Abstract
Accurate assessment of renal function is critical for appropriate drug dosing of renally excreted compounds. Glomerular filtration rate (GFR) is considered the best marker of kidney function. Inulin clearance forms the gold standard for measuring GFR, both in adults and in children. The method is invasive, cumbersome, and smaller children require urinary catheterization for accurate timed urine collections. Nuclear medicine methods replaced inulin clearance in the 1970s after 51Cr EDTA clearance was introduced. Inulin has no plasma protein binding, whereas all commonly used radioisotopes have a small amount of plasma protein binding that leads to lower values. Only iohexol does not have significant plasma protein binding. The underestimation due to plasma protein binding is partially offset by overestimation due to the use of non-compartmental pharmacokinetic modeling of the plasma disappearance of the radioisotope. The problem could be overcome with a urinary nuclear medicine clearance method, but these have not been validated in children. Endogenous markers of GFR include serum creatinine and low molecular weight proteins such as cystatin C and beta-trace protein. Of these, estimation of GFR using cystatin C appears to be the most promising, although its accuracy in pregnancy and in the neonatal period may be limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adequate measurement of kidney function is important for the management of all children and adolescents. In the neonatal period, there is considerable ontogeny of drug disposition, largely due to developmental changes of nephron formation and recruitment [1]. This was shown elegantly for ceftazidime [2] and for famotidine [3]. Accurate measurement of kidney function is also important for drugs excreted by the kidneys across all other ages. With many kidney diseases, intervention often depends on whether kidney function is normal or abnormal. Further, when there is impaired kidney function, accurate assessment of kidney function is important for initiation of renal replacement therapy [4], listing for renal transplant [5], evaluating interventions, and monitoring changes of function over time [6].
For drug dosing, tubular secretion is perhaps more important than glomerular filtration rate (GFR) [7]. Tubular secretion capability cannot easily be measured and requires assessment of both GFR and renal plasma flow [8]. GFR may be inappropriately high due to glomerular hyperfiltration [9]. Glomerular hyperfiltration is a phenomenon that can occur in various clinical conditions including kidney disease. No single definition of glomerular hyperfiltration has been agreed upon [10]. However, it is thought that glomerular hyperfiltration can be caused by afferent arteriolar vasodilation as seen in patients with diabetes or after a high-protein meal, and/or by efferent arteriolar vasoconstriction owing to activation of the renin-angiotensin-aldosterone system, thus leading to glomerular hypertension [10]. GFR may therefore be inappropriately high for a given nephron endowment. Despite this limitation, it is widely accepted that kidney function is best measured as GFR [11].
GFR cannot be measured directly. The most common method of measuring GFR is based on the concept of clearance. The renal clearance of substance x (Cx) is calculated as:
where V is the urine flow rate (ml/min), Ux is urine concentration of substance x, and Px is the plasma concentration of substance x. Cx is expressed in milliliters per minute. If the substance is freely permeable across the glomerular capillary and is not synthesized, transported, or metabolized by the kidney, Cx is equal to GFR [12].
Gold-standard measurement of GFR
If a substance in stable concentration in the plasma is physiologically inert, freely filtered at the glomerulus, and is not secreted, reabsorbed, synthesized, or metabolized by the kidney, the amount of that substance filtered at the glomerulus is equal to the amount excreted in the urine [13]. The gold standard for the measurement of GFR is inulin clearance. In 1934, while studying water reabsorption in the renal tubule of amphibians, Richards found that the polysaccharide inulin (a fructose polymer made from the Jerusalem artichoke) is freely filtered through collodion membranes, not absorbed. Inulin is one of the few molecules that satisfies the requirements of an ideal marker of glomerular filtration, namely exclusive elimination by glomerular clearance and no tubular secretion and no non-renal excretion [13].
When considering inulin clearance, a few concepts have to be introduced:
-
1.
Single nephron GFR vs. total GFR: The level of GFR is the product of the single-nephron GFR multiplied by the number of functioning nephrons in both kidneys. In the case of CKD, GFR can be decreased because of a reduction in filtration rate of each nephron and/or a drop in nephron number. Factors leading to decreased renal perfusion may cause a drop in the single nephron GFR. The total GFR serves as the most reliable marker of functioning renal mass [12].
-
2.
Single bolus vs. infusion technique: GFR with an exogenous marker can be measured either by infusion technique [14] or single bolus [15]. The gold standard is an infusion technique that involves a bolus injection, followed by a steady infusion, blood samples at 2, 3, and 4 h and timed urine collection [16]. Small children require catheterization. This is obviously very invasive. The single bolus injection method with its plasma disappearance approach is more convenient and eliminates the need for timed urine collection. However, there are principal disadvantages with an infusion technique. After injections of inulin or other GFR markers, the marker is first distributed in the intravascular space. The volume of distribution is the extracellular space [17]. Time needs to pass to allow for equilibration between both compartments. Only infusion technique with timed urine collection after 3 or 4 h will have saturated both compartments for certain. If samples after single bolus injection of the GFR marker are drawn before equilibration occurs, GFR will be overestimated [18]. The median equilibration time is usually considered to be 20 min in adults, but there is wide inter-individual variability [19]. Children may have longer equilibration times than adults because of the different body composition [20]. The authors are unaware of any studies that have determined equilibration time between the various compartments of inulin in children. Especially in situations such as nephrotic syndrome, edema, and increased extracellular volume, the equilibration time may be much longer. Studies in dogs suggest considerable impact of hydration status and hemodynamic changes [21]. It is not always possible to predict the equilibration time and protocols used for single bolus GFR measurement use fixed sampling times without accommodating to the hydration status of the patient. The difference between infusion versus single bolus injection inulin clearance is on average 9.7 ml/min/1.73 m2 in children, with a tendency to overestimate GFR with the single bolus technique [18]. For a patient with a true GFR of 20 ml/min/1.73 m2, this could mean a delay in transplant listing.
