Abstract
Background
There is still poor evidence about the safety and feasibility of laparoscopic liver resection (LLR) for huge (> 10 cm) hepatocellular carcinomas (HCC). The aim of this study was to assess the short- and long-term outcomes of LLR versus open liver resection (OLR) for patients with huge HCC from real-life data from consecutive patients.
Methods
Data regarding all consecutive patients undergoing liver resection for huge HCC were retrospectively collected from a Korean referral HPB center. Primary outcomes were the postoperative results, while secondary outcomes were the oncologic survivals.
Results
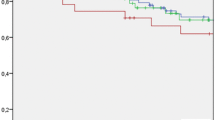
Sixty-three patients were included in the study: 46 undergoing OLR and 17 LLR. Regarding postoperative outcomes, there were no statistically significant differences in estimated blood loss, operation time, transfusions, postoperative bile leak, ascites, severe complications, and R1 resection rates. After a median follow-up of 48.4 (95% CI 8.9–86.8) months, there were no statistically significant differences in 3 years OS (59.3 ± 8.7 months vs. 85.2 ± 9.8 months) and 5 years OS (31.1 ± 9 months vs. 73.1 ± 14.1 months), after OLR and LLR, respectively (p = 0.10). Similarly, there was not a statistically significant difference in both 3 years DFS (23.5% ± 8.1 months vs. 51.6 ± months) and 5 years DFS (15.7 ± 7.1 months vs. 38.7 ± 15.3 months), respectively (p = 0.13), despite a potential clinically significant difference.
Conclusion
LLR for huge HCC may be safe and effective in selected cases. Further studies with larger sample size and more appropriate design are needed to confirm these results.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) represents the most common primary liver malignancy, accounting for the seventh most common cancer worldwide and the third leading cause of cancer-related death [1]. Despite the tremendous medical advances, HCC prognosis is still poor, not exceeding the 5 years overall survival rates (OS) of approximately 20% [2]. Nonetheless, early-staged HCC can benefit from surgical therapy, leading up to 50–70% of 5 years OS [3]. Thus, surgery represents the cornerstone treatment for HCC, including both liver transplantation (LT) and liver resection (LR) [4]. LT aims to treat both HCC and underlying chronic liver disease, but must face the problem of organ shortage, with a consequent risk of dropout from waiting list and tumor progression [5]. Thus, LT is mainly reserved for patients who are not candidates for LR due to impaired liver function or for patients with negative prognostic factors on specimen examination after a previous resection [6]. Accordingly, LR still represents the most performed treatment for early stages. Furthermore, the Milan criteria by Mazzaferro et al. restrict LT in adults to patients with tumor smaller than 5 cm, not more than three and each one not exceeding 3 cm, without angioinvasion, without extrahepatic involvement [7].
Previous data have shown that often HCC patients are often diagnosed at symptomatic and advanced disease stage, with large tumors (> 5 cm) or huge HCC (defined as lesions bigger than 10 cm) [8]. Such huge HCC cannot benefit from LT [9]. Similarly, they are not suitable for thermal ablation in the light of the impossibility to achieve complete tumor necrosis of large lesions [10]. However, according to current guidelines, patients with a solitary HCC and preserved liver function may benefit from liver resection, when preserving a sufficient FRL [11]. Indeed, an extended hepatectomy may be required for such cases, carrying out a non-negligible risk of postoperative morbidity and mortality, mainly related to post-hepatectomy liver failure (PHLF) [12, 13]. Nonetheless, LR has shown to provide significant survival benefits when compared to trans-arterial chemoembolization for huge HCC [14]. Indeed, the effectiveness of TACE in patients with huge HCC may be impaired by the presence of extrahepatic collaterals that makes difficult to achieve complete tumor embolization [15]. Even in cases with type I and II portal vein tumor thrombus, LR has been reported to be superior to TACE [16].
International guidelines have officially approved the use of laparoscopy for HCC treatment, in the light of less intraoperative blood loss, fewer complications, faster postoperative recovery, and equivalent long-term outcomes than open liver resection (OLR), as well as a decreased risk for postoperative decompensation in fragile cirrhotic patients [17,18,19,20]. However, several limitations to the universal adoption of LLR for HCC still exist. In particular, the outcomes of laparoscopic liver resection (LLR) for huge HCC are still controversial.
