Abstract
Background
This study presents a novel laparoscopic modified overlapping oesophagojejunostomy anastomosis method which consists of self-pulling and latter transection to perform a safer anastomosis, describes the anastomosis technique in detail and reveals its short-term outcomes.
Methods
Forty-five patients underwent totally laparoscopic total gastrectomy using the self-pulling and oesophagus latter-cut overlap method anastomosis for gastric cancer from January 2019–2022. During the self-pulling phase, the oesophagus was ligated at the level of the gastroesophageal junction or above and dragged down by a ligature rope to mobilise up to 5–6 cm. An entry hole was created on the right side of the oesophagus, and a nasogastric tube was taken out through the hole and tip of the tube was used as a guide for the endoscopic linear stapler to decrease the risk of entering the false lumen and creating a side-to-side anastomosis. The oesophagus was then latter-transected by a second endoscopic linear stapler. The common entry hole was closed using a hand-sewing method. Clinicopathological characteristics and surgical outcomes were collected and retrospectively evaluated.
Results
The mean anastomosis duration was 27 min. The morbidity rate was 4.4%. Only two patients experienced postoperative complications but subsequently recovered conservatively. None of the patients suffered anastomotic leak or stricture.
Conclusions
Self-pulling and latter transection-based overlapping anastomosis is a simple and reliable approach that overcomes most of the limitations of standard overlap method and provides satisfactory surgical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intracorporeal oesophagojejunostomy (EJ) is a challenging step in totally laparoscopic total gastrectomy (TLTG) [1, 2]. A range of approaches have been described to facilitate oesophagojejunal reconstruction [3, 4]. The use of linear staplers is becoming increasingly common due to its technical ease of application.
Therefore, overlap and functional end-to-end (FETE) methods have become more preferred among surgeons when performing EJ anastomosis. Compared with this method, the overlap method offers a larger sized and reduced tensioned anastomosis [5]. Although favourable outcomes have been reported with this approach [6, 7], there are still some limitations in overlap methods, such as difficulties obtaining oesophageal stump traction, technical difficulties when closing the common entry hole within the mediastinum, an increased risk of entering the false lumen of the oesophagus and unintentional diaphragmatic crus stapling during anastomosis.
We presented a modified overlap method of self-pulling and latter transection to overcome these major limitations and perform a safer anastomosis. In this report, we aim to describe the anastomosis technique in detail and reveal its short-term outcomes.
Materials and methods
Patients
Forty-five patients underwent TLTG using the Self-Pulling and Oesophagus Latter-Cut Overlap Method (SPLCOM) anastomosis for gastric cancer by the same surgeon from January 2019 to 2022. All subjects provided written informed consent. This study aimed to reveal the technical details of the modified EJ anastomosis. The local ethics committee approved the study; the Unique Identifying Number is 2022-20. The treatment approach for the patients was planned in accordance with the Enhanced Recovery after Surgery (ERAS) protocol recommendations. Preoperative assessments were conducted through endoscopy and contrast-enhanced computed tomography (CT). Gastroscopy was reapplied by the surgeon prior to surgery to evaluate the exact location of the tumour and to decide the level of oesophageal transection to obtain sufficient negative surgical margins.
The indications for SPLCOM-TLTG were as follows: (1) histologically confirmed gastric adenocarcinoma; (2) tumour located in the middle or upper part of the stomach, no more than 2 cm above the gastroesophageal junction; (3) no peritoneal involvement observed by laparoscopy; and (4) no distant metastasis detected.
Clinicopathological characteristics and surgical outcomes such as age, sex, body mass index (BMI), tumour size, TNM stage based on the American Joint Committee on Cancer staging system, surgical margin status, anastomosis duration, time to start oral intake, anastomosis-related complications, morbidity, and mortality were evaluated.
The data of 43 patients who also underwent TLTG for gastric cancer by the same surgical team and different EJ anastomosis techniques for reconstruction were retrospectively scanned. Clinicopathological features and surgical outcomes of these patients were analysed, and the data were compared with the results of the SPLCOM anastomosis technique.