-
3.
The third important concept is the appropriate use of pharmacokinetic modeling for the excretion of inulin. Most centers do not consider this when using either infusion or single bolus injection methods. Agents used to measure glomerular filtration rate (GFR) give a biexponential plasma disappearance curve on multiple peripheral venous sampling between 20 min and 4 h after intravenous injection. These two exponentials are generally regarded to represent equilibration of agent throughout the extracellular fluid (ECF) space and renal clearance, respectively. The arterial plasma clearance curve of GFR agents is triexponential; the first exponential reflects equilibration of agent between plasma and the interstitial space of carcass tissue (mainly muscle and skin). The second exponential is minor compared with the first; it is not clear what it represents. Only the third exponential reflects renal clearance [19]. To adequately address this issue, proper two-compartmental pharmacokinetic modeling needs to be utilized as shown by Van Rossum et al. [22]. In this paper, the need for a late sample at 240 min when using the single bolus injection method is stressed [22]. We recommend at least 3 sampling times and a start no earlier than 90 min. Delayed sampling is important in patients with severe renal function impairment.
If inulin clearance is utilized, we recommend a bolus injection of 5,000 mg of inulin per 1.73 m2, with a maximum dose of 5,000 mg, using Inutest from Fresenius. The inulin has to be infused with a constant rate over 30 s. Extravasation must be excluded as this would result in overestimation of GFR. There have to be 3 or more sampling times, ideally with samples extending to 240 min. For the evaluation, a two-compartmental model using the concentrations at the actual time points must be utilized, ideally with NONMEM or other appropriate pharmacokinetic software programs like WINNONLIN [18]. The limited availability of Inutest and the issues outlined in this chapter may significantly restrict the use of this method. Therefore, alternate methods of measuring GFR have been developed.
GFR methods using radiolabeled isotopes and iohexol
Since the 1970s, radiolabeled isotope techniques for estimation of GFR have replaced inulin clearance [23]. The main advantage of using a radiolabeled compound with characteristics similar to inulin is its immediate determination by counting the radioactivity. Today’s nuclear medicine methods with easily assayed, radiolabeled, and stable compounds that meet the criteria of a marker of GFR, namely clearance only by glomerular filtration without tubular secretion and non-renal elimination, form a new standard. Unfortunately, the widely used iothalamate does undergo tubular secretion [24]. The most widely used single bolus-injection techniques utilize the intravenous injection of a suitable compound at a precisely known amount. Similar to inulin clearance, extravasation will cause significant overestimation of GFR [25]. After the injection, the plasma must be sampled from the opposite arm and concentration is plotted against time.
A number of substances are used for nuclear medicine GFR measurement. They are listed in Table 1. An important concept to introduce is that tracers used for GFR measurement by nuclear medicine show a small amount of plasma protein binding, which may affect their ultrafiltration (see Table 1) [26]. As a consequence, there is slight underestimation of GFR with nuclear medicine techniques by about 10 % compared to inulin [27]. It is also known that the negatively charged glomerular basement membrane forms a restriction to the ultrafiltration of 51Cr-ethylenediamine tetra-acetic acid (EDTA) and 99Tc-diethylenetriamine penta-acetic acid (DTPA) [26]. As a consequence, disorders affecting the serum albumin concentration or the glomerular basement charge, such as nephrotic syndrome, may affect the accuracy of GFR measurement [26].
Most of these nuclear medicine GFR measurements are used as single-bolus injection methods. The same limitations that apply for inulin also apply to these substances. It is generally recognized that at least three sampling points are required. Again, a 240-min sample is necessary, and for lower GFR even further extended sampling times are required. Unfortunately, there is poor standardization among most centers, and only two-point sampling time points are often chosen to reduce the number of venipunctures in children. Similar to the limitations raised with inulin, most centers utilize only one-compartmental models, instead of appropriate pharmacokinetic two-compartmental non-linear models. As with inulin, a single compartmental model results in some overestimation of GFR [28]. Late sampling (4 and/or 5 h concentrations after injection) could overcome some of the problems related to the mathematical models, but this is rarely implemented [29]. For a more accurate assessment of the extracellular water (especially important in children with altered fluid status, for instance if they are on long-term diuretics), an early sampling point is required [30]. Also, if one-compartmental models are employed, appropriate corrections for the overestimation should be employed [28].
Of course, nuclear medicine GFR scans involve radiation exposure. Beta-particles from 51Cr-EDTA are clearly carcinogenic. There are low and high dose methods for 99mTc DTPA GFR scans [31]. While most of the radiation is quickly excreted through the kidneys, the whole long-term body radiation exposure of 99mTc DTPA GFR scans in adults was 2–3 % of the administered radioactivity, probably reflecting the protein-bound fraction. Also, while the radiation exposure is low, 4 % is retained by various tissues at 24 h, 69 % is eliminated by the kidneys with a half time of 1.73 h in healthy adults [26]. The radiation exposure clearly forms a limiting factor towards repeated nuclear medicine GFR scans. The non-ionic low osmolar contrast medium iohexol has become the most commonly used contrast medium for GFR measurements in Europe and may form a good alternative to the radiolabeled GFR markers (Table 1), especially as it has a much lower protein binding.