In this scenario, we retrospectively analyzed the clinical and oncologic outcomes after LLR for patients with huge HCC.
Materials and methods
Patients and data
Data from consecutive patients undergoing LR for huge HCC from January 2003 to June 2022 at a tertiary referral HPB center (Seoul National University Bundang Hospital, South Korea) were retrospectively collected from a prospectively established database.
The inclusion criteria were as follows: male or female patients aged > 18 years, at least one nodule with histopathological confirmation of HCC larger than 99 mm, no extrahepatic metastasis, no tumor thrombus in portal vein or other major vessels, adequate future remnant liver volume according to current literature [13]. The exclusion criteria were as follows: liver resection extended to other abdominal organs other than gallbladder, histological finding of combined HCC–CCA.
According to the different surgical approach, patients were divided into two cohorts: the LLR group and the OLR group. Primary endpoints were the perioperative outcomes, while secondary endpoints were the long-term oncological outcomes.
This study was conducted according to the Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines of the EQUATOR network [21]. Informed consent was obtained prior to every surgical procedure. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board (B-2407-912-101).
Preoperative management
Patients ‘management was decided by the institutional multidisciplinary team meeting including hepatologists, oncologists, and radiologists. Preoperative evaluations were similar in both groups and included routine blood tests, liver function, coagulation examinations, serum tumor markers, ICG clearance tests, and triphasic enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). The definition of resectable HCC was based on multidisciplinary team decision, according to comprehensive evaluation of liver function test, ICG clearance test, remnant liver volume, and liver compensation status.
After surgeons fully informed patients about the pros and cons of the two approaches, the final decision was made by surgeons’ and patients’ preferences.
Surgical procedures
The laparoscopic procedures have been described in detail elsewhere [22,23,24]. The proximity of major vessels with the subsequent risk of ischemia of the remnant liver or R1 resection was an important factor to decide if a major liver resection was needed. Briefly, patients under intravenous total anesthesia were positioned in supine position, with the primary operator standing between patients’ legs, and the assistant and scopist standing on his sides. Carbon dioxide pneumoperitoneum was established with a pressure of 12–14 mmHg. A 12 mm port was used for the laparoscope, whereas two 12 mm ports and two 5 mm ports were inserted under vision and applied for the operation. Laparoscopic ultrasonography was routinely performed to confirm the positions of tumors, prevent the omissions of additional lesions, and guide the transection lines. An extracorporeal Pringle maneuver was prepared to eventually help controlling blood loss. The liver parenchyma was transected by a combination of a harmonic scalpel (Ethicon, Endo-Surgery, USA) and a laparoscopic cavitron ultrasonic surgical aspirator (CUSA, Integra-France) or Ligasure (Medtronic, USA). Intraparenchymal vascular and biliary vessels were secured by clips or sutures. The specimen was placed into a retrieval bag and extracted through a suprapubic incision. After hemostasis, a drainage tube was routinely placed near the surgical bed.
For the open procedure, a reverse L-incision was conducted with patients who underwent the same anesthesia in the supine position. The operating procedure was similar to LLR, and CUSA or clamp crushing was used as the main method for liver parenchyma transection.
Postoperative management and follow‑up
Postoperative follow-up data were analyzed. Postoperative complications were classified according to the Clavien–Dindo classification [25]. PHLF, post-hepatectomy bile leakage (PHBL), and post-hepatectomy hemorrhage (PHH) were diagnosed and classified according to the International Study Group of Liver Surgery (ISGLS) guidelines [26,27,28]. Ascites was defined according to the International Ascites Club definition [29]. Surgical procedure was classified according to Brisbane classification [30].
All patients were examined in outpatients’ clinics within one month after discharge, undergoing clinical, biological, and imaging evaluations every 3 months after discharge for the first 2 years, according to the oncological protocols. Following controls were scheduled every 12 months if no relapse was found. In case of tumor recurrence, the case was re-examined by a multidisciplinary team (MDT) with the aim of carrying out curative treatment as much as possible. First therapeutical strategy for localized recurrent HCC was repeat hepatectomy, according to previous literature that have showed the same OS and DFS as primary liver resection [31]. In case liver resection was not indicated because of liver, as well as because of tumor or patient status, other locoregional therapies represented the second choice.
