Abstract
Background
Limited data exist on the performance of cystatin C-based glomerular filtration rate (GFR) equations in pediatric transplant recipients and other high-risk patients. The aim of our study was therefore to evaluate the performance of current cystatin C-based equations in this population.
Methods
This was a retrospective, cross-sectional study of 141 consecutive patients (58 % post-transplant) who received a nuclear medicine GFR (NucGFR) examination using 99mTc- diethylenetriaminepentaacetic acid at our institution. Subjects included children receiving liver, kidney or hematopoietic stem cell transplants and patients with oncologic or urologic disease. GFR estimates using published GFR estimating equations, including those based on cystatin C (Filler, Zappitelli, Larsson, Hoek, Rule and Le Bricon equations, respectively) and on both cystatin C and creatinine (Zappitelli, Bouvet and Schwartz equations, respectively), were evaluated and compared to the NucGFR measurement using Bland–Altman analysis.
Results
The mean NucGFR was 95 (interquartile range 76–111) ml/min/1.73 m2. Of the cystatin C-based equations, the Rule, Hoek, Zappitelli and Schwartz (2009 CKiD equation) formulas provided the closest agreement to the NucGFR estimate. All other formulas overestimated the GFR in our cohort.
Conclusion
Cystatin C-based GFR formulas can provide an accurate estimation of NucGFR in a pediatric population with a high proportion of transplant recipients and oncology patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accurate assessment of kidney function is essential in the care of children at high risk for renal injury and chronic kidney disease (CKD), such as those receiving a transplant. Careful attention to the glomerular filtration rate (GFR) ensures proper medication dosing and allows kidney function to be followed over time. While serum creatinine is currently used to monitor renal function, cystatin C is emerging as a potentially superior alternative [1]. Cystatin C, produced by all nucleated cells, is a 13-kDa protein inhibitor of cysteine proteases. It is freely filtered by the glomerulus without tubular secretion and is generated at a constant rate, making it an ideal marker to estimate GFR [2].
Several studies have compared cystatin C to serum creatinine for estimating GFR in children. Unlike serum creatinine, which increases with growth and development, cystatin C has been shown to be independent of age, height and body composition [3, 4]. Therefore, serum cystatin C levels parallel renal maturation, attaining peak values in the neonatal period and then decreasing during the first few months of life [5]. Despite these advantages, cystatin C has not been shown to consistently perform better than creatinine in the estimation of GFR. While some studies have suggested that cystatin C is a more accurate and sensitive marker of GFR decline [6–8], others have failed to demonstrate a significant difference [9, 10].
Many cystatin C-based equations have been published, including some which include serum creatinine and other covariates to improve GFR estimation. Most recently, Schwartz et al. reported that combining cystatin C with serum creatinine, blood urea nitrogen, height and gender provided the best estimate of GFR in 349 children in the Chronic Kidney Disease in Children (CKiD) cohort [11]. However, limited data exist on the use of cystatin C to estimate GFR in pediatric transplant recipients and other children at high risk of renal dysfunction. The primary aim of this study was therefore to evaluate cystatin C-based formulas in children whose primary disease increased the risk of renal injury.
Patients and methods
This study was a retrospective, cross-sectional analysis of 142 consecutive children and young adults who received a nuclear medicine GFR (N5ucGFR) examination using 99mTc- diethylenetriaminepentaacetic acid (99mTc-DTPA) at Cincinnati Children’s Hospital Medical Center from June to August 2010. All patients undergoing NucGFR testing for clinical indications during this time period were included. Patients were excluded if they had previously received NucGFR testing during the study period to avoid repeated measurements. Patients with more than one NucGFR examination during the study period only had their first test included in the analysis. Cystatin C values were obtained as a quality improvement effort in the Division of Nephrology clinical laboratory to evaluate the accuracy of the GFR estimation.
Examination of a residual plot of cystatin C and NucGFR revealed one significant outlier from a subject with a very high cystatin C concentration (3.34 mg/L) on the day of NucGFR testing that was inconsistent with subsequent values (0.75–1.61 mg/L). Furthermore, serum creatinine levels in this 17-year-old subject were normal, ranging from 0.6 to 0.9 mg/dL, and the NucGFR was only slightly decreased (85 ml/min/1.73 m2). This patient was therefore excluded from the analysis, leaving a final cohort of 141 subjects.
