Abstract
Background
This retrospective study compared the short- and long-term outcomes of laparoscopic liver resection (LLR) and open liver resection (OLR) and identified patients who might gain more benefits from LLR.
Methods
The demographic and perioperative data, short-term surgical outcomes, and long-term oncological results of all 313 patients who received elective liver resection for hepatocellular carcinoma (HCC) between January 2010 and June 2017 were analyzed. The patients were then divided into stage-specific subgroups according to the TNM staging system for comparison.
Results
LLR was performed in 153 patients and OLR in 160 patients. LLR is associated with less blood loss (p < 0.001), shorter surgical time (p = 0.001), shorter length of hospital stay (p < 0.001), and lower morbidity rate (p = 0.034). The 5-year overall survival (OS) rates in the LLR group were higher than those in the OLR group (78.1 vs. 57.6%; p = 0.002). Stage-specific subgroup analysis revealed similar 5-year OS in the two groups (stage I: 82.8 vs. 82.6%, p = 0.845; stage II: 80.3 vs. 69.2%, p = 0.638; stage III: 55.6 vs. 34.8%, p = 0.681), as did the 5-year recurrence-free survival. Moreover, the short-term outcomes were better in the LLR group in terms of surgical time, blood loss, and length of hospital stay, and these benefits attenuated with advancing tumor stage.
Conclusions
LLR for HCC is a safe and feasible procedure that does not compromise long-term oncological outcomes. In early tumor stages, LLR might be better in terms of short-term surgical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second leading cause of cancer-related death in Taiwan. Advancements in surgical and anesthetic techniques and better intensive care have decreased the surgical mortality rates of liver resection [1], but hepatic surgery continues to have a relatively high morbidity rate (4.09–47.7%), with fever, hemorrhage, bile leakage, liver failure, pulmonary complications, and subphrenic infection as potential complications [2]. Laparoscopic surgery has yielded better postoperative outcomes than the open method in many gastrointestinal surgeries. However, it is not widely accepted as a standard procedure by hepatobiliary surgeons owing to technical difficulties in hemostasis during liver resection, risk of gas embolism, inadequate tumor clearance, and possible intra-abdominal tumor spreading. The technical difficulty of laparoscopic liver resection (LLR) is gradually being eased by improvements in laparoscopic instruments and the increase in surgical experience. Even lesions located in posterosuperior segments have shown favorable results [3, 4]. LLR has yielded improved short-term outcomes, specifically shorter hospital stay, lesser blood loss and blood transfusion, and wider resection margins [5]. Furthermore, the long-term survival rate in LLR is noninferior to open liver resection (OLR) [5,6,7]. In the literature, data on randomized controlled trials comparing LLR and OLR for HCC are lacking, and the sample sizes were relatively small in most retrospective studies. The objectives of this study were to compare the outcomes of LLR and OLR and to identify patients who might gain more benefits from LLR in terms of clinical, surgical, and pathologic outcomes. Our retrospective study comprised relatively large LLR and OLR groups, with specific emphasis on the stage-specific short-term outcomes, long-term overall survival (OS), and recurrence-free survival (RFS).
Methods
Data sources
The medical records of all patients with HCC who underwent liver resection in our institute between January 2010 and June 2017 were collected retrospectively from a prospectively established database. The inclusion criteria were (1) age 20–85 years, (2) pathologic confirmation of HCC, and (3) underwent surgeries with radical resection intent (at least partial hepatectomy with adequate tumor-free margin). The exclusion criteria were (1) emergent surgeries, (2) benign liver tumor, (3) malignancy other than HCC (cholangiocarcinoma, metastases, and angiosarcoma), and (4) underwent single-incision laparoscopic method or robot-assisted laparoscopic surgeries.