Statistical analysis
Data were analysed by using SPSS version 22.00. Kolmogorov Smirnov and Levene tests were performed for homogeneity and normality analysis of the scaled data. The Pearson chi-square and Fisher exact tests were used in the evaluation of categorical data. One-way analysis of variance (ANOVA) and Kruskal–Wallis tests were used in the analysis of multi-group scale (Scale) data, and Posthoc multiple comparison (Bonferroni) tests were used in the analysis of the relationship between groups.
Operative procedure of SPLCOM
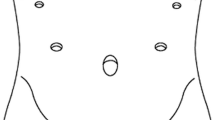
Patients were placed in a 15-degree reverse Trendelenburg position with their legs spread. The primary operator was positioned on the right side of the patient, and the first assistant was on the left side and the cameraman stood between the legs of the patient. After pneumoperitoneum was established at the umbilicus, four additional ports and a liver retractor were placed in two sides of the upper abdomen (Fig. 1). The liver retractor was placed from the epigastric area. Right upper quadrant ports were used by the surgeon and left upper quadrant ports were used for assistance. During the procedure, the 5 mm upper left port was used to drag down the oesophagus, the endoscopic linear stapler device was inserted through the 12–15 mm lower left port to perform the side-to-side EJ anastomosis, and oesophageal latter transection was performed via the 12–15 mm lower right port. A standard D2 lymphadenectomy was conducted with stomach mobilisation. Vagus nerves were removed around the oesophagus and the oesophagus was ligated at the level of gastroesophageal junction (or above the upper margin of the tumour) to avoid the spillage of gastric juice. The assistant held a ligature rope to drag down the oesophagus and allow easier detachment from the hiatal and mediastinal structures before the oesophagus was routinely mobilised up to 5–6 cm. An entry hole was created on the right side of the oesophagus, 2 cm above the ligature rope (Fig. 2a, b). Oesophageal contents were aspirated. A nasogastric tube was inserted and taken out through the hole opened on the oesophagus into the abdomen for later use (Fig. 3a, b). The jejunum was transected 20–25 cm distal to the ligament of Trietz with a 60 mm endoscopic linear stapler (Covidien™ Endo GIA™ Ultra Universal Stapler). Jejunojejunal side-to-side anastomosis was formed 40–50 cm beneath the planned EJ anastomosis. A small enterotomy was performed on the antimesenteric side of the efferent jejunum 5–6 cm from the end of the jejunum to serve as the entrance of the 60 mm endoscopic linear stapler (Covidien™ Endo GIA™ Ultra Universal Stapler). The thick blade of the endoscopic linear stapler was inserted into the jejunum to avoid unintentional perforation during the anastomosis. The assistant held the ligature rope to drag down the oesophagus to allow easy intraabdominal reconstruction. The tip of the nasogastric tube was used as a guide for the thin blade of the endoscopic linear stapler to decrease the risk of entering the false lumen of the oesophagus (Fig. 4a, b). After removing the nasogastric tube to decrease the risk of unintended stapling during the anastomosis, a length of 4–5 cm side-to-side EJ was performed through two holes, forming a common entry hole. Overlap method was modified in a latter-transected fashion, which means the oesophagus was transected by a second endoscopic linear stapler (Covidien™ Endo GIA™ Ultra Universal Stapler) at the level of the common hole margin on the side of oesophagus (Fig. 5a, b). The common entry hole was closed using the hand-sewing method (Fig. 6a, b). An air leak test was performed to ensure sufficient closure. The umbilical port site incision was extended up to 30–40 mm (according to the size of the tumour). The specimen was finally removed using a specimen bag through the extended umbilical port site. A drainage tube was placed behind the EJ anastomosis. Nasogastric drainage was not routinely necessary.
Postoperative management
On postoperative day one, patients were allowed to consume a clear liquid diet and then gradually advanced to a soft diet. An upper gastrointestinal water-soluble contrast radiograph was performed to identify anastomosis leakage (on postoperative day 3). The abdominal drainage tube was mostly removed on postoperative day four unless the drainage character was abnormal. Patients were discharged when they were able to ingest sufficient soft diet without discomfort.
Results
The demographic and clinicopathological characteristics of the 88 patients are presented in Table 1. The cohort consisted of 48 (54.5%) males and 40 (45.5%) females with a mean age of 63.13 ± 10.9 years (range: 37–89 years). Anastomosis techniques applied to the patients were as follows: SPLCOM 51.1%, Overlap 35.2%, Orvil 13.6%. The distribution of clinicopathological characteristics of patients according to anastomosis techniques groups is shown in Table 2.
