Abstract
Background
Valvuloplastic esophagogastrostomy (VEG) using the double flap technique (DFT) after proximal gastrectomy (PG) represents a promising procedure for the prevention of reflux oesophagitis. We aimed to retrospectively investigate the efficacy of minimally invasive PG followed by VEG-DFT in preventing reflux oesophagitis among patients who require intra-mediastinal anastomosis.
Methods
A total of 80 patients who underwent reconstruction with DFT after LPG from November 2013 to January 2021 were enrolled in the present study. Data were obtained through a review of our prospectively maintained database. At 1 year after surgery, multivariate analyses were performed to identify risk factors for gastroesophageal reflux disease of Los Angeles (LA) classification grade B or higher.
Results
The incidence of LA grade B or higher reflux oesophagitis 1 year after surgery was 10%. Multivariate analyses revealed that the longitudinal length of the resected oesophagus of > 20 mm was the only significant risk factor for reflux oesophagitis. Patients with a longitudinal length of the resected oesophagus > 20 mm (group-L, n = 35) had a significantly longer total operative time and a higher rate of complications within 30 days of surgery than those with a length of ≤ 20 mm (group-S, n = 45). LA grade B or higher reflux oesophagitis was significantly higher in group-L than in group-S (20% vs. 2.2%; P = 0.011).
Conclusions
There is a need for surgical procedures with improved efficacy for the prevention of reflux oesophagitis in patients requiring oesophageal resection of > 20-mm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Proximal gastrectomy (PG) has recently been adopted as a function-preserving surgery for selected patients with early proximal gastric cancer or adenocarcinoma of the oesophagogastric junction [1]. After PG, the lower oesophageal sphincter and the acute angle of His are lost, increasing the postoperative risk of reflux oesophagitis [2]. Therefore, reconstruction with preservation of the anti-reflux mechanism should be emphasised after PG. Valvuloplastic esophagogastrostomy (VEG) using the double flap technique (DFT) represents a promising procedure for the prevention of gastroesophageal reflux [3]. Several studies have demonstrated the efficacy of VEG using DFT, with reported incidences of reflux oesophagitis at 1 year postoperatively ranging between 0 and 6% [4,5,6]. In addition, studies using 24 h impedance–pH monitoring have demonstrated the anti-reflux efficacy of this procedure [7]. However, the use of minimally invasive surgery (MIS) for this procedure is technically demanding due to the presence of an anastomotic leakage and/or stricture [4,5,6].
In cases of oesophagogastric junction (EGJ) cancer < 6 cm in diameter, lower oesophagectomy (LE) and PG are currently regarded as the most suitable surgical procedures due to the need for lymph node dissection [8]. However, optimal reconstructive procedures after LE and PG have yet to be established. In addition to the loss of the lower oesophageal sphincter and the acute angle of His, the negative thoracic pressure of the mediastinal space increases the postoperative risk of reflux oesophagitis [4]. A recent multicentre retrospective study reported deteriorated clinical efficacy of intra-mediastinal VEG-DFT in preventing reflux oesophagitis compared to intra-abdominal VEG-DFT among patients with EGJ cancer [4].
At our institute, MIS is the standard radical procedure for GC and EGJ cancer [9, 10]. We previously demonstrated comparable short- and long-term outcomes between laparoscopic D2 gastrectomy and open D2 gastrectomy [11, 12]. Minimally invasive PG followed by VEG-DFT was introduced in 2013, with the feasibility of robotic VEG-DFT previously demonstrated [13]. However, studies on the efficacy of these approaches in preventing reflux oesophagitis have been limited, particularly regarding intra-mediastinal VEG-DFT. In the present study, we aimed to investigate the efficacy of minimally invasive PG followed by VEG-DFT in preventing reflux oesophagitis in patients requiring intra-mediastinal anastomosis.