Surgical methods
The LLR technique can be divided into three main categories based on the Louisville statement, namely pure laparoscopy, hand-assisted laparoscopy, and the hybrid technique [8]. In LLR, a CO2 pneumoperitoneum is established, with intra-abdominal pressure controlled at 12–15 mmHg. The laparoscopic camera is inserted through the transumbilical trocar site. Laparoscopic ultrasound is routinely used for checking tumor margin, synchronous tumor, or intrahepatic metastases. However, in the total or pure laparoscopic method, the entire liver resection procedure is performed using laparoscopic instruments. A laparoscopic cavitron ultrasonic surgical aspirator and an energy device are used for liver parenchymal resection. The specimen is then placed into a plastic bag and extracted through an enlarged transumbilical port or a suprapubic transverse incision.
In the hand-assisted laparoscopic method, a 10-cm hand-access port is placed in the upper midline position. A wound protector is applied into the hand port using a surgical glove to prevent air leak. Liver parenchymal resection is performed using laparoscopic instruments as in the total laparoscopic method.
In the hybrid or laparoscopy-assisted liver resection technique, the liver is mobilized laparoscopically and then delivered into the midline. A 12–15-cm incision is then made in the upper midline position. The technique used for parenchymal resection is the same as the open surgery.
For open hepatectomy, all patients are placed in a supine position and a reverse L-incision is performed or a midline wound used. The Cavitron ultrasonic surgical aspirator is mainly used for parenchymal transection. Bipolar electrocoagulation is usually performed for hemostasis.
Data collection of patients
This study is a retrospective analysis of 313 consecutive patients who underwent elective hepatectomy for HCC between January 2010 and June 2017. The study protocol was reviewed and approved by Taipei Medical University Joint Institutional Review Board. All cases were analyzed using medical records showing demographic and clinical characteristics, including age, sex, body mass index (BMI), hepatitis profile, Child–Pugh score, cirrhosis, comorbidity index, and operative and pathologic characteristics such as surgical time, estimated blood loss, main tumor size, tumor stage, tumor margin, major resection rate, length of stay (LOS), complications, and mortality. Patients were divided into two groups: LLR and OLR; the LLR group included those who underwent total (pure) laparoscopic, hand-assisted laparoscopic, and hybrid (laparoscopy-assisted) liver resection, as were patients who were converted from a laparoscopic procedure to the open procedure.
Outcomes
The primary outcomes were short-term postoperative outcomes, including postoperative complications, 30-day mortality, estimated blood loss, and LOS. Complications were assessed up to 30 days following surgery and included specific hepatectomy-related morbidity (bile leak, liver failure, and ascites) and general postoperative complications (intra-abdominal infection, respiratory complications, cardiac events, neurologic events, and gastrointestinal complications). Liver failure was defined by the “50–50 criteria,” [9] that is, the persistent rise in serum bilirubin > 50 µmol/L and decrease in prothrombin time ratio < 50% at postoperative day 5. Secondary outcomes were long-term OS and RFS. Vital statuses at the last follow-up were checked and classified as alive with HCC, alive without HCC, or deceased. OS was defined as the interval between the date of surgery and death for any reason, with censoring at the date of last follow-up. RFS was calculated as the interval between the date of surgery and date on which recurrence or death was recorded, with censoring at the date of last follow-up. Recurrence was defined as intra- or extrahepatic pathology-proven HCC or lesions deemed highly suspicious on dynamic cross-sectional images. The patients were further categorized into stage-specific subgroups per their TNM tumor stage, and the outcomes as well as OS and RFS in the LLR and OLR groups were compared.
Statistical analysis
SPSS statistical software (IBM SPSS, Inc., Chicago, IL, version 20) was used to perform the analyses. Continuous variables were expressed as mean ± standard deviation. Differences in continuous variables between groups were determined using Student’s t test. Categorical variables were expressed as absolute numbers (percentage) and compared between groups using the χ2 test. Overall survival and RFS curves were obtained using the Kaplan–Meier method and compared using means of the log rank test. p < 0.05 was considered statistically significant.
Results
Short-term outcomes
During the study period, 313 patients underwent elective liver resection for HCC, of which 160 patients underwent OLR and 153 patients LLR. Of the patients who underwent LLR, 39, 4, and 110 patients received the hybrid, hand-assisted, and pure laparoscopic methods, respectively. We performed more hybrid and hand-assisted methods in the earlier period of the surgery and transferred to pure laparoscopy since October 2015. Five patients required OLR, yielding a conversion rate of 3.3%.
