m2 [43]. Most recently, the Generation R study of over 6000 Dutch children followed prospectively since birth used TKV as a marker of kidney health and have shown subtle but significant differences in kidney function at 6 years of age [86, 87].
When the Gaussian distribution of TKV/m2 of the normal population of 624 infants, children and adults was superimposed on that of 60 preterm infants at birth from our cohort, the remarkable observation was that they were similar [17, 85]. This corroborates previous studies that nephron mass and presumed nephron endowment is normally distributed and similar in preterm infants at birth (Fig. 3). The implications are significant in that, if active nephrogenesis can be supported to fruition post-natally, then the preterm infant could potentially achieve a nephron endowment similar to that of a term infant. It also suggests that individuals whose TKV/m2 falls below the 10th percentile have an inherent risk for “oligonephropathy” and should be monitored for hypertension and progression to CKD.
The Gaussian distribution of total kidney volume (TKV) factored by body surface area (BSA) in milliliters (ml) per meter2 (ml/m2). The predominant distribution is derived from the data of 624 healthy German individuals from <1 month to 18 years of age [85]. The mean and 90 % confidence intervals define the normal distribution of kidney mass and, by inference, “nephron endowment” since kidney volume correlates closely with kidney weight [54]. An individual whose TKV/m2 falls below the 10th percentile (<90 ml/m2) should be observed closely for development of chronic kidney disease (CKD) due to nephropenia. The darker curve within the larger German cohort is data from our cohort of 60 preterm infants [17]. This demonstrates that preterm infants have a similar normal distribution of nephron endowment at birth and suggests that they have the potential for normal attainment of nephron mass in the extra-uterine environment. Most importantly, preterm infants should be followed sequentially for kidney growth by TKV/m2 throughout childhood
In seeking more accurate and alternative estimates of GFR in infants and children, it has become evident that TKV should be considered in the baseline and longitudinal assessment of neonatal kidney function and has implications for the individual’s renal longevity [13, 14, 16, 17].
Conclusions
In summary, it is well documented that being born prematurely confers an increased risk of CKD in adulthood. This review has highlighted the key factors likely underlying this phenomenon, namely the importance of the congenital nephron endowment. The extra-uterine environment of the preterm infant is often taxed with various insults such as nephrotoxic drug exposure and hemodynamic instability that may negatively affect the short window of ongoing nephrogenesis. Subsequently, obesity, recurrent AKI, and the natural aging process will all negatively impact the nephron endowment. Hence, early identification and periodic screening of these at-risk preterm individuals should allow for mitigation of injury and preservation of their nephron endowment and renal function over the life span. As more data accumulate, especially in larger diverse cohorts, such as the Neonatal Kidney Collaborative (NKC), NCT02443389, a scoring system may be forthcoming that incorporates the degree of prematurity, AKI, renal size, and other relevant factors to be validated for better stratification of future risk of CKD among these infants [19]. In a recent review, Carmody and Charlton proposed a screening algorithm including measuring blood pressure, renal function, and proteinuria for these at-risk patients [83]. Providers could consider screening these patients every 6 months to 1 year with well child visits and refer to specialists when appropriate. Future research efforts should focus on prospective longitudinal studies of preterm infants to identify more specific screening timelines and areas for early therapeutic intervention to mitigate the morbidity of CKD. Finally, as in most circumstances, the onset of cardiovascular and renal complications of being born preterm and/or of low birth weight will likely not manifest until early adulthood. Mechanisms for effective transition of care to adult providers must include highlighting these concerns.
Questions
-
1.
The human kidney forms from which sequential developmental stages?
-
a.
Pronephros, metanephros, mesonephros
-
b.
Antinephros, neonephros, humanonephros
-
c.
Metanephros, mesonephros, pronephros
-
d.
Pronephros, mesonephros, metanephros
-
a.
-
2.
What is considered the “gold standard” for measurement of GFR in infants?
-
a.
Iohexol
-
b.
Inulin
-
c.
Creatinine clearance
-
d.
Para-aminohippurate clearance
-
a.
-
3.
An endogenous marker of GFR must fulfill all of the following criteria except it:
-
a.
Must be freely filtered by the glomerular barrier
-
b.
Must be secreted and reabsorbed by the renal tubule at an equal rate
-
c.
Must be produced at a constant rate
-
d.
Must not be protein bound
-
a.
-
4.
The nephron endowment is determined by which of the following:
-
a.
Intra-uterine environment
-
b.
Genetic polymorphisms of intra-uterine drivers of nephrogenesis
-
c.
Prematurity and post-natal completion of nephrogenesis
-
d.
All of the above
-
a.
-
5.
Which of the following subjects does not have oligonephronia?