Materials and methods
Patients
The present study comprised 1256 consecutive patients who underwent gastrectomy for primary gastric malignancies including gastric cancer, gastric gastrointestinal stromal tumour (GIST) and adenocarcinoma of the EGJ with oesophageal invasion of less than 3 cm at Fujita Health University Hospital between November 2013 and January 2021. During the present study period, 123 patients underwent PG. A total of 80 patients were enrolled in the present study after the exclusion of 43 patients for the following reasons: open surgery (n = 1); other reconstructive procedures, including the overlap method (n = 16), double tract reconstruction (n = 9) or modified side overlap with fundoplication by Yamashita (n = 11) [14]; gastric GIST (n = 1) and follow-up period of < 1 year (n = 5). The patient selection process is summarised in Fig. 1. Clinical tumour staging was determined according to the 15th edition of the Japanese Classification of Gastric Carcinoma [1]. Adenocarcinoma of the EGJ was diagnosed according to the Nishi classification [1]. Cancer staging was performed based on the findings of contrast-enhanced computed tomography, oesophago-gastrography, esophagogastroduodenoscopy and endosonography prior to the initiation of treatment and, when applicable, after the completion of chemotherapy as previously described [10, 13]. Neoadjuvant chemotherapy (80-mg/m2 S−1 on days 1–21 + 60-mg/m2 cisplatin on day 8 or 80-mg/m2 S−1 on days 1–14 + 100-mg/m2 oxaliplatin on day 1) was performed in patients with clinical T ≥ 2, tumour size ≥ 5 cm, and/or swollen locoregional lymph nodes ≥ 1.5 cm [15]. The extent of systematic lymph node (LN) dissection was determined based on the Japanese Gastric Cancer Treatment Guidelines [1, 8, 16]. Standard clinical procedures at our institution including detailed indications for radical gastrectomy, assessment of physical function, perioperative management of radical gastrectomy, extent of gastric resection and LN dissection and postoperative chemotherapy in addition to oncologic follow-up are described elsewhere [10, 13, 15, 17, 18]. We routinely perform the preoperative nutritional screening, using the serum albumin levels from the initial visit to our department. When the serum albumin levels are ≤ 3.5 g/dl, the nutritional support team initiates nutritional guidance and dietary counselling. The present study was approved by the Institutional Review Board of the Fujita Health University. Patients were fully involved in the decision-making process regarding their surgical procedures, and informed consent was obtained from all patients.
Decisions regarding procedure selection
Patients were involved in all stages of decision-making process and informed consent was obtained from all patients. However, decision-making regarding patient procedures during the study period was affected by the chronologically changed circumstances surrounding national medical insurance coverage. RG was not included in national medical insurance coverage in Japan between January 2009 and March 2018, during which period patients were charged 2,200,000 JPY upon admission for RG [19]. All patients were equally offered robotic surgery regardless of background, including physical and oncological status. Hence, 26 patients who agreed to uninsured use of the da Vinci Surgical System (DVSS) underwent RG, whereas the remaining 33 patients who refused uninsured use of the DVSS underwent LG with health insurance coverage. Between October 2014 and January 2017, we conducted a multi-institutional, single-arm prospective clinical study approved for Advanced Medical Technology (‘Senshiniryo’) B [20]. Accordingly, two patients with cStage I/II GC who were enrolled in our institution’s Senshiniryo B trial were also included in the present analysis. Since its approval for national medical insurance coverage based on the outcomes of the Senshiniryo B trial in April 2018 [20], RG has been the first choice as the standard radical gastrectomy for GC at our institution [22]. Insured RPG has been performed in all patients (19 patients) requiring PG.
Surgical operator selection
Criteria for selecting the surgical operator were determined according to our basic policy as previously described [21]. All surgeons performing the minimally invasive PG procedure had qualifications from the Endoscopic Surgical Skill Qualification System (ESSQS), which was launched in 2004 by the Japan Society for Endoscopic Surgery (JSES) to assess surgical skill by two separate judges using an unedited operative video in a double-blinded fashion according to strict criteria. In addition, applicants must fulfil the following requirements: certification by the Japan Surgical Society Board; attendance at a JSES scientific meeting, educational seminars or workshops using animals (> 14 points); conducted > 50 simple laparoscopic surgical procedures or > 20 complex laparoscopic procedures; have recommendations from two instructors; and have authorship on more than two original studies in endoscopic surgery and have presented more than three reports at scientific meetings [22]. I.U., who had performed over 1500 LG and 500 RG procedures, selected surgical operators based on the skill level of the surgical operator and patient condition and supervised all LG and RG procedures.
Surgical procedure
The entire process of laparoscopic or robotic PG with nodal dissection was performed using a five-port system with Nathanson hook liver retractors (Yufu Itonaga, Tokyo, Japan) as previously described [13]. To further widen the operative field around the oesophageal hiatus, the hepatic left lateral segment was mobilised and the oesophageal hiatus was dissected if necessary [23]. PG was performed as previously described [13].
Trans-hiatal lower mediastinal dissection and oesophageal resection
After the PG procedure, trans-hiatal lower mediastinal dissection was performed in patients diagnosed with EGJ cancer according to the Japanese Gastric Cancer Treatment Guidelines [1, 8, 16]. After circumferentially dissecting around the oesophagus, the central tendon of the diaphragm and bilateral crura were divided. Then, adipose tissues containing the no. 111 LNs of the anterior side of the oesophagus were dissected until the pericardium was revealed (Fig. 2a). The adipose tissue of the dorsal side of oesophagus (no. 112ao LNs) was dissected from the muscle fibres of the oesophageal hiatus along the surface layer of the aorta while ensuring preservation of the thoracic duct and azygous vein (Fig. 2b). The adipose tissue containing the no. 110 + 112pulR LNs was dissected along the right parietal pleura, which was preserved as much as possible (Fig. 2c). Then, the left parietal pleura was intentionally incised and the left thoracic cavity was widely opened to the mediastinal space. After then, the left pulmonary ligament was incised and the adipose tissue containing the no. 112pulL LNs was dissected (Fig. 2d). After the dissected adipose tissue was separated from the oesophagus, the tumour location was confirmed by intraoperative plain radiography to detect the endoscopic clip marking the oral margin of the tumour. Finally, the lower oesophagus was transected using a linear stapler through the right lower abdominal port (Fig. 2e) or the additional port inserted into the left 7th intercostal space (at the level of the xiphoid process; Fig. 2f). The tumour-negative status was confirmed in the cut end of a frozen Section [24].