The lack of standardization of nuclear medicine methods needs to be addressed. Apart from the issues named above, there ought to be a mandatory image of the injection site to assess for extravasation, and a plot of the radioactivity over time should be provided to assess for outliers of the equilibration, especially in hemodynamically unstable patients and patients with significant volume overload. Finally, non-linear modeling using a two-compartmental model should be standard.
In our opinion, if nuclear medicine GFR studies with the plasma disappearance method are employed, the non-compartmental Russell method can be used, but a two-compartmental model is preferable. The Russell method in short: Plasma 99mTc-DTPA is calculated, after correcting for the decay rate from the control sample, based upon a formula by Russell et al. [32]. The formula shown below uses a non-compartmental linear approach after log-transformation of the counts in each of the three samples. The median of the three-GFR values was then standardized by total body surface area. The first measurement should not occur earlier than 90 min.
Nuclear medicine GFR: plasma disappearance versus urinary clearance method
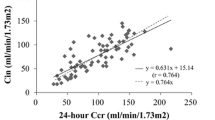
There are two methods typically employed to estimate the GFR: the commonly used plasma disappearance method described above, which examines the rate of decline of a radioisotope in timed plasma samples to determine clearance, and a more labor-intensive urinary method, which combines timed samples of both urine and plasma to determine clearance. Several studies have demonstrated that the accuracy of the plasma disappearance method of GFR estimation is limited when GFR is below 20–30 ml/min/1.73 m2 [33, 34]. Trials assessing GFR estimation equations have used the urinary clearance GFR method to overcome this limitation [35]. However, these studies are limited to advanced CKD in adults. We recently performed a study across all GFR ranges in adults that have not yet been published. We used the following method:
The urinary clearance of 99mTc-DTPA was calculated using urinary clearance equation:
where U i is the urinary concentration of 99mTc-DTPA adjusted for isotopic decay rate, V i is the urinary volume and P i is the plasma concentration of 99mTc-DTPA adjusted for the isotopic decay rate. While there was a strong correlation between both the urinary and plasma disappearance method (R2 = 0.86), the plasma disappearance method showed increasing overestimation with lower GFR. Among the 213 samples ranging from chronic kidney disease (CKD) stage 1 through 4, the mean (standard deviation) plasma iGFR of 60.7 (24.9) ml/min/1.73 m2 compared to urinary GFR of 52.0 (28.0) ml/min/1.73 m2, which was statistically significant (p value < 0.001). We concluded that the plasma clearance method of GFR estimation generally yielded higher results than the urinary method, with the bias increasing with advanced CKD stage.
The physician ordering a nuclear medicine GFR in a child needs to be fully aware of the limitations of these methods. Table 2 summarizes the few studies that were performed comparing inulin clearance with the various nuclear medicine methods. Unfortunately, none of them show complete agreement. The small number of patients in these studies points to the need for more studies. With regards to the bias, 51Cr EDTA appears to be the most favorable (Table 2), but its availability is largely restricted to Europe.
Magnetic resonance imaging (MRI) for assessment of GFR
Magnetic resonance with dynamic imaging of uptake of contrast agent using a two-compartment model has been proposed for GFR measurement [36], but more clinical studies are needed to establish if this method is sensitive to alterations in disease state, and it requires contrast administration. Functional magnetic resonance imaging enables non-invasive assessment of renal function without contrast media; recently it was reported that the renal blood oxygen-level dependent magnetic technique failed to discriminate between patients with different stages of chronic kidney disease [37]. At this point in time, it is too early to utilize this promising technique, and the use of gadolinium forms a significant limitation. In patients with advanced renal failure gadolinium can cause nephrogenic systemic fibrosis (NSF) characterized by fibrosis of the skin and connective tissue, and occasionally includes internal organs—it develops in days or over several weeks, and can be fatal. Patients with moderate renal disease or with acute kidney injury are at risk of developing NSF, and alternative tests or MRI without gadolinium should be considered.
Children-specific considerations
There are significant developmental changes (ontogeny) during childhood that have a significant effect on the use of any compound for the measurement of GFR. To accommodate for this change, GFR is normalized to body surface area, which is considered to be the best denominator, although the extracellular volume is also considered [28]. However, while all nephrons are terminally differentiated at birth, only the juxtamedullary glomeruli are used at birth. There is continuous recruitment of additional glomeruli until 18–24 months of age [38, 39]. Ideally, GFR should be reported as age-independent z-scores, rather than as absolute GFR or GFR/body surface area [40]. In the first year of life, and during puberty, there are growth spurts and rapid increase of muscle mass, requiring special considerations for the calculation of GFR [41], especially in adolescent males [42]. The most important determinant of GFR is height, which when taken into account led to the Schwartz formula [43], a GFR estimation model based on the height/creatinine ratio. Every nephrologist is familiar with it, and in its most recent iteration, the accuracy is much improved, especially in the range of 25–75 ml/min/1.73 m2 [44]. As outlined above, gold-standard measurements of GFR are cumbersome and invasive and involve the use of an exogenous compound. Those children at risk who require frequent GFR monitoring, such as transplant recipients [45], are already subject to numerous other tests. As such, accurate non-invasive measurements of GFR are essential and endogenous markers are required.