NucGFR testing was performed in the outpatient or inpatient setting, and the NucGFR was calculated according to the methods of Balachandran et al [12]. A single dose of 99mTc-DTPA was administered with direct visualization and intermittent aspiration of blood while injecting to ensure delivery of the isotope intravenously. If extravasation was suspected, the injection site was evaluated with gamma camera imaging. The NucGFR was then computed using plasma 99mTc-DTPA disappearance curves obtained from four time points at approximately 120, 150, 180 and 210 min. Each plasma disappearance curve had a correlation coefficient of >0.98, indicating intercompartmental equilibration had occurred. A quadratic correction factor was then used to adjust the slope–intercept GFR to a two-compartment model according to the methods of Brochner–Mortensen [13], as recommended for pediatric patients by Blaufox et al. [14]. 99mTc-DTPA has previously demonstrated good agreement with inulin clearance [15]. After the GFR was measured, samples from each subject were pooled and stored at −80 °C until radioisotope decay occurred prior to the measurement of cystatin C on the pooled serum samples from each subject. Cystatin C was measured using particle-enhanced immunonephelometry (Siemens Healthcare Diagnostics, Deerfield, IL) in the Cincinnati Children’s Hospital Division of Nephrology clinical laboratory.
GFR estimations from these measured serum cystatin C values were calculated according to equations published by Filler and Lepage, Zappitelli et al., Larsson et al., Hoek et al., Rule et al. and Le Bricon et al. [6, 8, 16–19]. In addition, GFR estimation from cystatin C and creatinine was performed using the equations published by Schwartz et al. (New CKiD equation), Zappitelli et al. and Bouvet et al. (Table 1) [8, 11, 20]. The Bouvet equation was corrected for body surface area.
Demographic and clinical covariates were obtained by chart review and included disease diagnosis, age, race, gender and serum creatinine. Serum creatinine values were included only if obtained on the same day of the NucGFR measurement, a criterion met by 87 subjects (62 %) from our cohort. Serum creatinine was measured locally by an isotope dilution mass spectrometry (IDMS)-traceable enzymatic assay (Fusion Chemistry Analyzer; Ortho Vitros Diagnostics, Raritan, NJ). The study was approved by the Institutional Review Board who waived the requirement for informed consent/assent as this study was a retrospective analysis of previously obtained serum samples.
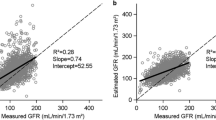
All analyses were conducted using SAS statistical software (ver. 9.2; SAS Institute, Cary, NC). Descriptive statistics for continuous variables were reported as medians and interquartile ranges (IQR). For the comparisons of cystatin C-based equations to NucGFR measurements, we calculated the mean bias (mean difference between estimated GFR and NucGFR) and 95 % limits of agreement (LOA) according to the methods of Bland–Altman [21]. Sensitivity and specificity for detecting a NucGFR of <90 ml/min/1.73 m2 (to define CKD) were also evaluated. Additionally, the proportion of the estimated (e) GFR for each formula within 10 and 30 %, respectively, of the NucGFR (accuracy) were reported, as well as the proportion of variability (R 2).
Results
Patient characteristics
A total of 141 patients were included in the analysis and their demographic and clinical characteristics are summarized in Table 2. Indications for obtaining NucGFR measurements included liver transplantation, malignancy, kidney transplantation, urologic abnormalities, hematopoietic stem cell transplantation (HSCT) and other miscellaneous conditions.
All liver transplant recipients (n = 62) were evaluated in the outpatient setting, and for 58 of these patients the post-transplant period was longer than 1 year. Only one subject showed evidence of significant liver dysfunction (increased prothrombin time and bilirubin) and was subsequently admitted 1 week after the GFR measurement for abdominal pain and ascites. Immunosuppressive medication use in the liver transplant recipients included tacrolimus (n = 51), mycophenolate mofetil (n = 12), cyclosporine (n = 6), steroids (n = 6), sirolimus (n = 4) and azathioprine (n = 1).