a–d Landmarks and representative findings for trans-hiatal lower mediastinal dissection. a The ventral border was the pericardium. b The dorsal border was the surface layer of the aorta. c The right border was the parietal pleura. d The left border was the left pulmonary ligament. e–f Transection of the oesophagus by a linear stapler. e Transection through the right lower abdominal port. f Transection through the intercostal port
VEG-DFT reconstruction
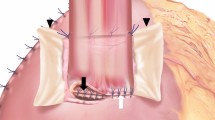
After PG, VEG-DFT reconstruction was performed according to our previous report [13]. Briefly, transverse H-shaped seromuscular flaps with 2.5 cm width and 3.5 cm height were extracorporeally created at the anterior wall of the remnant stomach. After the seromuscular flap was created, a mucosal window was opened 1 cm above the caudal end of the seromuscular flap. Then, the stomach was inserted into the abdomen and the posterior aspect of the oesophagus 4 cm above the cut end was fixed to the cranial end of the mucosal window (Fig. 3a). Next, the staple line at the cut end was removed and continuous suturing using an absorbable barbed suture was performed between the posterior wall of the oesophagus and the cranial opening of the mucosa of the remnant stomach. Then, continuous full-thickness sutures were placed between the anterior wall of the oesophagus and the caudal opening of the remnant stomach (Fig. 3b). Finally, the anastomosis was covered by the seromuscular flaps (Fig. 3c). After completion of the anastomosis, the oesophagus was fixed firmly to the hiatus. When the anastomosis had been created in the mediastinum, the diaphragm was closed with the body of the stomach fixed circumferentially to the hiatus using the nonabsorbable sutures to avoid postoperative artificial hiatal hernia (Fig. 3d). To avoid postoperative artificial hiatal hernia, A closed drain was routinely inserted near the anastomosis. Additional pyloroplasty was not performed in any cases included in the present study.
Intra-mediastinal VEG using the double flap technique. a Fixation of the posterior wall of the oesophagus and the superior end of the mucosal window. b Full-thickness sutures between the oesophagus and the remnant stomach. c Coverage by the seromuscular flaps. d Circumferential fixation of the anastomotic site into the hiatus
Postoperative management
A nasogastric tube was kept overnight in patients who had intra-mediastinal anastomosis but not in patients who had intra-abdominal anastomosis. Postoperative care was provided to all patients according to the same clinical protocol. Walking and drinking water were resumed on postoperative day (POD) 1, soft meals were resumed on POD 3 after examination of the anastomosis using a water-soluble contrast (50 mL of meglumine sodium amidotrizoate), the closed drain was removed on POD 4 and hospital discharge was permitted on POD 7 for patients with a favourable postoperative course. All patients who underwent VEG-DFT were routinely administered an oral proton pump inhibitor (PPI) for several months after surgery. Discontinuation of PPI administration was determined according to symptoms related to reflux oesophagitis and at patient request.
Postoperative endoscopic follow-up
Postoperative esophagogastroduodenoscopy was performed at 6-month intervals for the first year after surgery and every year thereafter to evaluate the anastomotic passage and the grade of reflux oesophagitis and to monitor for local recurrence and metachronous multicentre or multiple cancers. Patients complaining of dysphagia or heartburn were offered additional esophagogastroduodenoscopy. The severity of reflux oesophagitis was determined according to the modified Los Angeles (LA) Classification System. [25].
Retrospective video review
To identify surgical risk factors for reflux oesophagitis, non-edited videos were retrospectively reviewed by two surgeons (K.M. and S.S.). The following parameters were investigated: (1) incision of the pleura; (2) success rate of fixation at 4 cm from the distal oesophageal stump; and (3) success rate for insertion of a sufficient volume of the remnant stomach into the opened intra-mediastinal space for formation of the pseudo-fornix to stabilise the His angle. Two expert ESSQS-qualified surgeons (I.U. and K.S.) supervised these investigations.
Measurements
Data were obtained by review of our prospectively maintained database. The primary outcome of this single-centre retrospective analysis was the incidence of gastroesophageal reflux disease LA grade B or severer at one year postoperatively. Clinicopathological characteristics and surgical outcomes, including operative time, estimated blood loss, LN dissection extent, number of dissected lymph nodes, rate of conversion to laparotomy, mortality rate, complication rate within 30 days and over 30 days after surgery, length of postoperative hospitalisation and symptoms at one year postoperatively, were evaluated as secondary endpoints. All postoperative complications of grade IIIa or higher based on the Clavien–Dindo classification were recorded [26] and were classified in accordance with the Japan Clinical Oncology Group Postoperative Complication Criteria [27]. The total operative time was defined as the time from the first abdominal incision until complete wound closure. Blood loss was estimated by weighing suctioned blood and gauze pieces with absorbed blood. The longitudinal length of the resected oesophagus was measured from intraoperative videos and resected specimens.