Endogenous surrogate markers of GFR
Because of this, it has become a preferred practice to use an endogenous marker that is produced at a constant rate, shares the features of inulin, and thus eliminates the need for a compound injection. Popper and Mandel proposed the use of serum creatinine in 1937 [46], which remains the most widely used marker for GFR estimation in spite of its shortcomings. Whereas serum endogenous markers are considered as suitable markers of GFR, the National Kidney Foundation guidelines recommend the use of serum marker-based prediction equations for GFR estimation and rules against the use of serum markers alone in the assessment of renal function, as discussed in [47]. However, the limitations of creatinine in children are far greater than that in adults, which has lead to significant research in this field for years.
Limitations of serum creatinine as endogenous marker of GFR
Serum creatinine remains the most widely used endogenous marker to predict GFR. Creatinine is a metabolic product of creatine and phosphocreatine found in muscle, and as such reflects muscle mass and varies little from day to day [48]. Serum creatinine measurement is widely available at low cost. In Canada, the cost of a single serum measurement is 40 cents US, whereas cystatin C is approximately $11.60 for the reagents and standards. On a population basis, creatinine may be a feasible marker of GFR in populations with near normal GFR, but this marker has multiple limitations for individuals. There is substantial inter- and intra-patient variability due to differences in muscle mass [49]. In childhood, there is age and muscle mass dependency of serum creatinine, and accurate assessment of normal GFR even with the use of body length/creatinine ratios remains difficult. In certain pediatric patient clientele, such as patients with spina bifida [50], neuromuscular disease, anorexia nervosa, or liver cirrhosis [51], serum creatinine is completely unusable because of the abnormal muscle mass in these children, who are often wheelchair bound [50]. In addition, the production of creatinine is not constant; the rate of turnover is also variable [52]. It is also known that creatinine undergoes tubular secretion that increases with lower GFR [53]. Creatinine is secreted in the proximal convoluted tubule by active transport similar to that of organic cations. Tubular secretion of creatinine divided by inulin clearance increases progressively from 0.16 in adult patients with normal GFR to 0.92 in patients with GFR less than 40 ml/min [54]. Furthermore, there used to be considerable variability in the reference range for serum creatinine based on the method used for its determination [55]. Proficiency testing surveys, published periodically by the College of American Pathologists, evaluated the variability in creatinine measurement among various methods used by different institutions across the US and demonstrated substantial variability [56]. This will make the validation of older methods to estimate GFR based on serum creatinine, measured by different techniques, subject to debate. Only more recently, the traceability of the creatinine measurements to higher-order reference methods (isotope dilution-mass spectrometry (IDMS) reference method) has improved accuracy of creatinine measurements [57]. This paper established improved pediatric reference intervals that may be adopted by any laboratory serving a similar population (predominantly Caucasian). Clinical implications of creatinine standardization have recently been reviewed [58].
Overall, the method used for serum creatinine is of high importance. Calculation of GFR based on serum creatinine measured by the alkaline picrate method is limited because of method non-specificity, low values in children, particularly in infants, and lack of appropriate GFR formulae. Therefore, enzymatic methods are preferred, as widely suggested in the literature [59]. As outlined above, creatinine measurements have to be IDMS traceable.
Even when correcting for the analytical variability, the problem of tubular secretion of serum creatinine cannot be addressed with the methodology of measurement. Blockade with H2 antagonists revealed promising results that may overcome the significant problem of tubular secretion of serum creatinine [60]. The use of cimetidine protocols in children remains scarce [61]. Creatinine clearance determinations involving timed urine collections may provide greater accuracy, but are difficult for pediatric patients to perform, time-consuming, and impractical for routine use. Small molecular proteins have emerged as superior endogenous markers of GFR [62]. For the new Schwartz formula, cystatin C and urea as well as creatinine measurements are required [44].
Small molecular weight proteins as markers of GFR
Small molecular mass proteins have long been proposed as markers of GFR as they are normally almost freely filtered through the normal glomerular membrane [63]. In a normally functioning kidney these small molecular weight proteins should then be almost completely reabsorbed and degraded by the proximal tubular cells. Several proteins have been tested, such as beta-2 microglobulin and beta-trace protein (analyzed in children in [64]). Beta-2 microglobulin is an acute phase protein, associated with inflammation [65]. However, a recent study in unborn children with congenital obstructive uropathy has demonstrated good results for the measurement of GFR in the fetus [66]. Cystatin C is the best-studied small molecular weight protein as a surrogate GFR marker and its superiority for the detection of mildly impaired GFR was proven in meta-analyses [67].
Cystatin C
The features of cystatin C have been reviewed in detail elsewhere [12]. Earlier studies of the serum level of cystatin C in large patient cohorts have failed to correlate the serum level to any pathophysiological state besides those affecting the glomerular filtration rate, which also is compatible with a stable secretion of cystatin C from most human tissues [68]. However, very large doses of glucocorticoids have recently been described to increase the production of cystatin C [69], whereas low and medium doses of glucocorticoids do not seem to alter its production [70]. Also, thyroid dysfunction can affect cystatin C levels [71], but our group found no association with thyroid function markers in recent studies [72, 73].