Of the 37 malignancy patients, 31 were evaluated as outpatients and six were admitted when the GFR measurement was performed. All of these six patients were admitted either after their initial diagnosis and/or for chemotherapy, except one who was admitted with fever. Seventeen patients were actively receiving or had completed chemotherapy within 2 months of the time of GFR measurement. Twenty-seven patients were diagnosed with a malignancy within 1 year of the GFR measurement, while ten were over 1 year removed from the initial diagnosis.
All kidney transplant recipients were evaluated as outpatients, and all but one were over 1 year post-transplant. Immunosuppressive medication use included sirolimus (n = 8), tacrolimus (n = 5), cyclosporine (n = 2), mycophenolate mofetil (n = 10), steroids (n = 5) and azathioprine (n = 3). Five of six HSCT recipients received autologous stem cell transplants for neuroblastoma. Three patients were evaluated as outpatients and three as inpatients, two of whom had received chemotherapy for relapsed disease.
The remainder of our cohort was comprised of ten patients with urologic disease (six with cloacal anomalies or anorectal malformations) and 12 patients with other diseases (nine with underlying hematologic disease and undergoing evaluation prior to HSCT). Only three of these remaining patients were hospitalized at the time of GFR measurement.
Comparison of cystatin C-based equations in different disease categories
Cystatin C-based equations were compared to NucGFR measurements in patients with different underlying diseases (Table 3). Of previously published equations, the Rule formula performed best, accurately estimating GFR in all patients except liver transplant recipients, in whom there was about an 8 ml/min/1.73 m2 underestimation of GFR. The Zappitelli, Larsson, Hoek, and Le Bricon equations also performed reasonably well, although the GFR was significantly overestimated in urologic and oncology patients. The Filler equation consistently overestimated renal function in all patient categories. Among those equations including cystatin C and creatinine (Table 4), the New CKiD formula significantly outperformed the Bouvet and Zappitelli equations, which overestimated GFR in most patient categories.
Comparison of cystatin C-based equations and creatinine-based equations in the entire cohort
The performance of each formula based on mean bias, 95 % LOA, accuracy, sensitivity and specificity for estimating NucGFR and predicting renal insufficiency (NucGFR <90 ml/min/1.73 m2) in the entire cohort is summarized in Table 5. The only established formulas that did not have a significant mean bias were the Rule and Hoek formulas. The Zappitelli (including only cystatin C) and New CKiD equations also performed well, with mean biases of only 5.9 and 4.2 ml/min/1.73 m2, respectively. All other formulas more significantly overestimated GFR, especially the Filler equation and creatinine-based GFR formulas derived by Bouvet and Zappitelli (mean biases ranging from 15.8 to 27.5 ml/min/1.73 m2).
Discussion
We evaluated the accuracy and performance of cystatin C equations in a pediatric cohort at high risk of renal injury. The majority of our subjects did not have significant underlying renal disease at the time of assessment, as evidenced by the cohort median GFR of >90 ml/min/1.73 m2. We found that the Rule, Hoek, Zappitelli and New CKiD equations provided reasonably accurate assessments of GFR in our patients. In contrast, the Filler equation and the other creatinine-based equations (Bouvet and Zappitelli combined formula) significantly overestimated the GFR in our subjects.
The limitations of using only creatinine to estimate GFR are well documented and include variability due to gender, age, tubular secretion and muscle mass [22]. To improve the estimation of kidney function, other investigators have developed cystatin C-based equations in children and adults with known kidney disease [6, 8, 11, 16–20]. Many of these adult studies have rigorously assessed GFR estimating equations in liver and kidney transplant recipients, as well as patients with malignancy (Table 6). However, the formulas published to date have not been analyzed or validated in a large group of pediatric patients with similar high-risk conditions.
In the pediatric liver transplant population, Samyn et al. measured renal function in 62 children but did not report cystatin C-based GFR estimation [23]. Berding et al. reported on 48 pediatric liver transplant recipients and found reasonable performance of cystatin C, but assessed only the Filler equation [24]. Several additional pediatric studies have included a variable proportion of transplant recipients, including those of Grubb et al. [7] (unclear how many transplant patients), Filler et al. [6] (only 5.4 % received a kidney transplant), Bouvet et al. [20] (48 % of cohort were kidney transplant recipients) and Zappitelli et al. [8] (27 % kidney transplant, 7 % ‘other’ transplants). Finally, we identified one pediatric study comparing the Filler cystatin C equation to the new Schwartz formula in 68 children with malignancy [25].