Statistical analyses
All analyses were performed using IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY, USA). Between-group comparisons were performed using the χ2 test for categorial variables or the Mann–Whitney U test for continuous variables. Univariate analysis using the χ2 test was performed, followed by multivariate logistic regression of factors with a P value of < 0.05 during univariate analysis, to identify factors contributing to the occurrence of reflux oesophagitis and postoperative anastomotic stricture. The median value was used to define the cutoff values for each factor during univariate and multivariate analyses. There were more than seven events per confounder variable to avoid the problem of overfitting. Data were expressed as medians with ranges or odds ratios (OR) with 95% confidence intervals unless otherwise noted. Two-tailed P-values < 0.05 were considered statistically significant.
Results
Clinicopathological features and surgical outcomes after minimally invasive proximal gastrectomy, followed by VEG-DFT
Table 1 shows the patient characteristics and surgical outcomes for the entire cohort of this study and the comparison of robotic and laparoscopic procedures. In this cohort, 56 patients have comorbid conditions; hypertension in 28 (35%), diabetes mellitus in 23 (28.7%), hyperlipidemia in 12 (15%), ischemic heart disease in 11 (13.7%), arrythmia in 6 (7.5%), pulmonary disease in 8 (10%), and cerebrovascular disease in 5 (6.2%). Five (6.2%) patients with preoperative serum albumin levels < 3.5 g/dl received preoperative nutritional support from the nutritional support team. All patients completed R0 resection. There were no statistically significant differences in clinicopathological features between the two groups, including the longitudinal length of the resected oesophagus (entire cohort, 20 [0–80] mm; robotic vs. laparoscopic, 20 [0–60] vs. 20 [0–40], P = 0.074; Fig. 4). The total operative time in the robotic group was significantly higher (448 [285–736] vs. 358 [284–566], P = 0.001), whereas there were no significant differences in other surgical outcomes (Table 1), including early and late complications (Table 2). The incidence of LA grade B or higher reflux oesophagitis 1 year after the surgery was 10%. Univariate and multivariate analyses were performed to determine the risk factors for LA grade B or higher reflux oesophagitis 1 year after the surgery. The resected oesophagus of > 20 mm was the only significant risk factor for LA grade B or higher reflux oesophagitis 1 year after the surgery (OR = 11.000, 1.284–94.263; P = 0.011, Table 3). Accordingly, we divided patients into two groups according to a cutoff value for the longitudinal length of the resected oesophagus of 20 mm. Among the 80 patients, 45 and 35 were assigned to the short longitudinal length of resected oesophagus (0–20 mm, group-S) and long longitudinal length of resected oesophagus (> 20 mm, group-L) groups, respectively. Although no differences in age, sex, BMI, comorbidity, ASA grade, tumour size or surgical procedure approach were observed between group-S and group-L, significant differences were observed in history of laparotomy, use of neoadjuvant chemotherapy, tumour location, and clinical and pathological stage (Table 1). Group-L had a significantly greater total operative time (group-S, 370 [284–663] min; group-L, 491 [307–736] min; P < 0.001) and a greater rate of complications within 30 days of surgery (group-S, 0%; group-L, 11.4%; P = 0.033; Tables 1, 2). No significant differences were observed in estimated blood loss, number of dissected lymph nodes, reoperation rate or length of postoperative hospital stay (Table 1). No patients required conversion to an open procedure, and no mortality within 30 days after surgery was observed. There were no significant differences in the rate of complications within 30 days of surgery, including anastomotic stricture, between the two groups (Table 2).