The demographic, clinical, and surgical parameters are summarized in Table 1. The two groups did not differ significantly in terms of age, sex, BMI, type of hepatitis, Child–Pugh grade, liver cirrhosis, Charlson comorbidity index [10], and surgical margin. Surgical time in the LLR group was significantly shorter than in the OLR group (175.1 ± 84.9 vs. 202.2 ± 59.4 min; p = 0.001). Furthermore, LLR was associated with significantly less blood loss (363.1 ± 579.4 vs. 839.3 ± 866.7 mL; p < 0.001) and shorter postoperative hospital stay (7.3 ± 4.4 vs. 11.4 ± 7.0 d; p < 0.001). The tumor size and rate of major liver resection were significantly smaller in the LLR group (3.9 ± 2.6 vs. 7.2 ± 5.3 cm; p < 0.001, and 22.2 vs. 45.6%; p < 0.001). The TNM stage distributions of the patients in the LLR group compared with the OLR group were 52.3 versus 26.2% in stage I, 32.7 versus 31.2% in stage II, 15.0 versus 38.8% in stage III, and zero versus 3.8% in stage IV (p < 0.001).
The 30-day operative mortality rate of all patients was 1.92%, with 2.6% in the OLR group and 1.3% in the LLR group (p = 0.689). The surgical morbidity rate of all patients was 14.1%, with 18.1% in the OLR group and 9.8% in the LLR group (p = 0.034). The surgical morbidities included bile leak, abdominal abscess or infection, ascites, liver failure, pulmonary complications, congestive heart failure, neural complications (stroke or transient ischemic attack), and gastrointestinal complications (bleeding or ileus). The mortality rate of the two groups did not differ significantly. However, morbidity rate and severe complications were lower in the LLR group than in the OLR group (Clavien–Dindo classification grade 3–4: 2.6 vs. 10.6%; p = 0.005) [11].
Long-term outcomes
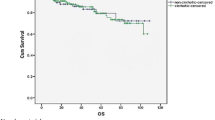
The OS rate of patients at all stages treated with LLR was significantly higher than those treated with OLR (Fig. 1A), with 1-, 3-, and 5-year OS rate of 90.3, 82.9, and 78.1% for the LLR group versus 85.0, 63.6, and 57.6% for the OLR group, respectively (p = 0.002).
There was no difference in the RFS of patients at all stages who received LLR compared with those who received OLR (Fig. 2A), with 1-, 3-, and 5-year RFS of 72.9, 49.2, and 37.9% for the LLR group versus 60.8, 43.0, and 31.1% for the OLR group, respectively (p = 0.153).
Stage-specific subgroup analysis
The cohort was subdivided according to TNM staging, and the short-term outcomes of the two groups were compared (Table 2). In the subgroup analysis, age, sex, BMI, type of hepatitis, liver cirrhosis, Charlson comorbidity index, surgical margin, complication, surgical mortality, as well as the rate of major liver resection of the two groups did not differ significantly. In the subgroup analysis of stage I, LLR was associated with a significantly smaller tumor size (3.1 ± 2.0 vs. 4.7 ± 4.3 cm; p = 0.025), shorter surgical time (152.5 ± 67.3 vs. 183.9 ± 54.6 min; p = 0.010), less blood loss (286.3 ± 463.8 vs. 550.7 ± 431.0 mL; p = 0.003), and shorter LOS (6.8 ± 3.6 vs. 9.1 ± 4.1 days; p = 0.002). In the stage II subgroup analysis, the surgical time and tumor size in the groups did not differ significantly, whereas blood loss (338.6 ± 476.0 vs. 623.6 ± 601.0 mL; p = 0.010), hospital stay (7.6 ± 4.6 vs. 12.3 ± 9.9 days; p = 0.003), and severe complications (Clavien–Dindo classification grade 3–4: 2.0 vs. 16.0%; p = 0.014) were lower in the LLR group. In stage III patients, laparoscopic resection was only associated with smaller tumor size (6.5 ± 3.7 vs. 10.2 ± 5.1 cm; p = 0.001) and shorter LOS (8.5 ± 5.9 vs. 11.7 ± 4.6 days; p = 0.012). No stage IV patient received LLR.