Recently, Bacchetta et al. published a well-designed analysis validating cystatin C-based equations in 252 children at high risk of renal injury [26]. However, in contrast to our cohort, most of these children had primary renal disease, and only 26 subjects (10 %) received a non-renal organ transplant. The authors concluded that the Le Bricon, Larsson, Rule and both Zappitelli formulas provided the most accurate agreement with inulin clearance, whereas the Filler equation overestimated GFR and the Bouvet formula underestimated GFR. Similarly, in our study, the Rule and Zappitelli equations performed reasonably well, although the Larsson and Le Bricon formulas significantly overestimated GFR (mean bias of 10.2 and 8.0 ml/min/1.73 m2, respectively).
Equations including cystatin C and creatinine have demonstrated improved accuracy and precision compared to those including only cystatin C [8, 20]. The Bouvet and combined Zappitelli formulas, however, markedly overestimated GFR in our cohort. Systematic biases evident in these formulas may be secondary to variation in creatinine assay methods or endogenous creatinine production between study populations. For example, creatinine-based GFR estimations have overestimated renal function in pediatric liver transplant recipients and children with malignancy [25, 27], possibly due to low serum creatinine from muscle wasting. Additionally, Bouvet et al. used a non-compensated Jaffe method for creatinine measurement, whereas all others (including the present study) used an enzymatic assay. Smaller creatinine values yielded by enzymatic techniques likely contributed to the overestimation of GFR by the Bouvet formula. These limitations of creatinine notwithstanding, the New CKiD formula performed quite well, demonstrating the best 30 and 10 % accuracy among all formulas. Our study is the first, therefore, to validate its use in a cohort composed primarily of children with malignancy or solid organ transplantation.
The strengths of our analysis include the use of an established nuclear medicine technique as the gold standard and measuring cystatin C on the same sample as the NucGFR, thereby reducing intra-patient variability which is often a concern with these measurements [28]. Furthermore, we used an accurate nephelometric assay for the measurement of cystatin C, which has been shown to be more reliable than other techniques, such as those based on turbidimetry [1]. In addition, we used an IDMS-traceable, enzymatic assay to measure serum creatinine, similar to those used by Zappitelli et al. and the 2009 CKiD study. Our patient population had a wide range of GFR (17–147 ml/min/1.73 m2), whereas prior studies have been limited by including patients with narrower ranges of renal function (Table 6). Finally, we included all patients having a formal GFR measurement at our institution, reducing selection bias. As these tests were ordered for clinical indications, our cohort represented a heterogeneous group of patients at high risk for kidney dysfunction.
Several limitations of our study deserve consideration. First, our cohort was composed of many liver transplant recipients and patients with a history of malignancy. In these subjects, variations in endogenous cystatin C production may have affected the performance of cystatin C-based estimations. For example, elevated cystatin C levels have been demonstrated in patients with active liver disease (cirrhosis, hepatitis) and leukemia [29–31], potentially underestimating GFR when cystatin-C based equations are used. However, cystatin-C based equations have not demonstrated a consistent bias in clinically stable liver transplant recipients and patients with malignancy, although variability in performance has been demonstrated among individual formulas [24, 25, 32–34]. Second, we used 99mTc-DTPA plasma disappearance curves as our ‘gold standard’ of GFR measurement, thereby differing from previous studies that measured GFR by iohexol or iothalamate clearance. However, GFR estimation using 99mTc-DTPA has demonstrated close agreement with iohexol, iothalamate and inulin [15, 35, 36]. Lastly, our last plasma sample was drawn at 210 min post-injection, whereas other studies have used longer intervals of up to 300 min [37]. Shorter sampling intervals can cause an overestimation of GFR in patients with severe renal dysfunction. However, as only one patient in our cohort had a GFR of <30 ml/min/1.73 m2, this effect was likely to be insignificant.