Statistical analysis
Continuous data were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), depending on whether they had a normal distribution or not. Group comparisons were performed using Student’s T test or Wilcoxon’s rank test, depending on the distribution of the variable. Categorical data were expressed as frequencies and associated percentages. Comparisons between groups were performed using Pearson’s chi-squared test or Fisher’s exact test, depending on the expected value of the variable of interest. Overall and recurrence-free survival analyses were performed using the Kaplan–Meier method to calculate the median and 95% confidence interval (CI), and comparisons were performed using the log-rank method. The median follow-up was analyzed using the reverse Kaplan–Meier method.
All statistical analyses were performed using SPSS software version 28.1 (IBM SPSS Inc. Chicago, IL).
Results
Patients and tumor characteristics
During the study period, the institutional database included 3799 liver resections performed at Seoul National University Bundang Hospital. After removing lesions smaller than 10 cm and not matching the inclusion criteria, 63 patients were included in the study: 46 on the open liver resection (OLR) group and 17 in the laparoscopic liver resection (LLR) group (Fig. 1).
Mean age was 58.1 (± 13.3), with 12.6% of females (n = 8), and a median BMI of 24.1 (IQR = 3.8). Right hepatectomy was performed in 28 patients (44.5%), left hepatectomy in 10 patients (15%), a right trisectionectomy in 6 cases (9%), a mesohepatectomy in 4 cases (6.2%), a sectionectomy in 8 cases (12.6%), a bisegmentectomy in 2 patients (3.1%), a segmentectomy in 1 case (1.5%), and a wedge resection in 2 cases (3.1%).
No significant differences were found between the two groups in all the preoperative characteristics (Table 1).
Postoperative outcomes
Intraoperative results showed no differences between OLR and LLR in mean operative time (287.5 ± 146 vs. 290 ± 250, respectively; p = 0.69), median estimated blood loss (800 vs 680, respectively; p = 0.53), and rate of intraoperative transfusions (41% vs 23.5%, respectively; p = 0.11).
Similarly, postoperative results showed no differences in the rate of PHLF (6.5% vs. 5.9%, respectively; p = 0.92), severe postoperative complications (8.7% vs. 5.9%, respectively; p = 0.71), and in-hospital mortality (2.2% vs. 0%, p = 0.54). All perioperative outcomes are found in Table 2.
Survival analysis
After a median follow-up of 48.4 (95% CI 8.9–86.8) months, there were no statistically significant differences in 3 years OS (59.3 ± 8.7 months vs. 85.2 ± 9.8 months) and 5 years OS (31.1 ± 9 months vs. 73.1 ± 14.1 months), after OLR and LLR, respectively (p = 0.10) (Fig. 2a).
Similarly, both 3 years DFS (23.5% ± 8.1 months vs. 51.6 ± months) and 5 years DFS (15.7 ± 7.1 months vs. 38.7 ± 15.3 months) were similar in the open and laparoscopic groups, respectively (p = 0.13) (Fig. 2b).
Discussion
This was the largest study to deal with LLR for huge HCC, as well as the first one to specifically compare the short- and long-term results of the open and laparoscopic group for huge HCC. Our data showed that LLR may be safe and feasible in selected cases of huge HCC.