Endoscopic findings and clinical symptoms at one year postoperatively
The results of endoscopic evaluations and clinical symptoms at one year postoperatively are shown in Table 4. Eight (10%) patients had a grade B or higher reflux oesophagitis. The incidence of grade B or higher reflux oesophagitis in the robotic group was significantly higher than in the laparoscopic group (10% vs. 0%; P = 0.011). Furthermore, the incidence of LA grade B or higher reflux oesophagitis in group-L was significantly higher than in group-S (20 vs. 2.2%; P = 0.011). The rate of PPI use (group-S, 22.2%; group-L, 48.5%; P = 0.013) and symptoms related to reflux oesophagitis such as acid swallowing and heartburn (group-S, 4.4%; group-L, 22.8%; P = 0.016) were significantly higher in group-L than in group-S. In addition, body weight loss was significantly greater in group-L than in group-S (group-S, 11.0%; group-L, 13.0%; P = 0.006, Table 4). A total of 21 (26.2%) patients underwent endoscopic dilation due to an anastomotic stricture in the present study. Anastomotic stricture occurred at a median interval of 60 (34–112) days after the surgery. Most patients required repeated endoscopic dilation, with a median of two dilation procedures (1–8, Table 4). There were no significant differences in LA grade B or higher reflux oesophagitis, PPI use, and symptoms related to reflux oesophagitis after endoscopic dilation between the robotic and laparoscopic groups and between group-S and group-L (Table 4). On the other hand, body weight loss did not differ significantly between group-S and group-L, but it was significantly lower in the robotic group than in the laparoscopic group (Table 4). Among patients who did not develop anastomotic stricture, LA grade B or higher reflux oesophagitis was significantly higher in the robotic group than in the laparoscopic group, whereas there were no significant differences in PPI use, symptoms related to reflux oesophagitis, and body weight loss (Table 4). PPIs and body weight loss, on the other hand, were significantly higher in group-L than in group-S (Table 4). There was a trend toward higher rates of LA grade ≥ B reflux oesophagitis and symptoms related to reflux oesophagitis in group-L compared to group-S; however, this difference did not achieve statistical significance (Table 4). In terms of adjuvant chemotherapy, a total of 15 patients received it, with three receiving the SP regimen, six receiving the SOX regimen, six receiving S1 alone, and two receiving others. There was no significant difference in the rate of LA grade B reflux esophagitis between patients who did (3/15; 20%) and did not (5/65; 7.6%) receive adjuvant chemotherapy (P = 0.166).
Surgical risk factors for anastomotic stricture
The incidence of anastomotic stricture was significantly higher in each operator’s initial five procedures performed than in subsequent procedures (initial five procedures, 41.6%; subsequent procedures, 13.6%; P = 0.005). Univariate analysis identified three significant risk factors for anastomotic stricture, including male, ASA score 2 or higher, and initial five cases of each operator. Multivariate analysis demonstrated that the initial five cases of each operator were a significant independent risk factor for anastomotic stricture (OR 4.388, 1.325–14.529; P = 0.015; Table 5).
Surgical risk factors for reflux oesophagitis
To identify surgical risk factors for LA grade B or higher reflux oesophagitis, we performed univariate analysis of factors related to patient background and surgical procedure in group-L (Table 4). Univariate and multivariate analyses identified incomplete pseudo-fornix formation as the only significant risk factor for LA grade ≥ B reflux oesophagitis (OR, 20.833; CI, 2.735–158.715; P = 0.003; Table 6).
Discussion
The results of this retrospective, single-centre study examined the safety and anti-reflux effect of minimally invasive PG followed by VEG-DFT in 80 patients. The rates of early complications and LA grade B or higher reflux oesophagitis were 5% and 10%, respectively. These findings appear comparable with the previous studies [4, 5, 28]. Therefore, we consider that minimally invasive PG followed by VEG-DFT could be performed safely and effectively for reflux oesophagitis. In contrast, the anastomotic stricture rate was 26.3%, suggesting that it was higher than in previous studies [4, 5, 28]. Therefore, this is still a problem to be solved. The present study has four major findings.
First, limited to patients with resection length of the oesophagus ≤ 20 mm, the incidence of LA grade ≥ B reflux oesophagitis was only 2.2%, irrespective of the presence or absence of anastomotic stricture. This finding indicates that the clinical efficacy of minimally invasive PG followed by VEG-DFT to prevent reflux oesophagitis was excellent when limited to patients who required resection of ≤ 20 mm of the oesophagus. These finding appears to corroborate a previous multicentre retrospective study reported by Kuroda et al. that observed LA grade ≥ B reflux oesophagitis in 6.0% (28/464) of patients [4], and the combined rate of LA grade ≥ B reflux oesophagitis among all previous DFT studies is reportedly 2.7% (3/112) [4]. Simple EG without additional anti-reflux procedures has been reported to cause reflux oesophagitis in 9.1%–35.3% of patients [29]. The efficacy of other EG reconstruction procedures with additional fundoplication has been limited or insufficient [30,31,32,33]. In addition, the incidence of reflux oesophagitis following jejunal interposition, jejunal pouch interposition and double tract method is reportedly 0%–33.3%, 4%–27.8% and 0%–25%, respectively [29, 34], which appears to be considerable. Accordingly, we believe that the anti-reflux effect of VEG-DFT is highly reliable in patients undergoing PG with oesophageal resection of ≤ 20 mm.