SPLCOM anastomosis
Of the patients who underwent SPLCOM anastomosis, 28 were male and 17 were female, with a median age of 64 years and a median BMI of 25.7. Clinical staging of the patients was as follows: Stage 1 disease 15.5%, Stage 2 disease 37.7% and Stage 3 disease 46.6%. The mean tumour size was 4 cm and the mean distance from the proximal surgical margin to tumour was 1.9 cm.
Other anastomosis techniques
Of the 42 patients who had different EJ anastomosis techniques to SPLCOM, 31 had traditional overlap and 12 had circular anastomosis with a DST Series™ EEA™ OrVil™ Device. In the overlap anastomosis group, the median age was 61 years and the median BMI was 25.4. In the DST Series™ EEA™ OrVil™ Device anastomosis group, the median age was 65 years and the median BMI was 25.5. The mean distance to the proximal surgical margin was 2.2 cm in the overlap group and 2.05 cm in the DST Series™ EEA™ OrVil™ Device group.
We found a statistically significant difference in the distribution of anastomosis time according to the groups of anastomosis techniques (p < 0.001). This significant difference is due to the relationship of the SPLCOM group with the overlap anastomosis and circular anastomosis with the DST Series™ EEA™ OrVil™ Device groups, according to the post hoc Bonferroni analysis. The median anastomosis time was 27 min in the SPLCOM group, 48 min in the Overlap group and 38 min in the circular group.
Anastomosis-related complications
The distribution of anastomosis-related complications according to anastomosis technique groups is listed in Table 3. There was no statistically significant difference in the distribution of postoperative anastomosis-related complication rates according to the anastomosis technique groups (p = 0.299). The morbidity rates of anastomosis techniques were as follows: SPLCOM 4.4%, Overlap 6.4%, Orvil 8.3%. Complications occurred in 2 of the 45 patients who underwent TLTG using SPLCOM anastomosis. One patient experienced decreased oral intake on postoperative day seven, and the other patient was re-hospitalised 10 days after surgery due to mechanical bowel obstruction.
Two patients experienced complications in the overlap anastomosis group; one patient experienced bleeding from the anastomotic stapler line and the other patient experienced decreased oral intake. One patient in the circular anastomosis group was readmitted to the hospital due to stenosis in the anastomosis and treated endoscopically.
Discussion
TLTG is more widely used in the treatment of proximal gastric cancers. The technical difficulties of the intracorporeal EJ anastomosis are still a challenge. This anastomosis can be performed via circular and linear stapling. Endoscopic linear staplers are less likely to have anastomotic complications than circular staplers [8, 9]. Overlap and FETE methods are the most common linear stapler approaches that have become more preferred among surgeons when performing EJ anastomosis.
Inaba et al. introduced overlap method for EJ anastomosis in 2010 [10]. Although the method offers wide, tension-free and easy-to-apply anastomosis, it still has some limitations. The main limitations include (1) retraction of the oesophageal stump towards the mediastinum after oesophageal transection, (2) difficulty in obtaining oesophageal stump traction which may result in unnecessary oesophageal injury, (3) mis-insertion of the linear stapler into the false lumen of the oesophagus, (4) striving for tension-free delivery of the efferent jejunum to the EJ anastomosis, (5) the risk of unintended diaphragmatic crus stapling during the high level EJ anastomosis, (6) technical difficulties during the closure of the common entry hole and (7) the risk of anastomotic stenosis.
In this context, surgeons have suggested many modifications to make the overlap method more feasible. Kim et al. applied two stitches at the oesophagostomy site of the stump to guide the insertion of the linear stapler [11]. Sun et al. transected the oesophagus in two consecutive steps with an endoscopic linear stapler and harmonic ultrasonic scalpel to avoid unnecessary oesophageal injury during latter opening on the stapled line of the oesophageal stump. The common entry hole closure was performed via suturing instead of using a linear stapler to avoid anastomotic stricture [12]. Yamamoto et al. transected the oesophagus while being rotated by 90 degrees in a clockwise direction to improve the visualisation during the closure of the common entry hole [13]. Son et al. demonstrated that opening the left side of the oesophageal stump for linear stapler insertion had a risk of unintended left crus stapling during the anastomosis and therefore preferred to create an entry hole at the centre of the oesophageal stump [14].