-
a.
Six-year-old born 36 weeks preterm with a serum creatinine of 0.4 mg/dl and a TKV/m2 = 160 ml/m2
-
b.
Fifteen-year-old obese male with unilateral renal agenesis with a TKV of 135 ml and a BSA = 2.00 m2
-
c.
Three-year-old born extremely preterm and low birth weight with serum creatinine of 0.6 mg/dl and a TKV/m2 = 85 ml/m2
-
d.
Six-year-old born at term with IUGR who is growing well along the 15th percentile for length and the 50th percentile for weight.
-
a.
References
Walker MW, Clark RH, Spitzer AR (2011) Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. J Perinatol 31:199–205
Thayyil S, Sheik S, Kempley ST, Sinha A (2008) A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J Perinatol 28:226–229
Potter EL, Thierstein ST (1943) Glomerular development in the kidney as an index of fetal maturity. J Pediatr 22:695–706
Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V (2012) Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol 227:1257–1268
Rodriguez M, Gomez A, Abitbol C, Chandar J, Duara S, Zilleruelo G (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25
Faa G, Gerosa C, Fanni D, Puddu M, Marinelli V, Zaffanello M, Fanos V (2010) Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern Fetal Neonatal Med 23:129–133
Veille JC, McNeil S, Hanson R, Smith N (1998) Renal hemodynamics: longitudinal study from the late fetal life to one year of Age. J Matern Fetal Investig 8:6–10
Ferraz MLF, dos Santos AM, Cavellani CL, Rossi RC, Correa RRM, dos Reis MA, Teixeira VPA, Castro ECC (2008) Histochemical and immunohistochemical study of the glomerular development in human fetuses. Pediatr Nephrol 23:257–262
Abitbol CL, Rodriguez MM (2012) The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol 8:265–274
Faa G, Sanna A, Gerosa C, Fanni D, Puddu M, Ottonello G, Van Eyken P, Fanos V (2015) Renal physiological regenerative medicine to prevent chronic renal failure: should we start at birth? Clin Chim Acta 444:156–162
Rhone ET, Carmody JB, Swanson JR, Charlton JR (2014) Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 27:1485–1490
Toth-Heyn P, Drukker A, Guignard JP (2000) The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14:227–239
Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9:2036–2043
Weidemann DK, Weaver VM, Fadrowski JJ (2015) Toxic environmental exposures and kidney health in children. Pediatr Nephrol. doi:10.1007/s00467-015-3222-3
Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O’Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR, Nephrotic Syndrome Study Network (2015) Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol. doi:10.1681/ASN.2014111131
Filler G (2015) A step forward towards accurately assessing glomerular filtration rate in newborns. Pediatr Nephrol 30:1209–1212
Abitbol CL, Seeherunvong W, Galarza MG, Katsoufis C, Francoeur D, Defreitas M, Edwards-Richards A, Master Sankar Raj V, Chandar J, Duara S, Yasin S, Zilleruelo G (2014) Neonatal kidney size and function in preterm infants: what is a true estimate of glomerular filtration rate? J Pediatr 164:1026–1031.e2
Treiber M, Pečovnik Balon B, Gorenjak M (2015) A new serum cystatin C formula for estimating glomerular filtration rate in newborns. Pediatr Nephrol 30:1297–1305
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL (2015) Neonatal acute kidney injury. Pediatrics 136:e463–473
Askenazi DJ, Ambalavanan N, Goldstein SL (2009) Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol 24:265–274
Jetton JG, Askenazi DJ (2014) Acute kidney injury in the neonate. Clin Perinatol 41:487–502
Deng F, Finer G, Haymond S, Brooks E, Langman CB (2015) Applicability of estimating glomerular filtration rate equations in pediatric patients: comparison with a measured glomerular filtration rate by iohexol clearance. Transl Res 165:437–445
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Pottel H, Hoste L, Delanaye P (2015) Abnormal glomerular filtration rate in children, adolescents and young adults starts below 75 ml/min/1.73 m2. Pediatr Nephrol 30:821–828
Kuure S, Vuolteenaho R, Vainio S (2000) Kidney morphogenesis: cellular and molecular regulation. Mech Dev 92:31–45
Fanos V, Loddo C, Puddu M, Gerosa C, Fanni D, Ottonello G, Faa G (2015) From ureteric bud to the first glomeruli: genes, mediators, kidney alterations. Int Urol Nephrol 47:109–116
Crobe A, Desogus M, Sanna A, Fraschini M, Gerosa C, Fanni D, Fanos V, Van Eyken P, Faa G (2014) Decreasing podocyte number during human kidney intrauterine development. Am J Physiol Ren Physiol 307:F1033–F1040