In summary, our results demonstrate that the Rule, Hoek and Zappitelli cystatin C-based equations and the New CKiD formula provided a good estimation of the GFR in children at high risk of developing renal insufficiency, such as after transplant. While these GFR estimating equations are unlikely to replace gold standard methods in all clinical situations, they offer reasonable accuracy, improved cost and patient convenience compared to formal isotope techniques.
References
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A (2005) Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem 38:1–8
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J (1998) Cystatin C–a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101:875–881
Filler G, Witt I, Priem F, Ehrich JH, Jung K (1997) Are cystatin C and beta 2-microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem 43:1077–1078
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J (1998) Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12:125–129
Filler G, Lepage N (2003) Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol 18:981–985
Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A (2005) Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan–Barratt prediction equations for children. Clin Chem 51:1420–1431
Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L (2006) Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis 48:221–230
Filler G, Priem F, Vollmer I, Gellermann J, Jung K (1999) Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol 13:501–505
Martini S, Prevot A, Mosig D, Werner D, van Melle G, Guignard JP (2003) Glomerular filtration rate: measure creatinine and height rather than cystatin C! Acta Paediatr 92:1052–1057
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Balachandran S, Toguri AG, Petrusick TW, Abbott LC (1981) Comparative evaluation of quantitative glomerular filtration rate measured by isotopic and nonisotopic methods. Clin Nucl Med 6:150–153
Brochner-Mortensen J, Haahr J, Christoffersen J (1974) A simple method for accurate assessment of the glomerular filtration rate in children. Scand J Clin Lab Invest 33:140–143
Blaufox MD, Aurell M, Bubeck B, Fommei E, Piepsz A, Russell C, Taylor A, Thomsen HS, Volterrani D (1996) Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med 37:1883–1890
Barbour GL, Crumb CK, Boyd CM, Reeves RD, Rastogi SP, Patterson RM (1976) Comparison of inulin, iothalamate, and 99mTc-DTPA for measurement of glomerular filtration rate. J Nucl Med 17:317–320
Hoek FJ, Kemperman FA, Krediet RT (2003) A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 18:2024–2031
Larsson A, Malm J, Grubb A, Hansson LO (2004) Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64:25–30
Le Bricon T, Thervet E, Froissart M, Benlakehal M, Bousquet B, Legendre C, Erlich D (2000) Plasma cystatin C is superior to 24-h creatinine clearance and plasma creatinine for estimation of glomerular filtration rate 3 months after kidney transplantation. Clin Chem 46:1206–1207
Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS (2006) Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69:399–405
Bouvet Y, Bouissou F, Coulais Y, Seronie-Vivien S, Tafani M, Decramer S, Chatelut E (2006) GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol 21:1299–1306
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38:1933–1953
Samyn M, Cheeseman P, Bevis L, Taylor R, Samaroo B, Buxton-Thomas M, Heaton N, Rela M, Mieli-Vergani G, Dhawan A (2005) Cystatin C, an easy and reliable marker for assessment of renal dysfunction in children with liver disease and after liver transplantation. Liver Transpl 11:344–349
Berding G, Geisler S, Melter M, Marquardt P, Luhr A, Scheller F, Knoop BO, Pfister ED, Pape L, Bischoff L, Knapp WH, Ehrich JH (2010) Estimation of glomerular filtration rate in liver-transplanted children: comparison of simplified procedures using 51Cr-EDTA and endogenous markers with Sapirstein’s method as a reference standard. Pediatr Transplant 14:786–795
Blufpand HN, Tromp J, Abbink FC, Stoffel-Wagner B, Bouman AA, Schouten-van Meeteren AY, van Wijk JA, Kaspers GJ, Bokenkamp A (2011) Cystatin C more accurately detects mildly impaired renal function than creatinine in children receiving treatment for malignancy. Pediatr Blood Cancer 57:262–267
Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L (2011) Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol 6:552–560