In the present study, we described a modified overlap anastomosis technique with recommendations focussed on overcoming technical difficulties. The oesophagus was ligated by a rope at the level of gastroesophageal junction (or above the upper margin of the tumour) to avoid the spillage of gastric juice and tumour spread-out. The ligature rope was held by the assistant to drag down the oesophagus to allow easy mobilisation of the oesophagus by 4–6 cm. Hong et al. similarly stated that the ligated rope can block gastric fluid spillage and tumour spread-out and mobilisation of the oesophagus from the posterior mediastinum is easier with the self-pulling method [15]. This manoeuvre also allowed the avoidance of technical difficulties associated with the oesophageal stump mentioned above. An entry hole was created on the right side of the oesophagus 2 cm above the ligated rope to have a sufficient clear surgical margin. Despite the perspective that utilising a nasogastric tube as a guide to the lumen in the oesophagus increases the risk of pollution [12], we inserted a nasogastric tube and the tip of the tube was taken out through the entry hole into the abdomen to decrease the risk of entering the false lumen of the oesophagus during anastomosis. We did not observe any increase in infectious complications in our postoperative results. A side-to side EJ anastomosis was performed on the right side of the oesophagus under direct visualisation of the right diaphragmatic crus. Self-pulling and latter transection manner provided by a rope facilitates this phase and shortens the procedure. Similarly, it has been demonstrated that EJ anastomosis performed with the self-pulling and latter transection methods reduce surgical difficulties by creating a wider working space and shorten the anastomosis time [15, 16]. The oesophagus is transected after anastomosis by the endoscopic linear stapler at the level of the common entry hole margin on the side of the oesophagus. The common entry hole was closed using hand-sewing to avoid stenosis.
In the Japanese gastric cancer treatment guidelines 2021 (sixth edition), a proximal resection margin of at least 3–5 cm is recommended. A resection margin > 5 cm is not necessarily required, but frozen section examination of the resection line is preferable to ensure an R0 resection, especially for tumours invading the oesophagus [17]. Due to the lack of an intraoperative frozen section to determine the resection margin, oncological safety remains a concern in self-pulling and latter transection reconstruction because the specimen can be obtained after the entire anastomosis process is completed. For this reason, surgeons who perform self-pulling latter transection reconstruction have made different attempts to determine the proximal margin. Jun Hong et al. ligated the pulling rope above the clamped margin and made a larger oesophageal hole above to identify the true lumen; a safe surgical margin was then checked through the hole [15]. Xian-tu Qui et al. believed that mobilisation of the lower oesophagus up to 8–10 cm above the cardia and ligation of the rope above the tumour provide an adequate safe distance for anastomosis. They also compared the length of proximal resection margins in tumours located in the cardia with oesophageal invasion of less than 2 cm between the conventional and self-pulling group. The data showed no significant difference between the two groups, indicating the oncological safety of self-pulling reconstruction [18]. Considering the importance of the resection margin, we also had some attempts to provide oncologically safe anastomosis. Gastroscopy was reapplied by the surgeon prior to surgery to evaluate the location of the tumour and to decide the level of oesophageal transection. The lower oesophagus was routinely mobilised up to 5–6 cm, the oesophagus was ligated above the upper margin of the tumour and the oesophageal hole was made at least 2 cm above the ligatured rope to have a sufficient result. In the present study, the mean distance from the proximal surgical margin to the tumour was 1.9 cm. Proximal resection margin positivity was not observed in any patients. Similarly, studies providing safe surgical margins with self-pulling latter transection reconstruction have been previously reported [15, 18,19,20,21]. We believe that SPLCOM can be applied with rational patient selection in gastric tumours even with limited oesophageal invasion. However, we do not recommend SPLCOM when frozen section examination is indispensable to determine the proximal resection margin, especially for tumours invading the oesophagus.