Many studies have previously reported that hepatic resection is the best available option for HCC larger than 10 cm, when compared to other therapeutic strategies [32]. Long-term recurrence is the main problem to face in these patients, and several prognostic risk factors have been identified, such as T4 status, macrovascular portal invasion, and the use of intraoperative transfusion by Yamashita and al., or serum alpha fetoprotein ≥ 100 ng/mL, hypermetabolic uptake on positron emission tomography, satellite nodules, and microvascular invasion by Hwang et al. [33]. In case of recurrence, timely and aggressive treatment is able to significantly improve long-term survival of HCC patients, with recurrent surgery always to be chosen in the optic of a hierarchic strategy and a personalized management of HCC patients [34, 35]. Within the entire cohort of 63 patients treated in our institution since the start of the HPB program, the 3 years OS was 65.8 (± 7.2) and the 5 years OS was 40% (± 8.5), while the 3 years and the 5 years DFS were 32.8 (± 7.4) and 23.2 (± 7). Such encouraging results are in line with previous literature and confirm the leading role of surgery for huge HCC patients. Furthermore, despite not showing statistically significant differences in OS between OLR and LLR groups, the OS was twice as high after LLR than after OLR, potentially reflecting clinically relevant differences. Further appropriated studies should investigate this aspect.
Regarding the role of LLR for the surgical treatment of HCC, many previous meta-analyses and propensity score matched studies have reported reduced bleeding, shorter hospital stays, and fewer postoperative complications, without affecting long-term results [19, 20, 36]. On these bases, previous consensus meetings have stated its safety and effectiveness in selected cases [37, 38]. Nonetheless, there are still some scenarios in which the role of LLR is still debated, such as for huge HCC, multiple HCC, or difficult located HCC [39].
There are several reports concerning laparoscopic hepatectomy for large liver cancer and these confirm that it can be performed safely, although the operative time is extended [40, 41]. Indeed, Goh et al. have reported that tumor size does not affect both short- and long-term outcomes [42]. However, laparoscopic liver resection for large liver tumors is technically challenging and is currently performed only by experienced surgeons in referral HPB centers. The difficulty of LLR for large tumors is due to the limited surgical view, together with the limited possibility of handling the underlying fibrotic or cirrhotic liver, and the proximity to blood and biliary structures [3]. Indeed, tumor size is one of the main parameters of the most used difficulty scores for LLR, and an interesting recent study by Xiaocui et al. reports a correlation between technical difficulty and long-term results after minimally invasive liver resection [43].
In this scenario, reporting our real-life data about the outcomes of LLR for huge HCC may add important evidence about its safety and effectiveness. There were no significant differences in both intraoperative and postoperative outcomes, including in-hospital, short-term, and long-term survival. Similarly, also the rate of R0 resection was similar. It is interesting to note that there were no differences also in the operative time, despite previous studies reporting longer operative time [44]. Probably, the surgeons’ and center experience play a key role in this aspect, as previously reported in literature [45]. Another interesting result was the rate of blood transfusions, that was almost double in the OLR when compared to LLR, despite non-significant (41% vs. 23.5%, p = 0.11). Further studies may focus on such aspect, given the prognostic importance of blood transfusions during liver resection for HCC [46, 47].
Furthermore, our population included all consecutive cases of huge HCC undergoing surgery at Seoul National University Bundang Hospital since the start of the HPB program. This reflects the real everyday life scenario in a HPB referral center, but, on the other hand, the results of the LLR case may even be partially influenced by the learning curve effect, given the progressive increase in number and technical difficulty of laparoscopic liver procedures in our center, which is notoriously accompanied by an improvement in the results [45]. Thus, results after LLR may be even better. Further studies may clear such aspect.
This study has some limitations. Firstly, its retrospective and single-center nature which is liable to selection bias. Nonetheless, to reduce selection bias, all consecutive patients meeting the selection criteria were included. Secondly, the small sample size that may affect the statistical results, due to the reduced statistical power that could make it difficult to achieve statistical significance (despite statistical significance is different from clinical significance) [48]. Indeed, it is well known that the absence of evidence is not evidence of absence, and results should not be misinterpreted in this sense [49]. When the question is if whether the absence of evidence is a valid enough justification for changing clinical practice, we must contextualize the clinical scenario. In our case, we included patients who already underwent LLR for huge HCC, as already reported in other centers, and, since patients with huge HCC are not always eligible for surgery, it is difficult to include larger sample size. We do not suggest changing clinical practice based on our results, but we believe it is important to report first available results from everyday practice in referral centers, in order to build further studies. Furthermore, we believe it is important to focus on how patients with huge HCC may be selected for LLR in referral centers. To this aim, a future strategy may be to design a multicenter study, but with the following risk of additional heterogeneity. This is still the largest monocentric series of LLR for huge HCC, so far. Thirdly, the patients were not matched according to the patients’ and tumors’ characteristics.