Second, in patients with resection of > 20 mm of the oesophagus, incidence of LA grade ≥ B reflux oesophagitis at one year postoperatively was considerable at 20%. This finding corroborates the results of a previous multicentre retrospective study that reported an anastomotic site located in the mediastinum or intra-thorax was an independent risk factor for reflux oesophagitis [4]. When the resected oesophagus was over 20 mm in longitudinal length, the need for intra-mediastinal anastomosis or insertion of the remnant stomach into the narrow intra-mediastinal space may increase. However, retrospective video review to identify surgical risk factors for reflux oesophagitis indicated that pseudo-fornix formation to stabilise the His angle was incompletely performed in patients with LA grade ≥ B reflux oesophagitis due to failure to insert a sufficient volume of the remnant stomach into the opened intra-mediastinal space. The main mechanism by which VEG-DFT prevents reflux oesophagitis is that the anterior side of the anastomotic site is fully covered by the seromuscular double flap under intragastric pressure to create a pressure gradient between the oesophagus and stomach, thereby acting as a one-way valve [13]. In cases where creation of a pseudo-fornix is incomplete, the optimal intragastric pressure may not be achieved, thereby reducing the clinical efficacy of VEG-DFT in preventing reflux oesophagitis. In addition, the negative intra-thoracic pressure may further worsen the regurgitation of gastric secretions into the thoracic oesophagus. In this study, we may not have paid enough attention to the lower mediastinal space suitable for insertion of the sufficient volume of the remnant stomach to form the pseudo-fornix, especially in the craniocaudal direction, while we opened the hiatus based on the width of the short axis of the remnant stomach. We consider that this might lead to a relatively high incidence of reflux oesophagitis in Group-L. Therefore, we consider that it’s crucial to both widely open the hiatus and slightly more extensively mobilize the dorsal part of the lower esophagus in the direction of the head so that enough remnant stomach can be introduced to construct the pseudo-fornix during intra-mediastinal VEG-DFT. Further, we would like to continue working on improving the anti-reflux mechanisms of intra-mediastinal VEG-DFT.
Third, anastomotic stricture occurred in 26.3% of the patients examined in this study, which is greater than the previous study, indicating an 8.3% (12/147) incidence of anastomotic stricture after laparoscopic VEG-DFT [5]. In the present study, the incidence of anastomotic stricture was significantly higher in the first five cases performed by each operator. This was the only significant risk factor for anastomotic stricture. Previously, we reported that six cases are required to achieve a learning plateau for VEG-DFT; however, this finding was limited to robotic surgery [13]. Therefore, we posit that the number of procedures performed by an individual operator may be a risk factor for anastomotic stricture. On the other hand, anastomotic leakage occurred in only one (1.3%) patient in the present study. In addition, we previously reported that a greater number of stitches were used in patients with anastomotic stricture than in patients who did not develop anastomotic stricture [13]. Therefore, we postulate that inexperienced operators tend to perform hand-sewn anastomosis too tightly with an excessive number of stitches. The anastomotic orifice ratio of the oesophagus/stomach may also contribute to anastomotic stricture. The reason is that the ratio ≥ 1 tended to be higher in the anastomotic stricture group (OR: 5.106, P = 0.087), although not significantly different. As a result, we hypothesize that making the anastomotic aperture in the stomach larger than the one in the oesophagus may avoid anastomotic stricture. To determine the anastomotic stricture’s technical weakness, more research is necessary. Conversely, the severity of reflux oesophagitis was not worsened even after repeated balloon dilation procedures in either group in the present study. Hence, endoscopic balloon dilatation may preserve the anti-reflux mechanism created by the seromuscular double flap and intragastric pressure.
Fourth, the rate of early complications was significantly higher (11.2% vs. 0%) and operative duration was significantly longer in group-L compared to group-S. We believe this difference is predominantly attributable to an increased requirement for trans-hiatal procedures during both dissection and reconstruction among patients in group-L. Trans-hiatal procedures are considered to be technically demanding, predominantly due to an inadequate operative view, narrow working space and risk of injury to important visceral organs including the aorta, inferior vena cava, pericardium and pulmonary veins during dissection. In fact, a previous prospective, nationwide, multicentre study reported the rate of grade-III or higher complications following trans-hiatal gastrectomy for EGJ cancers was 18.7% [35], although approximately 90% of patients underwent open surgery in this study. Although it has not been clarified whether dissection or reconstruction procedures are most associated with the risk of complications, at least three out of four (75%) early complications in group-L appeared to be associated with reconstruction procedures in the present study. Accordingly, when trans-hiatal VEG-DFT is performed, we consider it to be important to enhance the precision of each procedure and avoid adjacent organ injury by securing a wide and stable operative view and widening the working space, thereby decreasing the risk of complications.