Mention K, Lahoche-Manucci A, Bonnevalle M, Pruvot FR, Declerck N, Foulard M, Gottrand F (2005) Renal function outcome in pediatric liver transplant recipients. Pediatr Transplant 9:201–207
Borrows R, Cockwell P (2007) Measuring renal function in solid organ transplant recipients. Transplantation 83:529–531
Takeuchi M, Fukuda Y, Nakano I, Katano Y, Hayakawa T (2001) Elevation of serum cystatin C concentrations in patients with chronic liver disease. Eur J Gastroenterol Hepatol 13:951–955
Demirtas S, Akan O, Can M, Elmali E, Akan H (2006) Cystatin C can be affected by nonrenal factors: a preliminary study on leukemia. Clin Biochem 39:115–118
Chu SC, Wang CP, Chang YH, Hsieh YS, Yang SF, Su JM, Yang CC, Chiou HL (2004) Increased cystatin C serum concentrations in patients with hepatic diseases of various severities. Clin Chim Acta 341:133–138
Boudville N, Salama M, Jeffrey GP, Ferrari P (2009) The inaccuracy of cystatin C and creatinine-based equations in predicting GFR in orthotopic liver transplant recipients. Nephrol Dial Transplant 24:2926–2930
Chew JS, Saleem M, Florkowski CM, George PM (2009) Estimating renal function in oncology patients using cystatin C-based equations. Clin Oncol (R Coll Radiol) 21:425–426
Gerhardt T, Poge U, Stoffel-Wagner B, Ahrendt M, Wolff M, Spengler U, Palmedo H, Sauerbruch T, Woitas RP (2006) Estimation of glomerular filtration rates after orthotopic liver transplantation: evaluation of cystatin C-based equations. Liver Transpl 12:1667–1672
Stake G, Monn E, Rootwelt K, Monclair T (1991) The clearance of iohexol as a measure of the glomerular filtration rate in children with chronic renal failure. Scand J Clin Lab Invest 51:729–734
Houlihan C, Jenkins M, Osicka T, Scott A, Parkin D, Jerums G (1999) A comparison of the plasma disappearance of iohexol and 99mTc-DTPA for the measurement of glomerular filtration rate (GFR) in diabetes. Aust N Z J Med 29:693–700
Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A (2006) Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69:2070–2077
Ling Q, Xu X, Li J, Wu J, Chen J, Xie H, Zheng S (2008) A new serum cystatin C-based equation for assessing glomerular filtration rate in liver transplantation. Clin Chem Lab Med 46:405–410
Risch L, Herklotz R, Blumberg A, Huber AR (2001) Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem 47:2055–2059
Qutb A, Syed G, Tamim HM, Al Jondeby M, Jaradat M, Tamimi W, Al Ghamdi G, Al Qurashi S, Flaiw A, Hejaili F, Al Sayyari AA (2009) Cystatin C-based formula is superior to MDRD, Cockcroft–Gault and Nankivell formulae in estimating the glomerular filtration rate in renal allografts. Exp Clin Transpl 7:197–202
White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2007) Chronic kidney disease stage in renal transplantation classification using cystatin C and creatinine-based equations. Nephrol Dial Transplant 22:3013–3020
White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2005) Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol 16:3763–3770
Maillard N, Mariat C, Bonneau C, Mehdi M, Thibaudin L, Laporte S, Alamartine E, Chamson A, Berthoux F (2008) Cystatin C-based equations in renal transplantation: moving toward a better glomerular filtration rate prediction? Transplantation 85:1855–1858
Zahran A, Qureshi M, Shoker A (2007) Comparison between creatinine and cystatin C-based GFR equations in renal transplantation. Nephrol Dial Transplant 22:2659–2668
Poge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP (2006) Cystatin C-based calculation of glomerular filtration rate in kidney transplant recipients. Kidney Int 70:204–210
Poge U, Gerhardt T, Woitas RP (2008) Equations to estimate GFR using serum cystatin C in kidney transplant recipients. Am J Kidney Dis 52:383–384
Huang SH, Macnab JJ, Sontrop JM, Filler G, Gallo K, Lindsay RM, Clark WF (2011) Performance of the creatinine-based and the cystatin C-based glomerular filtration rate (GFR) estimating equations in a heterogenous sample of patients referred for nuclear GFR testing. Transl Res 157:357–367
Conflict of Interest statement
There are no conflicts of interest to disclose.
Financial statement
No financial support was received.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nehus, E.J., Laskin, B.L., Kathman, T.I. et al. Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol 28, 453–461 (2013). https://doi.org/10.1007/s00467-012-2341-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2341-3