Conclusion
Laparoscopic liver resection for giant tumors (larger than 10 cm) may be safely performed in selected cases in referral centers. Such results need to be confirmed by further studies with larger sample size.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Bruix J, Cheng AL, Meinhardt G et al (2017) Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 67(5):999–1008
Cassese G, Han HS, Lee B, Lee HW, Cho JY, Troisi R (2022) Leaping the boundaries in laparoscopic liver surgery for hepatocellular carcinoma. Cancers (Basel). https://doi.org/10.3390/cancers14082012
Graf D, Vallbohmer D, Knoefel WT, Kropil P, Antoch G, Sagir A et al (2014) Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med 25(5):430–437
Herrero A, Boivineau L, Cassese G, Assenat E, Riviere B, Faure S et al (2022) Progression of AFP SCORE is a preoperative predictive factor of microvascular invasion in selected patients meeting liver transplantation criteria for hepatocellular carcinoma. Transpl Int 35:10412
Tribillon E, Barbier L, Goumard C, Irtan S, Perdigao-Cotta F, Durand F et al (2016) When should we propose liver transplant after resection of hepatocellular carcinoma? A comparison of salvage and de principe strategies. J Gastrointest Surg 20(1):66–76
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334(11):693–699
Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N et al (2015) Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol 7(20):2274–2291
Furuta T, Sonoda T, Matsumata T, Kanematsu T, Sugimachi K (1992) Hepatic resection for a hepatocellular carcinoma larger than 10 cm. J Surg Oncol 51(2):114–117
Nault JC, Sutter O, Nahon P, Ganne-Carrie N, Seror O (2018) Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol 68(4):783–797
Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76(3):681–693
Faber W, Sharafi S, Stockmann M, Denecke T, Sinn B, Puhl G et al (2013) Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery 153(4):510–517
Cassese G, Han HS, Al Farai A, Guiu B, Troisi RI, Panaro F (2022) Future remnant liver optimization: preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg 77(4):368–379
Bogdanovic A, Bulajic P, Masulovic D, Bidzic N, Zivanovic M, Galun D (2021) Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: a propensity score matched analysis. Sci Rep 11(1):4493
Chung JW, Kim HC, Yoon JH, Lee HS, Jae HJ, Lee W et al (2006) Transcatheter arterial chemoembolization of hepatocellular carcinoma: prevalence and causative factors of extrahepatic collateral arteries in 479 patients. Korean J Radiol 7(4):257–266
Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY (2012) Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 118(19):4725–4736
Franken C, Lau B, Putchakayala K, DiFronzo LA (2014) Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg 149(9):941–946
Morise Z (2018) Laparoscopic liver resection for the patients with hepatocellular carcinoma and chronic liver disease. Transl Gastroenterol Hepatol 3:41
Witowski J, Rubinkiewicz M, Mizera M, Wysocki M, Gajewska N, Sitkowski M et al (2019) Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc 33(5):1491–1507
Xiangfei M, Yinzhe X, Yingwei P, Shichun L, Weidong D (2019) Open versus laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc 33(8):2396–2418
Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth 13(Suppl 1):S31–S34
Montalti R, Rompianesi G, Cassese G, Pegoraro F, Giglio MC, De Simone G et al (2023) Role of preoperative 3D rendering for minimally invasive parenchyma sparing liver resections. HPB (Oxford) 25(8):915–923
Cassese G, Han HS, Lee B, Lee HW, Cho JY, Troisi RI (2023) Minimally-invasive anatomical liver resection for hepatocellular carcinoma: a literature overview with technical and anatomical tips and tricks. Mini-invasive Surg 7:5
Torzilli G, McCormack L, Pawlik T (2020) Parenchyma-sparing liver resections. Int J Surg 82S:192–197
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149(5):713–724
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149(5):680–688
Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ et al (2011) Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 13(8):528–535
Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F et al (2003) The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 38(1):258–266