The comparison of OS of patients with stage I, II, and III disease is shown in Fig. 1B–D. Five-year OS rates in stage I, II, and III were 82.8, 80.3, and 55.6% for LLR versus 82.6, 69.2, and 34.8% for OLR (p = 0.845, 0.638, and 0.681, separately) in the stage-specific subgroups, respectively.
Furthermore, 5-year RFS in stage I, II, and III disease was 45.8, 32.7, and 17.1% for LLR versus 58.5, 28.2, and 15.8% for OLR (p = 0.403, 0.801, and 0.465, separately) in the stage-specific subgroups (Fig. 2B–D), respectively.
Discussion
In this retrospective study, we compared the short-term and long-term outcomes of LLR with OLR. The results showed that laparoscopic surgery not only improved the short-term outcomes of patients with HCC in terms of surgical time, blood loss, length of hospital stay, and complication rate but also yielded a higher 5-year OS rate. However, the group categorization did have some bias because of the earlier stage of HCC (stage I + II: 85.0 vs. 57.4%; p < 0.001) and lower rate of major resection (22.2 vs. 45.6%; p < 0.001) in the LLR group compared with the OLR group. The patients were further categorized into subgroups based on the tumor stage (Table 2); the rate of major resection did not differ significantly between the LLR and OLR groups in the stage-specific analysis. Long-term oncological outcomes of the LLR and OLR group, assessed in terms of stage-specific OS and RFS, did not differ significantly. Regarding short-term outcomes, the LLR group had a smaller tumor size (3.1 ± 2.0 vs. 4.7 ± 4.3 cm; p = 0.025), shorter surgical time (152.5 ± 67.3 vs. 183.9 ± 54.6 min; p = 0.010), less blood loss (286.3 ± 463.8 vs. 550.7 ± 431.0 mL; p = 0.003), and shorter LOS (6.8 ± 3.6 vs. 9.1 ± 4.1 days; p = 0.002) in stage I disease. Blood loss (338.6 ± 476.0 vs. 623.6 ± 601.0 mL; p = 0.010), hospital stay (7.6 ± 4.6 vs. 12.3 ± 9.9 days; p = 0.003) and severe complications (Clavien–Dindo classification grade 3–4: 2.0 vs. 16.0%; p = 0.014) were less in the LLR group of stage II disease, while only smaller tumor size (6.5 ± 3.7 vs. 10.2 ± 5.1 cm; p = 0.001) and shorter LOS (8.5 ± 5.9 vs. 11.7 ± 4.6 days; p = 0.012) were noted in the LLR group of stage III patients.
The survival rate is clearly influenced by the tumor stage; thus, theoretically, better intervention should improve the outcomes at every disease stage. Therefore, tumor stage-based subgroup analysis was performed. In the subgroup analysis, the stage-specific OS rate as well as the RFS did not differ significantly (Figs. 1B–D, 2B–D). LLR had no impact on the long-term oncological outcomes, and the results are consistent with other reports on long-term survival [12,13,14]. These findings might clarify the oncological effect in LLR. In addition, the rate of major liver resection directly influences the rate of surgical complications, surgical time, and blood loss. The rate of major resection did not differ significantly in the stage-specific subgroup analysis, whereas some short-term outcomes differed between the LLR and OLR groups. In stage I disease, LLR had the advantages of less surgical time, less blood loss, and shorter LOS. The advantage of less surgical time could not be observed in the stage II population, as LLR still had less blood loss and shorter LOS. However, only the advantage of shorter LOS continued into stage III. The complication rate did not differ in the stage-specific subgroups; less severe complications were observed in the LLR group of stage II patients. Most importantly, this study demonstrated a trend of a slight reduction in the advantage of LLR with advancing tumor stage. Stage IV data could not be compared because no patient at this stage received laparoscopic surgery.