The reference values for cystatin C obtained in a carefully selected population are 0.75 ± 0.09 mg/l for children aged 4–19 years, 0.74 ± 0.10 mg/l for males and 0.65 ± 0.09 mg/l for females (aged 20–59 years), and 0.83 ± 0.10 mg/l for older individuals (> or =60 years) [74]. In the first year of life, renal function matures physiologically. Accordingly, much higher cystatin C values up to 2.8 mg/l were found at birth. These are subject to a rapid decline after birth reflecting maturation of kidney function [1]. Age dependency also has to be considered in adults. New reference intervals with more detailed age distribution from central Europe have recently been published on 985 healthy subjects over 25 years old [75].
Studies of the handling of human cystatin C in rats have shown that the plasma renal clearance of cystatin C is 94 % of that of the generally used GFR-marker 51Cr-EDTA and that cystatin C thus is practically freely filtered in the glomeruli [76]. At least 99 % of the filtered cystatin C is degraded in the tubular cells. When the GFR of a set of rats was variably lowered by constricting their aortas above the renal arteries, the renal plasma clearance of cystatin C correlated strongly with that of 51Cr-EDTA, with a linear regression coefficient of 0.99 and with the y-intercept not being statistically different from 0 [77].
After these encouraging studies, additional studies suggested that the reciprocal of cystatin C correlates better with a gold-standard GFR measurement than the reciprocal of serum creatinine [78]. Cystatin C as a marker of GFR was found to be independent of body composition [79]. Development of automated and rapid particle-enhanced immunoturbidimetric and immunonephelometric methods, also more precise than the original radioimmuno- or enzyme-linked immunosorbant assays [80], has allowed large-scale use of serum cystatin C as a clinically useful GFR-marker.
The diagnostic performance of cystatin C in comparison with serum creatinine was first analyzed in 2002 with a meta-analysis of 46 studies, in both adults and children [67]. The pooled data analysis compared correlation coefficients between GFR and the reciprocals of serum creatinine and cystatin C in 3,703 individuals and found significantly better correlations (mean r = 0.816 [95 % confidence interval: 0.804–0.826] versus mean r = 0.742 [95 % confidence interval: 0.726–0.758]). ROC plots were available for a pooled sample size of 997 individuals, again showing a significantly better area under the [ROC] curve (mean = 0.926 [95 % confidence interval: 0.892–0.960] versus mean r = 0.837 [95 % confidence interval: 0.796–0.878]). This meta-analysis suggests that cystatin C is superior to serum creatinine for the detection of impaired GFR in cross-sectional studies. More recently, another meta-analysis of 24 studies (n = 2,007) also confirmed that the diagnostic accuracy favored cystatin C [81], although in this more recent study, the diagnostic odds ratios started to overlap, most probably due to the IDMS testing of serum creatinine. Cystatin C measurement is more expensive than creatinine.
The need for standardization of cystatin C, similar to the IDMS traceability of serum creatinine
It should be highlighted that problems due to the lack of standardization of cystatin C lead to similar problems as highlighted above with regards to serum creatinine and the need for IDMS traceability [57]. Results obtained with the two main assays that are commercially available, namely the DAKO kit (turbidimetric, PETIA) and the Siemens Healthcare assay (nephelometric, PENIA), can be quite different, as recently demonstrated with the publication of reference intervals for healthy term and preterm infants. The results with the PENIA method in our study [82] were significantly lower than those reported by Harmoinen et al. [83]. Similar problems occurred when the results of the CKiD study were re-analyzed using PENIA, which led to substantially better results [44]. The difference in the results between these assays is partially due to different calibrators; DAKO uses recombinant cystatin C and Siemens human cystatin C from patients with tubulopathy. Recently, Anders Grubb reported the first certified standardized reference material for cystatin C [84]. Worldwide standardization, as demonstrated above, is a powerful tool towards improving the diagnostic accuracy of GFR biomarkers, and the authors of this review are convinced that standardization of cystatin C similar to that of creatinine will further enhance the diagnostic performance of cystatin C.
Beta-trace protein
Another potential surrogate marker for GFR measurement is beta-trace protein (BTP) [64], a low molecular weight protein that has been traditionally used as a marker for cerebrospinal fluid leakage. Beta-trace protein shares many of the features of cystatin C as a marker of GFR [64]. Unlike cystatin C, which is strongly positively charged, beta-trace protein has an isoelectric point of 5.5 or lower [85]. Beta-trace protein appears to be a more favorable marker in pregnancy [86] and in newborns [87] and might be better than cystatin C in renal transplant children [88, 89]. Formulae for the estimation of GFR have been developed and validated for adults [90] and for children [91].