Jose PA, Fildes RD, Gomez RA, Chevalier RL, Robillard JE (1994) Neonatal renal function and physiology. Curr Opin Pediatr 6:172–177
Arant BS Jr (1987) Postnatal development of renal function during the first year of life. Pediatr Nephrol 1:308–313
Ingelfinger JR, Nuyt AM (2012) Impact of fetal programming, birth weight, and infant feeding on later hypertension. J Clin Hypertens (Greenwich) 14:365–371
Nigam SK (2013) Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs? Stem Cells Transl Med 2:993–1000
Hallows SE, Regnault TR, Betts DH (2012) The long and short of it: the role of telomeres in fetal origins of adult disease. J Pregnancy 2012:638476
Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ (2011) Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22:1365–1374
Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ (2009) Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Ren Physiol 297:F1668–F1677
Ingelfinger JR (2008) Disparities in renal endowment: causes and consequences. Adv Chronic Kidney Dis 15:107–114
Merlet-Bénichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelièvre-Pégorier M (1999) Nephron number: variability is the rule. Lab Investig 79:515–527
Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232:194–201
Bendtsen TF, Nyengaard JR (1992) The number of glomeruli in type 1 (insulin-dependent) and type 2 (non-insulin dependent) diabetic patients. Diabetologia 35:844–850
Keller G, Zimmer G, Mall G, Ritz E, Amann K (2003) Nephron number in patients with primary hypertension. N Engl J Med 348:101–108
McNamara BJ, Diouf B, Hughson MD, Douglas-Denton RN, Hoy WE, Bertram JF (2008) Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant 3:2576–2585
McNamara BJ, Diouf B, Hughson MD, Hoy WE, Bertram JF (2009) Associations between age, body size and nephron number with individual glomerular volumes in urban West African males. Nephrol Dial Transplant 4:1500–1506
Zimanyi MA, Hoy WE, Douglas-Denton RN, Hughson MD, Holden LM, Bertram JF (2009) Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol Dial Transplant 24:2428–2433
Zhang Z, Quinlan J, Hoy W, Hughson MD, Lemire M, Hudson T, Hueber PA, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Houghton F, Bertram J, Goodyer P (2008) A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol 19:2027–2034
Bueters RR, van de Kar NC, Schreuder MF (2013) Adult renal size is not a suitable marker for nephron numbers: an individual patient data meta-analysis. Kidney Blood Press Res 37:540–546
Charlton JR, Springsteen CH, Carmody JB (2014) Nephron number and its determinants in early life: a primer. Pediatr Nephrol 29:2299–2308
Delanaye P, Glassock RJ (2015) Lifetime risk of CKD: what does it really mean? Clin J Am Soc Nephrol 10:1504–1506
Crump C, Sundquist K, Sundquist J, Winkleby MA (2011) Gestational age at birth and mortality in young adulthood. JAMA 306:1233–1240
Puelles VG, Bertram JF (2015) Counting glomeruli and podocytes: rationale and methodologies. Curr Opin Nephrol Hypertens 24:224–230
Tsuboi N, Kanzaki G, Koike K, Kawamura T, Ogura M, Yokoo T (2014) Clinicopathological assessment of the nephron number. Clin Kidney J 7:107–114
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D (1991) Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Investig 64:777–784
Hinchliffe SA, Sargent PH, Chan YF, van Velzen D, Howard CV, Hutton JL, Rushton DI (1992) “Medullary ray glomerular counting” as a method of assessment of human nephrogenesis. Pathol Res Pract 188:775–782
Bertram JF, Cullen-McEwen LA, Egan GF, Gretz N, Baldelomar E, Beeman SC, Bennett KM (2014) Why and how we determine nephron number. Pediatr Nephrol 29:575–580
Baldelomar EJ, Charlton JR, Beeman SC, Hann BD, Cullen-McEwen L, Pearl VM, Bertram JF, Wu T, Zhang M, Bennett KM (2015) Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int. doi:10.1038/ki.2015.316
Jones TB, Riddick LR, Harpen MD, Dubuisson RL, Samuels D (1983) Ultrasonographic determination of renal mass and renal volume. J Ultrasound Med 2:151–154
van Venrooij NA, Junewick JJ, Gelfand SL, Davis AT, Crumb TL, Bunchman TE (2010) Sonographic assessment of renal size and growth in premature infants. Pediatr Radiol 40:1505–1508
Cheong B, Muthupillai R, Rubin MF, Flamm SD (2007) Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol 2:38–45
Guignard JP, Drukker A (1999) Why do newborn infants have a high plasma creatinine? Pediatrics 103:e49
Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G (2011) Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol 6:274–280
Doxiadis SA, Goldfinch MK (1952) Comparison of inulin and endogenous creatinine clearance in young children. J Physiol 118:454–460