The present study had several limitations that need consideration. First, the present study employed a single-centre, retrospective, small-scaled, and non-randomised design. Therefore, several sources of patient bias, particularly patient selection bias, could not be excluded. This study included laparoscopic and robotic procedures, and there were significant differences in patient characteristics, particularly tumour location. In contrast to the previous report [36], the advantages of robotic procedures for technically demanding procedures are unknown despite comparing clinical outcomes between these two procedures in this study. In addition, the robotic group had a higher rate of LA grade B or higher than the laparoscopic group. We speculate on two possible reasons. First, as shown in Fig. 4, robotic surgery was more favourably performed in patients who required a greater longitudinal length of the resected esophagus. Second, more working space is required for laparoscopic surgery to compensate for its ergonomic limitations when performing hand-sawn anastomosis with straight forceps with a limited range of motion. As a result, laparoscopic surgery may have succeeded in creating enough space to insert a sufficient volume of the remnant stomach to form the pseudo-fornix, resulting in a reduced incidence of reflux oesophagitis. However, we were unable to perform multivariate analysis using the robotic versus laparoscopic factor because no patients in the laparoscopic group had grade B or higher reflux oesophagitis. This is an important limitation. Further studies, including prospective trials and large-scaled studies, are required to determine the impact of robotic surgery in this procedure. Second, this study may have included operator bias. In particular, all procedures were performed by ESSQS-qualified surgeons with extensive experience of laparoscopic gastrectomy. In addition, this procedure is considered to be technically demanding. Therefore, the safety and anti-reflux efficacy of minimally invasive VEG-DFT performed by non-ESSQS-qualified surgeons or inexperienced surgeons have yet to be demonstrated. A large-scale study is required to determine the association between surgical experience and the anti-reflux efficacy of VEG-DFT. Third, we did not compare VEG-DFT with other reconstruction techniques after PG, such as double tract reconstruction [37] and SOFY [14], as we performed other reconstruction procedures for initial experience only. Thus, further studies comprising a larger number of patients and prospective randomised controlled studies comparing VEG-DFT with other procedures are required to confirm the advantages of VEG-DFT. Fourth, the focus of this study was on the incidence of postoperative reflux oesophagitis, which we evaluated based on endoscopic findings, patient symptoms, and use or nonuse of PPIs. We did not, however, perform oesophageal manometry or pH monitoring, which are important objective indicators of reflux oesophagitis.
In conclusion, minimally invasive PG followed by VEG-DFT represents a promising procedure with efficacy in preventing reflux oesophagitis in patients with oesophageal resection of 20 mm or less. In contrast, the clinical efficacy of VEG-DFT in preventing reflux oesophagitis in patients requiring oesophageal resection of more than 20 mm remains unclear. The confirmation of complete pseudo-fornix formation may improve the anti-reflux efficacy of VEG-DFT.
References
Japanese Gastric Cancer Association (2021) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 24:1–21.
Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T et al (2008) Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg 32(7):1473–1477
Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S et al (2016) Double flap technique as an anti-reflux procedure in esophagogastrostomy after proximal gastrectomy. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2016.04.041
Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A et al (2019) Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann Gastroenterol Surg 3(1):96–103
Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T et al (2019) Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer 22(5):1036–1043
Saze Z, Kase K, Nakano H, Yamauchi N, Kaneta A, Watanabe Y et al (2021) Functional benefits of the double flap technique after proximal gastrectomy for gastric cancer. BMC Surg 21(1):392
Hosoda K, Yamashita K, Moriya H, Mieno H, Ema A, Washio M et al (2017) Laparoscopically assisted proximal gastrectomy with esophagogastrostomy using a novel “Open-Door” technique : LAPG with novel reconstruction. J gastrointest surg 21(7):1174–1180
Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T et al (2019) Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann surg. https://doi.org/10.2139/ssrn.3321496
Uyama I, Suda K, Satoh S (2013) Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J gastric cancer 13(1):19–25
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K et al (2020) Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: a single-center, retrospective and propensity score-matched analysis. World J Gastroenterol 26(11):1172–1184
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J et al (2013) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27(1):286–294
Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I (2016) Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc 30(10):4632–4639
Shibasaki S, Suda K, Nakauchi M, Kikuchi K, Kadoya S, Ishida Y et al (2017) Robotic valvuloplastic esophagogastrostomy using double flap technique following proximal gastrectomy: technical aspects and short-term outcomes. Surg Endosc 31(10):4283–4297
Yamashita Y, Tatsubayashi T, Okumura K, Miyamoto T, Ueno K (2022) Modified side overlap esophagogastrostomy after laparoscopic proximal gastrectomy. Ann Gastroenterol Surg 6(4):594–599
Nakauchi M, Suda K, Shibasaki S, Nakamura K, Kadoya S, Kikuchi K et al (2021) Prognostic factors of minimally invasive surgery for gastric cancer: does robotic gastrectomy bring oncological benefit? World J Gastroenterol 27(39):6659–6672
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 20(1):1–19
Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S (2012) Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 36(2):331–337
Suzuki K, Shibasaki S, Nakauchi M, Nakamura K, Akimoto S, Tanaka T et al (2021) Impact of routine preoperative sonographic screening with early intervention for deep venous thrombosis in lower extremities on preventing postoperative venous thromboembolism in patients with gastric cancer scheduled for minimally invasive surgery. Langenbeck’s arch surg. https://doi.org/10.1007/s00423-021-02315-5
Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I (2015) Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 29(3):673–685
Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S et al (2019) Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer 22(2):377–385
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Tanaka T, Kikuchi K et al (2021) Impact of the endoscopic surgical skill qualification system on the safety of laparoscopic gastrectomy for gastric cancer. Surg Endosc 35(11):6089–6100
Shibasaki S, Suda K, Kadoya S, Ishida Y, Nakauchi M, Nakamura K et al (2022) The safe performance of robotic gastrectomy by second-generation surgeons meeting the operating surgeon’s criteria in the Japan society for endoscopic surgery guidelines. Asian j endoscop surg 15(1):70–81
Nakamura K, Suda K, Shibasaki S, Nakauchi M, Kikuchi K, Inaba K et al (2020) The hepatic left lateral segment inverting method offering a wider operative field of view during laparoscopic proximal gastrectomy. J gastrointest surg 24(10):2395–2403
Nakauchi M, Suda K, Nakamura K, Shibasaki S, Kikuchi K, Nakamura T et al (2017) Laparoscopic subtotal gastrectomy for advanced gastric cancer: technical aspects and surgical, nutritional and oncological outcomes. Surg Endosc 31(11):4631–4640
Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP et al (1999) Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los angeles classification. Gut 45(2):172–180
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N et al (2016) Extended Clavien–Dindo classification of surgical complications: Japan clinical oncology group postoperative complications criteria. Surg Today 46(6):668–685
Omori T, Yamamoto K, Yanagimoto Y, Shinno N, Sugimura K, Takahashi H et al (2021) A Novel Valvuloplastic esophagogastrostomy technique for laparoscopic transhiatal lower esophagectomy and proximal gastrectomy for Siewert type II esophagogastric junction carcinoma-the tri double-flap hybrid method. J gastrointest surg 25(1):16–27
Nakamura M, Yamaue H (2015) Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg today. https://doi.org/10.1007/s00595-015-1185-4
Okabe H, Obama K, Tanaka E, Tsunoda S, Akagami M, Sakai Y (2013) Laparoscopic proximal gastrectomy with a hand-sewn esophago-gastric anastomosis using a knifeless endoscopic linear stapler. Gastric Cancer 16(2):268–274
Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K et al (2014) Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery 156(1):57–63
Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H et al (2009) Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 209(3):344–351
Yasuda A, Yasuda T, Imamoto H, Kato H, Nishiki K, Iwama M et al (2015) A newly modified esophagogastrostomy with a reliable angle of his by placing a gastric tube in the lower mediastinum in laparoscopy-assisted proximal gastrectomy. Gastric Cancer 18(4):850–858
Nunobe S, Ida S (2020) Current status of proximal gastrectomy for gastric and esophagogastric junctional cancer: a review. Ann Gastroenterol Surg 4(5):498–504
Mine S, Kurokawa Y, Takeuchi H, Terashima M, Yasuda T, Yoshida K et al (2022) Postoperative complications after a transthoracic esophagectomy or a transhiatal gastrectomy in patients with esophagogastric junctional cancers: a prospective nationwide multicenter study. Gastric Cancer 25(2):430–437
Shibasaki S, Nakauchi M, Serizawa A, Nakamura K, Akimoto S, Tanaka T et al (2022) Clinical advantage of standardized robotic total gastrectomy for gastric cancer: a single-center retrospective cohort study using propensity-score matching analysis. Gastric Cancer 25(4):804–816
Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N et al (2019) Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan clinical oncology group study JCOG1401. Gastric Cancer 22(5):999–1008
Acknowledgements
The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for English language editing.
Author information
Authors and Affiliations
Contributions
All authors have fully met the ICMJE authorship criteria and contributed to the present manuscript as follows: study design, KM, SS, IU and KS; data collection, KM, SS, KS, AS, MN and TT; statistical analysis and interpretation of results, KM, SS, MN, KI and KS; drafting of the manuscript, KM, SS and KS; critical revision of the manuscript for important intellectual content, SS, IU and KS. All authors read and approved the final manuscript. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosures
Kazuhiro Matsuo, Susumu Shibasaki, Kazumitsu Suzuki, Akiko Serizawa, Shingo Akimoto, Masaya Nakauchi, Tsuyoshi Tanaka, Kazuki Inaba, Ichiro Uyama and Koichi Suda have no commercial association with or financial involvement that might be construed as a conflict of interest in connection with the submitted article. Ichiro Uyama has received lecture fees from Intuitive Surgical, Inc. outside of the submitted work. Tsuyoshi Tanaka and Ichiro Uyama have been funded by Medicaroid, Inc. in relation to the Collaborative Laboratory for Research and Development in Advanced Surgical Technology, Fujita Health University. Koichi Suda has been funded by Sysmex Corp. in relation to the Collaborative Laboratory for Research and Development in Advanced Surgical Intelligence, Fujita Health University and has also received advisory fees from Medicaroid, Inc. outside of the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuo, K., Shibasaki, S., Suzuki, K. et al. Efficacy of minimally invasive proximal gastrectomy followed by valvuloplastic esophagogastrostomy using the double flap technique in preventing reflux oesophagitis. Surg Endosc 37, 3478–3491 (2023). https://doi.org/10.1007/s00464-022-09840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09840-4