Conclusions
In summary, inulin remains the gold standard GFR measurement. However, there is limited availability and its use remains cumbersome and invasive. Nuclear medicine GFR scans have replaced inulin clearance in most centers; however, they are not completely interchangeable. Iothalamate undergoes tubular secretion, which is a significant limitation. 51Cr EDTA and 99Tc DTPA have plasma protein binding that reduces their clearance in comparison to inulin. Iohexol may be the best marker on a theoretical basis because of the low plasma protein binding, and being non radioactive can be re-used in children without radiation burden; however, it demonstrates considerable scatter, and the clinical studies comparing inulin GFR with nuclear medicine scans favor 51Cr EDTA, which has showed the lowest bias and best agreement, both in transplant and non-transplant patients. Significant limitations apply if extravasation occurs, if samples are drawn too early before an equilibration with the entire extracellular volume occurs, and if no appropriate two-compartmental model is used for the analysis. Most centers just use log transformation and proper pharmacokinetic non-linear two-compartmental models with exact time points are rarely employed. The endogenous markers have far greater limitations. Low molecular weight proteins appear to be more favorable than creatinine. Reasonably good agreement between measured and estimated GFR can be achieved with cystatin C, urea and creatinine together. Cystatin C appears to be slightly superior to beta trace protein with the exception of during pregnancy and the neonatal period.
References
Filler GM (2011) The challenges of assessing acute kidney injury in infants. Kidney Int 80:567–568
Pedersen M, Karstoft K, Lodrup A, Jespersen B, Nyengaard JR (2011) Advantages and controversies in the era of intrarenal volumetry. Am J Nephrol 33(Suppl 1):40–45
Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M (1999) Cystatin C in healthy women at term pregnancy and in their infant newborns: relationship between maternal and neonatal serum levels and reference values. Am J Perinatol 16:287–295
Abbink FC, Laarman CA, Braam KI, van Wijk JA, Kors WA, Bouman AA, Spreeuwenberg MD, Stoffel-Wagner B, Bokenkamp A (2008) Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem 41:299–305
Herget-Rosenthal S, Bokenkamp A, Hofmann W (2007) How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem 40:153–161
Bokenkamp A, Herget-Rosenthal S, Bokenkamp R (2006) Cystatin C, kidney function and cardiovascular disease. Pediatr Nephrol 21:1223–1230
Beck M, Graf C, Ellenrieder B, Bokenkamp A, Huber A, Hecher K, Bartmann P (2005) Long-term outcome of kidney function after twin-twin transfusion syndrome treated by intrauterine laser coagulation. Pediatr Nephrol 20:1657–1659
Kort SA, Bouman AA, Blankenstein MA, Bokenkamp A (2005) Cystatin C can be measured reliably in capillary blood samples. Clin Chem 51:903–904
Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G (2011) Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol 6:274–280
Bokenkamp A (2005) Kidney function itself, and not cystatin C, is correlated with height and weight. Kidney Int 67:777–778, author reply 778–779
Filler G, Browne R, Seikaly MG (2003) Glomerular filtration rate as a putative ‘surrogate end-point’ for renal transplant clinical trials in children. Pediatr Transplant 7:18–24
Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A (2005) Cystatin C as a marker of GFR–history, indications, and future research. Clin Biochem 38:1–8
Filler G, Huang SH, Yasin A (2012) The usefulness of cystatin C and related formulae in pediatrics. Clin Chem Lab Med. doi:10.1515/cclm-2012-0257
Bokenkamp A, Grabensee A, Stoffel-Wagner B, Hasan C, Henne T, Offner G, Lentze MJ (2002) The beta2-microglobulin/cystatin C ratio–a potential marker of post-transplant lymphoproliferative disease. Clin Nephrol 58:417–422
Poge U, Gerhardt T, Bokenkamp A, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP (2004) Time course of low molecular weight proteins in the early kidney transplantation period–influence of corticosteroids. Nephrol Dial Transplant 19:2858–2863
Miller BF, Leaf A, Mamby AR, Miller Z (1952) Validity of the endogenous creatinine clearance as a measure of glomerular filtration rate in the diseased human kidney. J Clin Invest 31:309–313
Bokenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B (2002) Effect of corticosteroid therapy on serum cystatin C and beta2-microglobulin concentrations. Clin Chem 48:1123–1126
Bokenkamp A, Herget-Rosenthal S (2004) Urinary cystatin C as a marker of GFR? A word of caution. Pediatr Nephrol 19:1429
Bokenkamp A, Ozden N, Dieterich C, Schumann G, Ehrich JH, Brodehl J (1999) Cystatin C and creatinine after successful kidney transplantation in children. Clin Nephrol 52:371–376
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J (1998) Cystatin C–a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101:875–881
Bokenkamp A, Ciarimboli G, Dieterich C (2001) Cystatin C in a rat model of end-stage renal failure. Ren Fail 23:431–438
Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT (2010) Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. Am J Cardiol 106:1594–1601
Filler G, Sharma AP (2008) How to monitor renal function in pediatric solid organ transplant recipients. Pediatr Transplant 12:393–401
Odlind B, Hallgren R, Sohtell M, Lindstrom B (1985) Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int 27:9–16
Bertholet-Thomas A, Ranchin B, King LA, Bacchetta J, Belot A, Gillet Y, Collardeau-Frachon S, Cochat P (2011) Post-diarrheal haemolytic uremic syndrome: when shall we consider it? Which follow-up? Arch Pediatr 18:823–830