Filler G, Yasin A, Medeiros M (2014) Methods of assessing renal function. Pediatr Nephrol 29:183–192
Strauss J, Adamsons K, James LS (1965) Renal function of normal full-term infants in the first hours of extra-uterine life. Am J Obstet Gynecol 91:286–290
Guignard JP, Torrado A, Da Cunha O, Gautier E (1975) Glomerular filtration rate in the first three weeks of life. J Pediatr 87:268–272
Fawer CL, Torrado A, Guignard JP (1979) Maturation of renal function in full-term and premature neonates. Helv Paediatr Acta 34:11–21
Strauss J, Daniel SS, James LS (1981) Postnatal adjustment in renal function. Pediatrics 68:802–808
Coulthard MG (1983) Comparison of methods of measuring renal function in preterm babies using inulin. J Pediatr 102:923–930
Brion LP, Fleischman AR, McCarton C, Schwartz GJ (1986) A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr 109:698–707
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 34:571–590
van der Heijden AJ, Grose WF, Ambagtsheer JJ, Provoost AP, Wolff ED, Sauer PJ (1988) Glomerular filtration rate in the preterm infant: the relation to gestational and postnatal age. Eur J Pediatr 148:24–28
Wilkins BH (1992) Renal function in sick very low birthweight infants: 1. Glomerular filtration rate. Arch Dis Child 67:1140–1145
van den Anker JN, de Groot R, Broerse HM, Sauer PJ, van der Heijden BJ, Hop WC, Lindemans J (1995) Assessment of glomerular filtration rate in preterm infants by serum creatinine: comparison with inulin clearance. Pediatrics 96:1156–1158
Cole BR, Giangiacomo J, Ingelfinger JR, Robson AM (1972) Measurement of renal function without urine collection. A critical evaluation of the constant-infusion technic for determination of inulin and para-aminohippurate. N Engl J Med 287:1109–1114
Holliday MA, Heilbron D, al-Uzri A, Hidayat J, Uauy R, Conley S, Reisch J, Hogg RJ (1993) Serial measurements of GFR in infants using the continuous iothalamate infusion technique. Southwest Pediatric Nephrology Study Group (SPNSG). Kidney Int 43:893–898
Staples A, LeBlond R, Watkins S, Wong C, Brandt J (2010) Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25:2321–2326
Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82:445–453
Nehus EJ, Laskin BL, Kathman TI, Bissler JJ (2013) Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol 28:453–461
Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L (2006) Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis 48:221–230
Montini G, Cosmo L, Amici G, Mussap M, Zacchello G (2001) Plasma cystatin C values and inulin clearances in premature neonates. Pediatr Nephrol 16:463–465
Benlamri A, Nadarajah R, Yasin A, Lepage N, Sharma AP, Filler G (2010) Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol 25:485–490
Abbink FC, Laarman CA, Braam KI, van Wijk JA, Kors WA, Bouman AA, Spreeuwenberg MD, Stoffel-Wagner B, Bökenkamp A (2008) Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem 41:299–305
Filler G, Lopes L, Harrold J, Bariciak E (2014) β-trace protein may be a more suitable marker of neonatal renal function. Clin Nephrol 81:269–276
Pottel H, Hoste L, Martens F (2012) A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr Nephrol 27:973–979
Filler GM (2011) The challenges of assessing acute kidney injury in infants. Kidney Int 80:567–568
Carmody JB, Charlton JR (2013) Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 131:1168–1179
Adibi A, Adibi I, Khosravi P (2007) Do kidney sizes in ultrasonography correlate to glomerular filtration rate in healthy children? Australas Radiol 51:555–559
Scholbach T, Weitzel D (2012) Body-surface-area related renal volume: a common normal range from birth to adulthood. Scientifica (Cairo) 2012:949164
Voortman T, Bakker H, Sedaghat S, Kiefte-de Jong JC, Hofman A, Jaddoe VW, Franco OH, van den Hooven EH (2015) Protein intake in infancy and kidney size and function at the age of 6 years: the generation R study. Pediatr Nephrol 30:1825–1833
Bakker H, Kooijman MN, van der Heijden AJ, Hofman A, Franco OH, Taal HR, Jaddoe VW (2014) Kidney size and function in a multi-ethnic population-based cohort of school-age children. Pediatr Nephrol 29:1589–1598
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Answers to questions
1. D
2. B
3. B
4. D
5. A
Rights and permissions
About this article
Cite this article
Abitbol, C.L., DeFreitas, M.J. & Strauss, J. Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol 31, 2213–2222 (2016). https://doi.org/10.1007/s00467-016-3320-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3320-x