Anastomosis-related complications should be evaluated to measure the reliability of an anastomotic method. It has been shown that the TLTG overlap method is a reliable approach with a low anastomosis-related complication rate [5,6,7]. With the self-pulling approach, which reduces surgical difficulties and provides a stable anastomosis vision, the applicability of overlap or FETE anastomosis becomes easier. In a study comparing self-pulling anastomosis with laparoscopic-assisted total gastrectomy, the self-pulling group had a lower mean operative time, blood loss and hospital stay than the laparoscopic-assisted total gastrectomy group. There were no significant differences in overall and anastomosis-related complications between the two groups [18]. Jun Hong et al. compared the self-pulling method with conventional methods to clarify the clinical benefits. The mean duration of the operation and the anastomosis were significantly shorter in the self-pulling group. Self-pulling latter-cut anastomosis developed no complications beyond the conventional methods [15]. Similar favourable results were obtained in another study comparing half-transected and self-pulling oesophagojejunostomy with standard end-to-end and overlap oesophagojejunostomy [21]. Jianjun Du et al. described a simplified and feasible self-pulling intracorporeal circular stapled EJ anastomosis and mentioned its benefits [20]. Moreover, a recent study presented the DaVinci Xi-assisted minimally invasive technique of total gastrectomy and intracorporal reconstruction using the self-pulling latter-transected method [22]. Considering the previous studies, self-pulling has often been used to facilitate FETE anastomosis. We described a novel method of self-pulling latter-transected overlapping anastomosis. Moreover, we compared this anastomosis with other anastomosis techniques used by our group for reconstruction after TLTG. According to our experience, the SPLCOM technique is easier to apply than other anastomosis techniques that we have used in the past. Moreover, this method does not result in an increase in anastomosis-related complications. In the present study, the median duration of the whole SPLCOM anastomosis was 27 min, which was significantly shorter than the conventional overlap (48 min) and DST Series™ EEA™ OrVil™ Device (38 min) anastomosis duration. Postoperative complications of SPLCOM anastomosis were observed in two patients. One patient experienced decreased oral intake. Endoscopic examination and oral contrast-enhanced CT were performed on postoperative day seven. No mechanical obstruction or anastomotic stricture was observed and conservatively managed. The other patient was re-hospitalised 10 days after surgery due to mechanical bowel obstruction. No anastomotic stenosis was observed in endoscopic examination and conservatively managed. Postoperative results of the present study were similar when compared to other self-pulling studies.
In conclusion, SPLCOM is a simple and reliable approach that provides satisfactory surgical outcomes. The self-pulling method overcomes most of the limitations of the standard overlap method, such as difficulty in obtaining oesophageal stump traction, the increased risk of entering the false lumen of the oesophagus and unintentional diaphragmatic crus stapling during anastomosis.
References
Yang HK, Suh YS, Lee HJ (2013) Minimally invasive approaches for gastric cancer—Korean experience. J Surg Oncol 107(3):277–281
Kim MG, Kim KC, Yook JH, Kim BS, Kim TH, Kim BS (2011) A practical way to overcome the learning period of laparoscopic gastrectomy for gastric cancer. Surg Endosc 25(12):3838–3844
Kang SH, Cho Y-S, Min S-H, Park YS, Ahn S-H, Park DJ et al (2019) Intracorporeal oesophagojejunostomy using a circular or a linear stapler in totally laparoscopic total gastrectomy: a propensity-matched analysis. J Gastric Cancer 19(2):193–201
Kyogoku N, Ebihara Y, Shichinohe T, Nakamura F, Murakawa K, Morita T et al (2018) Circular versus linear stapling in oesophagojejunostomy after laparoscopic total gastrectomy for gastric cancer: a propensity score-matched study. Langenbecks Arch Surg 403(4):463–471
Ko CS, Gong CS, Kim BS, Kim SO, Kim HS (2021) Overlap method versus functional method for oesophagojejunal reconstruction using totally laparoscopic total gastrectomy. Surg Endosc 35(1):130–138
Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K et al (2016) Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc 30(9):4086–4091
Morimoto M, Kitagami H, Hayakawa T, Tanaka M, Matsuo Y, Takeyama H (2014) The overlap method is a safe and feasible for oesophagojejunostomy after laparoscopic-assisted total gastrectomy. World J Surg Oncol 12(1):1–10
Kawamura H, Ohno Y, Ichikawa N, Yoshida T, Homma S, Takahashi M et al (2017) Anastomotic complications after laparoscopic total gastrectomy with oesophagojejunostomy constructed by circular stapler (OrVil™) versus linear stapler (overlap method). Surg Endosc 31(12):5175–5182
Jeong O, Jung MR, Kang JH, Ryu SY (2020) Reduced anastomotic complications with intracorporeal oesophagojejunostomy using endoscopic linear staplers (overlap method) in laparoscopic total gastrectomy for gastric carcinoma. Surg Endosc 34(5):2313–2320
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S et al (2010) Overlap method: novel intracorporeal oesophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211(6):e25–e29
Kim HS, Kim BS, Lee S, Lee IS, Yook JH, Kim BS (2013) Reconstruction of oesophagojejunostomies using endoscopic linear staplers in totally laparoscopic total gastrectomy: report of 139 cases in a large-volume centre. Surg Laparosc Endosc Percutaneous Techn 23(6):e209–e216
Sun K-K, Wang Z, Peng W, Cheng M, Huang Y-K, Yang J-B et al (2021) Oesophagus two-step-cut overlap method in oesophagojejunostomy after laparoscopic gastrectomy. Langenbeck’s Arch Surg. 406(2):497–502
Yamamoto M, Zaima M, Yamamoto H, Harada H, Kawamura J, Yamaguchi T (2014) A modified overlap method using a linear stapler for intracorporeal oesophagojejunostomy after laparoscopic total gastrectomy. Hepatogastroenterology 61(130):543–548
Son SY, Cui LH, Shin HJ, Byun C, Hur H, Han SU et al (2017) Modified overlap method using knotless barbed sutures (MOBS) for intracorporeal oesophagojejunostomy after totally laparoscopic gastrectomy. Surg Endosc 31(6):2697–2704. https://doi.org/10.1007/s00464-016-5269-z
Hong J, Wang Y-P, Wang J, Bei Y-B, Hua L-C, Hao H-K (2017) A novel method of self-pulling and latter transected reconstruction in totally laparoscopic total gastrectomy: feasibility and short-term safety. Surg Endosc 31(7):2968–2976
Matsui H, Uyama I, Sugioka A, Fujita J, Komori Y, Ochiai M et al (2002) Linear stapling forms improved anastomoses during oesophagojejunostomy after a total gastrectomy. Am J Surg 184(1):58–60
Japanese Gastric Cancer Association (2023) Japanese Gastric Cancer Treatment Guidelines 2021, 6th ed. Gastric Cancer 26:1–25. https://doi.org/10.1007/s10120-022-01331-8.
Qiu XT, Zheng CY, Liang YL, Zheng LZ, Zu B, Chen HH et al (2022) Totally laparoscopic total gastrectomy using the “enjoyable space” approach coupled with self-pulling and latter transection reconstruction versus laparoscopic-assisted total gastrectomy for upper gastric cancer: short-term outcomes. Wideochir Inne Tech Maloinwazyjne 17(2):352–364. https://doi.org/10.5114/wiitm.2022.113568
Chen D, Yang F, Woraikat S, Tang C, Qian K (2022) Effectiveness and safety of self-pulling and latter transected Roux-en-Y reconstruction in totally laparoscopic distal gastrectomy. Front Oncol. https://doi.org/10.3389/fonc.2022.916692
Du J, Shuang J, Li J, Li J, Hua J (2014) Intracorporeal circular-stapled oesophagojejunostomy after laparoscopic total gastrectomy: a novel self-pulling and holding purse-string suture technique. J Am Coll Surg 218(3):e67–e72
Wan H, Xiong J, Chen Y, Wei H, Tang R, Chen C et al (2022) Application of half-transected and self-pulling oesophagojejunostomy in total laparoscopic gastrectomy for gastric cancer: a safe and feasible technique. Can J Gastroenterol Hepatol. 2022:1–8
Hoeppner J (2022) Robotisch assistierte totale Gastrektomie mit D2-Lymphadenektomie und intrakorporaler Rekonstruktion. Zentralblatt für Chirurgie-Zeitschrift für Allgemeine, Viszeral-, Thorax-und Gefäßchirurgie 147(5):427–429
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr Mehmet Aslan and Dr Koray Topgul have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 215738 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslan, M., Topgül, K. A novel, easier and safer alternative method for oesophagojejunal reconstruction after totally laparoscopic total gastrectomy. Surg Endosc 37, 4075–4083 (2023). https://doi.org/10.1007/s00464-023-09992-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-09992-x