In the English literature, randomized controlled trials regarding HCC comparing the surgical outcomes of LLR versus OLR are rare; one such study discussed the surgical outcomes of solitary small (< 5 cm) peripheral HCC [15] and concluded that LLR is superior to OLR given the former’s significantly shorter duration of hospital stay without any compromise of the oncological outcomes. Most related studies are retrospective with or without propensity score matching. In our HCC surgical cohort, the patients underwent laparoscopic and open hepatectomy in a 1:1 ratio, and the study cohort was analyzed retrospectively without propensity score matching. All other propensity score-matched studies have reported shorter hospital stay, less blood loss, lower blood transfusion rate, lower or similar complication rate, and comparable long-term oncological outcomes in the LLR group [12, 13, 16,17,18,19,20,21,22,23,24,25]. In the current study, similar results were found for all disease stages. However, the most prominent finding in the stage-specific analysis is that the benefit of LLR declines as the stage advances. Only the advantage of shorter length of hospital stay persisted in patients with stage III HCC who received LLR compared with OLR.
In the published literature, general conversion rates range from 0 to 19.4% [26], and the most frequent causes for conversion are uncontrolled bleeding, technical difficulties in exposure, and adhesion. Consistent with previous studies, the conversion rate to OLR in this study was 3.3%. In the series, we converted pure laparoscopy procedures to hand-assisted or hybrid procedures sequentially instead of direct conversion to OLR; this explains the relatively low conversion rate. A detailed comparative analysis of pure, hand-assisted, and hybrid between techniques could not be realized in this study, and the advantages and drawbacks of these approaches could not be demonstrated.
With the introduction of laparoscopic surgery, favorable surgical outcomes—such as less pain, less analgesic requirement, faster recovery of the gastrointestinal tract, and a shorter hospital stay—were demonstrated in abdominal surgeries. Meta-analyses have reported shorter hospital stay, less blood loss and blood transfusion, and wider resection margins in LLR compared with OLR [5, 27]. The Louisville and Morioka international consensus conferences have shown that laparoscopic “minor” liver resections can be undertaken routinely as part of standard practice [8, 28]. This study demonstrated that patients in the early tumor stages can obtain more benefits via laparoscopic liver surgery, which is somewhat consistent with the international consensus of LLR. Thus, LLR is suggested for patients with early-stage HCC. Whether LLR or OLR is effective for advance-stage HCC patients warrants further research. Selection of patient demographics, such as BMI, body type, background liver status, and comorbidities, should be considered together with technical aspects such as the surgeon’s experience and difficulty scoring system [29] to achieve better surgical outcomes.
Limitations
As this study was a nonrandomized trial, biases in the selection of patients for the LLR group were inevitable. Furthermore, more patients with early-stage cancer were present in the LLR group than in the OLR group, whereas those in the OLR group had larger tumors than patients in the LLR group. Moreover, hepatectomy surgery is complicated by the background texture of liver (cirrhotic or steatotic), remnant liver reservoir, tumor location, and tumor adjacent to vessel or bile duct. These factors were not analyzed in this retrospective study. In addition, tumor differentiation and vascular invasion, factors that have an oncological impact, were not examined in this study. The effectiveness of anatomical hepatectomy is controversial [30,31,32,33]; unfortunately, anatomical and nonanatomical liver resection could not be combatively evaluated in this study.
Conclusions
LLR for HCC is a safe and feasible procedure that does not compromise long-term oncological outcomes if performed by an experienced surgeon for well-selected patients. In the earlier stages of HCC, laparoscopic liver resection might be better than open method in terms of short-term surgical outcomes.