Rehling M, Nielsen LE, Marqversen J (2001) Protein binding of 99Tcm-DTPA compared with other GFR tracers. Nucl Med Commun 22:617–623
Rehling M, Moller ML, Thamdrup B, Lund JO, Trap-Jensen J (1984) Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci (Lond) 66:613–619
Peters AM (2004) The kinetic basis of glomerular filtration rate measurement and new concepts of indexation to body size. Eur J Nucl Med Mol Imaging 31:137–149
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Stevens LA, Levey AS (2009) Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20:2305–2313
Assadi M, Eftekhari M, Hozhabrosadati M, Saghari M, Ebrahimi A, Nabipour I, Abbasi MZ, Moshtaghi D, Abbaszadeh M, Assadi S (2008) Comparison of methods for determination of glomerular filtration rate: low and high-dose Tc-99m-DTPA renography, predicted creatinine clearance method, and plasma sample method. Int Urol Nephrol 40:1059–1065
Russell CD, Bischoff PG, Kontzen FN, Rowell KL, Yester MV, Lloyd LK, Tauxe WN, Dubovsky EV (1985) Measurement of glomerular filtration rate: single injection plasma clearance method without urine collection. J Nucl Med 26:1243–1247
Itoh K (2003) Comparison of methods for determination of glomerular filtration rate: Tc-99m-DTPA renography, predicted creatinine clearance method and plasma sample method. Ann Nucl Med 17:561–565
LaFrance ND, Drew HH, Walser M (1988) Radioisotopic measurement of glomerular filtration rate in severe chronic renal failure. J Nucl Med 29:1927–1930
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Tofts PS, Cutajar M, Mendichovszky IA, Peters AM, Gordon I (2012) Precise measurement of renal filtration and vascular parameters using a two-compartment model for dynamic contrast-enhanced MRI of the kidney gives realistic normal values. Eur Radiol 22:1320–1330
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81:684–689
Celik S, Doesch A, Erbel C, Blessing E, Ammon K, Koch A, Katus HA, Dengler TJ (2008) Beneficial effect of omega-3 fatty acids on sirolimus- or everolimus-induced hypertriglyceridemia in heart transplant recipients. Transplantation 86:245–250
Filler G, Lepage N (2003) Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol 18:981–985
Filler G, Liu D, Sharma AP, Grimmer J (2012) Are fibroblast growth factor 23 concentrations in renal transplant patients different from non-transplanted chronic kidney disease patients? Pediatr Transplant 16:73–77
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106:522–526
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82:445–453
Filler G (2011) Challenges in pediatric transplantation: the impact of chronic kidney disease and cardiovascular risk factors on long-term outcomes and recommended management strategies. Pediatr Transplant 15:25–31
Popper H, Mandel E (1937) Filiations- und reabsorptionsleitung in der nierenpathologie. Ergeb Inn Med Kinderheilkd 53:685–694
Thomas L, Huber AR (2006) Renal function–estimation of glomerular filtration rate. Clin Chem Lab Med 44:1295–1302
Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S (1983) Measurement of muscle mass in humans: validity of the 24-h urinary creatinine method. Am J Clin Nutr 37:478–494
Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A (1999) Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59:587–592
Pham-Huy A, Leonard M, Lepage N, Halton J, Filler G (2003) Measuring Glomerular Filtration Rate with Cystatin C and [beta]-Trace Protein in Children with Spina Bifida. J Urol 169:2312–2315
Gerhardt T, Poge U, Stoffel-Wagner B, Palmedo H, Sauerbruch T, Woitas RP (2011) Creatinine-based glomerular filtration rate estimation in patients with liver disease: the new Chronic Kidney Disease Epidemiology Collaboration equation is not better. Eur J Gastroenterol Hepatol 23:969–973
Fitch CD, Sinton DW (1964) A study of creatine metabolism in diseases causing muscle wasting. J Clin Invest 43:444–452
Shannon JA (1935) The renal excretion of creatinine in man. J Clin Invest 14:403–410
Shemesh O, Golbetz H, Kriss JP, Myers BD (1985) Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 28:830–838
Arant BS Jr (1984) Estimating glomerular filtration rate in infants. J Pediatr 104:890–893
(1995) Proficiency Testing Survey, Creatinine. Northfield Il: College of American Pathologists:29–30
Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, Panteghini M (2008) Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 54:559–566
Delanghe JR, Cobbaert C, Harmoinen A, Jansen R, Laitinen P, Panteghini M (2011) Focusing on the clinical impact of standardization of creatinine measurements: a report by the EFCC Working Group on Creatinine Standardization. Clin Chem Lab Med 49:977–982
Cobbaert CM, Baadenhuijsen H, Weykamp CW (2009) Prime time for enzymatic creatinine methods in pediatrics. Clin Chem 55:549–558
Olsen NV, Ladefoged SD, Feldt-Rasmussen B, Fogh-Andersen N, Jordening H, Munck O (1989) The effects of cimetidine on creatinine excretion, glomerular filtration rate and tubular function in renal transplant recipients. Scand J Clin Lab Invest 49:155–159
Hellerstein S, Erwin P, Warady BA (2003) The cimetidine protocol: a convenient, accurate, and inexpensive way to measure glomerular filtration rate. Pediatr Nephrol 18:71–72
Filler G (2006) How to measure renal function in children - What is the role of cystatin C? Curr Pediatr Rev 2:225–231
Jung K (1987) Low-molecular-mass proteins in serum and their relationship to the glomerular filtration rate. Nephron 47:160
Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, Keely E, Matzinger M, Akbari A, Althaus H, Jung K (2002) Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 48:729–736
Revillard JP, Vincent C, Clot J, Sany J (1982) beta 2-Microglobulin and beta 2-microglobulin-binding proteins in inflammatory diseases. Eur J Rheumatol Inflamm 5:398–405