Pang YY (2002) The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 4(2):99
Iaria M, Bianchi G, Fazio F, Ardito F, Perri P, Pontarolo N et al (2022) The largest western experience on salvage hepatectomy for recurrent hepatocellular carcinoma: propensity score-matched analysis on behalf of He.RC.O.Le. Study Group. HPB (Oxford) 24(8):1291–304
Yamashita Y, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K et al (2011) Outcomes of hepatic resection for huge hepatocellular carcinoma (>/= 10 cm in diameter). J Surg Oncol 104(3):292–298
Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY et al (2015) Long-term outcome after resection of huge hepatocellular carcinoma >/= 10 cm: single-institution experience with 471 patients. World J Surg 39(10):2519–2528
Herrero A, Toubert C, Bedoya JU, Assenat E, Guiu B, Panaro F et al (2024) Management of hepatocellular carcinoma recurrence after liver surgery and thermal ablations: state of the art and future perspectives. Hepatobiliary Surg Nutr 13(1):71–88
Vitale A, Cabibbo G, Iavarone M, Vigano L, Pinato DJ, Ponziani FR et al (2023) Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol 24(7):e312–e322
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63(3):643–650
Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R et al (2018) The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 268(1):11–18
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS et al (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261(4):619–629
Cassese G, Han HS, Lee E, Lee B, Lee HW, Cho JY et al (2024) Laparoscopic versus open liver resection for multiple hepatocellular carcinoma within and beyond the Milan criteria: an eastern–western propensity score-matched analysis. J Hepatobiliary Pancreat Sci 31(1):2–11
Zhou YM, Li B, Xu DH, Yang JM (2011) Safety and efficacy of partial hepatectomy for huge (>/=10 cm) hepatocellular carcinoma: a systematic review. Med Sci Monit 17(3):RA76-83
Dumronggittigule W, Han HS, Komoltri C, D’Silva M, Lee B, Cho JY (2023) Laparoscopic versus open hepatectomy for large hepatocellular carcinoma: a single center propensity-score-matching comparative analysis of perioperative outcomes and long-term survival. Surg Endosc 37(4):2997–3009
Goh BK, Chow PK, Teo JY, Wong JS, Chan CY, Cheow PC et al (2014) Number of nodules, Child-Pugh Status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg 18(8):1477–1485
Lv X, Zhang L, Yu X, Yu H (2023) The difficulty grade of laparoscopic hepatectomy for hepatocellular carcinoma correlates with long-term outcomes. Updates Surg 75(4):881–888
Kabir T, Syn NL, Guo Y, Lim KI, Goh BKP (2021) Laparoscopic liver resection for huge (>/=10 cm) hepatocellular carcinoma: a coarsened exact-matched single-surgeon study. Surg Oncol 37:101569
Cassese G, Han HS, Yoon YS, Lee JS, Lee B, Lee HW et al (2024) Evolution of laparoscopic liver resection in the last two decades: lessons from 2000 cases at a referral Korean Center. Surg Endosc 38(3):1200–1210
Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H et al (1994) Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 115(3):303–309
Giehl-Brown E, Geipel E, Lock S, Dehlke K, Schweipert J, Weitz J et al (2024) Transfusions of packed red blood cells in surgery for liver cancer: predictor of impaired overall survival but not recurrence-free survival—impact of blood transfusions in liver surgery. J Gastrointest Surg 28(4):402–411
Gikandi A, Hallet J, Koerkamp BG, Clark CJ, Lillemoe KD, Narayan RR et al (2024) Distinguishing clinical from statistical significances in contemporary comparative effectiveness research. Ann Surg 279(6):907–912
Altman DG, Bland JM (1995) Absence of evidence is not evidence of absence. BMJ 311(7003):485
Funding
Open Access funding enabled and organized by Seoul National University Hospital. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Gianluca Cassese, Ho-Seong Han, Boram Lee, Hae Won Lee, and Jai Young Cho have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cassese, G., Han, HS., Lee, B. et al. Laparoscopic versus open liver resection for huge hepatocellular carcinoma (≥ than 10 cm): a retrospective analysis from a high-volume referral center. Surg Endosc (2024). https://doi.org/10.1007/s00464-024-11091-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-024-11091-4