References
Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC (2006) Predictive indices of morbidity and mortality after liver resection. Ann Surg 243:373
Jin S, Fu Q, Wuyun G, Wuyun T (2013) Management of post-hepatectomy complications. World J Gastroenterol 19:7983
Yoon Y-S, Han H-S, Cho JY, Ahn KS (2010) Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 24:1630–1637
Cardinal J, Reddy S, Tsung A, Marsh J, Geller D (2013) Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepato-Biliary-Pancreat Sci 20:114–119
Jiang B, Yan X, Zhang JH (2018) Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res: Off J Jpn Soc Hepatol 48:635–663
Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102:82–86
Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, Tan W, Zhang L (2017) Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc 31:4790–4798
Buell JF, Cherqui D, Geller DA, O’rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS (2009) The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 250:825–830
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F (2005) The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 242:824–828 (discussion 828–829)
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, Hatano E, Tanahashi Y, Mizuguchi T, Kamiyama T, Ikeda T, Tanaka S, Taniai N, Baba H, Tanabe M, Kokudo N, Konishi M, Uemoto S, Sugioka A, Hirata K, Taketomi A, Maehara Y, Kubo S, Uchida E, Miyata H, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepato-Biliary-Pancreat Sci 22:721–727
Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW (2014) Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc 28:950–960
Kobayashi T (2015) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 262:e20
El-Gendi A, El-Shafei M, El-Gendi S, Shawky A (2017) Laparoscopic versus open hepatic resection for solitary hepatocellular carcinoma less than 5 cm in cirrhotic patients: a randomized controlled study. J Laparoendosc Adv Surg Tech A 28:302–310
Lee JJ, Conneely JB, Smoot RL, Gallinger S, Greig PD, Moulton CA, Wei A, McGilvray I, Cleary SP (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: a 2-to-1 matched pair analysis. HPB 17:304–310
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650
Xu HW, Li HY, Liu F, Wei YG, Li B (2017) Laparoscopic versus open liver resection for lesions adjacent to major vessels: a propensity score matched analysis. J Laparoendosc Adv Surg Tech A 27:1002–1008
Yoon SY, Kim KH, Jung DH, Yu A, Lee SG (2015) Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 29:2628–2634
Xu HW, Liu F, Li HY, Wei YG, Li B (2018) Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc 32:712–719
Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscara C, Scotti M, Romito R, Mariani L, Mazzaferro V (2016) Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 103:871–880
Badawy A, Seo S, Toda R, Fuji H, Fukumitsu K, Ishii T, Taura K, Kaido T, Uemoto S (2017) A propensity score-based analysis of laparoscopic liver resection for liver malignancies in elderly patients. J Investig Surg: Off J Acad Surg Res. https://doi.org/10.1080/08941939.2017.1373170
Ahn KS, Kang KJ, Kim YH, Kim TS, Lim TJ (2014) A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 24:872–877
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, Lo CM (2016) Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a Single Center. Ann Surg 264:612–620
Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, Jung DH, Park GC, Ahn CS, Moon DB, Ha TY, Song GW, Hwang S, Lee SG (2017) Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 265:856–863
Piardi T, Sommacale D, Baumert T, Mutter D, Marescaux J, Pessaux P (2014) Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience. Hepatobiliary Surg Nutr 3:60–72
Zhou YM, Shao WY, Zhao YF, Xu DH, Li B (2011) Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Digest Dis Sci 56:1937–1943
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Tanaka S, Kubo S, Kanazawa A, Takeda Y, Hirokawa F, Nitta H, Nakajima T, Kaizu T, Kaneko H, Wakabayashi G (2017) Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the Endoscopic Liver Surgery Study Group in Japan. J Am Coll Surg 225:249–258, e241
Kobayashi A, Miyagawa S, Miwa S, Nakata T (2008) Prognostic impact of anatomical resection on early and late intrahepatic recurrence in patients with hepatocellular carcinoma. J Hepato-Biliary-Pancreat Sci 15:515–521
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242:252
Li SQ, Huang T, Shen SL, Hua YP, Hu WJ, Kuang M, Peng BG, Liang LJ (2017) Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. BJS 104:118–127
Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, Ishikawa O, Sakon M (2015) Anatomical versus non-anatomical resection for hepatocellular carcinoma. BJS 102:776–784
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kuei-Yen Tsai, Hsin-An Chen, Wan-Yu Wang, and Ming-Te Huang have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Tsai, KY., Chen, HA., Wang, WY. et al. Long-term and short-term surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma: might laparoscopic approach be better in early HCC?. Surg Endosc 33, 1131–1139 (2019). https://doi.org/10.1007/s00464-018-6372-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6372-0