Trnka P, Hiatt MJ, Tarantal AF, Matsell DG (2012) Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr Res 72:446–454
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Grubb AO (2000) Cystatin C–properties and use as diagnostic marker. Adv Clin Chem 35:63–99
Jungers P, Skhiri H, Zingraff J, Muller S, Fumeron C, Giatras I, Touam M, Nguyen AT, Man NK, Grunfeld JP (1997) Benefits of early nephrological management in chronic renal failure. Presse Med 26:1325–1329
Foster J, Reisman W, Lepage N, Filler G (2006) Influence of commonly used drugs on the accuracy of cystatin C-derived glomerular filtration rate. Pediatr Nephrol 21:235–238
Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS (2009) Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75:652–660
Al-Malki N, Heidenheim PA, Filler G, Yasin A, Lindsay RM (2009) Cystatin C levels in functionally anephric patients undergoing dialysis: the effect of different methods and intensities. Clin J Am Soc Nephrol 4:1606–1610
Huang SH, Filler G, Yasin A, Lindsay RM (2011) Cystatin C reduction ratio depends on normalized blood liters processed and fluid removal during hemodialysis. Clin J Am Soc Nephrol 6:319–325
Galteau MM, Guyon M, Gueguen R, Siest G (2001) Determination of serum cystatin C: biological variation and reference values. Clin Chem Lab Med 39:850–857
Hannemann A, Friedrich N, Dittmann K, Spielhagen C, Wallaschofski H, Völzke H, Rettig R, Endlich K, Lendeckel U, Stracke S, Nauck M (2011) Age- and sex-specific reference limits for creatinine, cystatin C and the estimated glomerular filtration rate. Clin Chem Lab Med 50:919–926
Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H (1985) Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 218:499–503
Tenstad O, Roald AB, Grubb A, Aukland K (1996) Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 56:409–414
Filler G, Priem F, Vollmer I, Gellermann J, Jung K (1999) Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol 13:501–505
Sharma AP, Kathiravelu A, Nadarajah R, Yasin A, Filler G (2009) Body mass does not have a clinically relevant effect on cystatin C eGFR in children. Nephrol Dial Transplant 24:470–474
Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindstrom V, Grubb A (1994) Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 40:1921–1926
Roos JF, Doust J, Tett SE, Kirkpatrick CM (2007) Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children–a meta-analysis. Clin Biochem 40:383–391
Bariciak E, Abeeryasin HJ, Walker M, Lepage N, Filler G (2011) Preliminary reference intervals for cystatin C and beta-trace protein in preterm and term neonates. Clin Biochem 44:1156–1159
Harmoinen A, Ylinen E, Ala-Houhala M, Janas M, Kaila M, Kouri T (2000) Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol 15:105–108
Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I (2010) First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 48:1619–1621
Harrington MG, Aebersold R, Martin BM, Merril CR, Hood L (1993) Identification of a brain-specific human cerebrospinal fluid glycoprotein, beta-trace protein. Appl Theor Electrophor 3:229–234
Akbari A, Lepage N, Keely E, Clark HD, Jaffey J, MacKinnon M, Filler G (2005) Cystatin-C and beta trace protein as markers of renal function in pregnancy. BJOG 112:575–578
Filler G, Grimmer J, Huang SH, Bariciak E (2012) Cystatin C for the assessment of GFR in neonates with congenital renal anomalies. Nephrol Dial Transplant 27:3382–3384
White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2009) Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76:784–791
Poge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP (2008) Beta-trace protein-based equations for calculation of GFR in renal transplant recipients. Am J Transplant 8:608–615
White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2007) A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem 53:1965–1968
Benlamri A, Nadarajah R, Yasin A, Lepage N, Sharma AP, Filler G (2010) Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol 25:485–490
Barbour GL, Crumb CK, Boyd CM, Reeves RD, Rastogi SP, Patterson RM (1976) Comparison of inulin, iothalamate, and 99mTc-DTPA for measurement of glomerular filtration rate. J Nucl Med 17:317–320
Krutzen E, Back SE, Nilsson-Ehle P (1990) Determination of glomerular filtration rate using iohexol clearance and capillary sampling. Scand J Clin Lab Invest 50:279–283
Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS (2006) Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr 136:2391–2398
Favre HR, Wing AJ (1968) Simultaneous 51Cr edetic acid, inulin, and endogenous creatinine clearances in 20 patients with renal disease. Br Med J 1:84–86
Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G (1995) Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 6:257–263
Medeiros FS, Sapienza MT, Prado ES, Agena F, Shimizu MH, Lemos FB, Buchpiguel CA, Ianhez LE, David-Neto E (2009) Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transpl Int 22:323–331
Hernandez Ocampo J, Torres Rosales A, Rodriguez Castellanos F (2010) Comparison of four methods for measuring glomerular filtration rate by inulin clearance in healthy individuals and patients with renal failure. Nefrologia 30:324–330
Berg UB, Back R, Celsi G, Halling SE, Homberg I, Krmar RT, Monemi KA, Oborn H, Herthelius M (2011) Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. Am J Kidney Dis 57:55–61
Source of funding
None
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filler, G., Yasin, A. & Medeiros, M. Methods of assessing renal function. Pediatr Nephrol 29, 183–192 (2014). https://doi.org/10.1007/s00467-013-2426-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2426-7