Abstract
Laparoscopy-assisted proximal gastrectomy (LAPG) with esophagogastrostomy using a novel “open-door” technique was introduced recently, with the aim of preventing gastroesophageal reflux. However, quantitate assessment of gastroesophageal reflux after this surgery has not been performed till date. The aims of the current study were to investigate the safety and feasibility of this operation and to elucidate the postoperative reflux status. Twenty consecutive patients (18 men) with (y)cStage I gastric cancer in the upper third of the stomach who underwent LAPG at Kitasato University Hospital from May 2015 through September 2016 were retrospectively reviewed. We performed 24-h impedance-pH monitoring 3 months after surgery for the first eight patients and analyzed the postoperative reflux status. Median operation time was 333 min, while median anastomotic time was 81 min. None of the 20 patients experienced anastomotic leakage while two patients experienced anastomotic stricture requiring endoscopic balloon dilatation. No patient experienced heartburn without antacid drugs. During the 24-h impedance-pH monitoring, all but one patient had normal gastroesophageal acid reflux with the acid percent time of <1.1% and reflux percent time of <1.4%. One patient with marginally abnormal postoperative gastroesophageal reflux had a normal DeMeester score of 3.0. Our results showed that esophagogastrostomy using the “open-door” technique is a safe and feasible procedure for LAPG. The degree of gastroesophageal reflux was acceptable using this technique. Randomized controlled trials with long-term follow-ups are required to confirm that this technique would be superior to the others.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proximal gastrectomy is recognized as an alternative treatment to total gastrectomy for cT1 upper third gastric cancer that is beyond the indication for endoscopic submucosal dissection.1, 2 There are mainly three reconstruction methods: esophagogastrostomy, double-tract reconstruction, and jejunal interposition. These methods have both advantages and disadvantages, because of which there is no standard reconstruction method for this operation. In Japan, where almost half of the patients with gastric cancer are diagnosed with cT1 cancer,3 laparoscopic surgery is performed for these patients who are also beyond the scope of performing endoscopic submucosal dissection. For patients with upper third cT1 gastric cancer, laparoscopy-assisted proximal gastrectomy (LAPG) is occasionally performed in institutions that are well experienced in laparoscopic surgery. In such cases, esophagogastrostomy is preferred because of its simplicity in anastomosis. However, the procedure of esophagogastrostomy has an intrinsic disadvantage of reflux esophagitis caused by gastroesophageal reflux. In fact, we had sometimes performed esophagogastrostomy without any method of preventing the reflux for technical reasons. Almost all these patients suffered from severe reflux esophagitis graded as D in the Los Angeles classification with standard dose administration of proton pump inhibitors. Therefore, a method for preventing the reflux should be added to this reconstruction procedure. We have actively performed LAPG for cT1 upper third gastric cancer.4, 5 Furthermore, we previously reported that, although the incidence rate of anastomotic stricture was higher in LAPG, it might be superior to LATG in terms of postoperative nutritional status such as hemoglobin levels.5 Thus, LAPG may be acceptable if anastomotic stricture can be circumvented without the increase in gastroesophageal reflux.
More recently, Kamikawa procedure or the “open-door” technique was introduced and performed at certain institutions for esophagogastrostomy during LAPG.6, 7 It is technically challenging, but gastroesophageal reflux was reported to be nearly non-existent after this operation. However, functional analysis of gastroesophageal reflux is yet to be adequately performed using multichannel intraluminal impedance-pH testing after this operation.
Therefore, we aimed to investigate the safety and feasibility of this operation as well as elucidate the postoperative reflux status.
Methods
This study was conducted in accordance with the 1995 Declaration of Helsinki (as revised in Edinburgh 2000) and was approved by the of Kitasato University School of Medicine Research Ethics Committee. The requirement for informed consent was waived because of the study’s retrospective design.
Patients
Twenty consecutive patients (18 men) underwent LAPG at Kitasato University Hospital from May 2015 through September 2016. All patients were diagnosed with (y)cStage I gastric cancer in the upper third of their stomach. The diagnosis was based on preoperative examinations including endoscopy, barium swallow, and computed tomography. All operations were performed by KH or HM (qualified surgeons, certified by Japanese society of endoscopic surgery).
Surgical Techniques
Proximal Gastrectomy
A 12-mm umbilical camera port was inserted. The abdominal cavity was insufflated with carbon dioxide to maintain an intra-abdominal pressure of 8 mmHg. A flexible 3D fiber optic laparoscope with a 10-mm tip (OLYMPUS ENDOEYE FLEX 3D; Olympus Optical Co., Ltd., Tokyo, Japan) was inserted through this port. A suture placed around the falciform ligament was pulled out of the abdomen to elevate the liver. Trocars of size 12 mm were placed in the upper-left side and lower-right side of the abdomen, while 5-mm trocars were placed in the lower-left side and upper-right side of the abdomen. The Nathanson liver retractor was placed to elevate the left lobe of liver (Fig. 1).
The extent of lymph node dissection was determined according to the 2010 Japanese gastric cancer treatment guidelines.8 The lymph nodes along the greater and lesser curvatures of the stomach (the right pericardial lymph nodes, left pericardial lymph nodes, lymph nodes along the lesser curvature, lymph nodes along the short gastric vessels, and the lymph nodes along the left gastroepiploic vessels) were dissected. In addition, the lymph nodes along the left gastric artery (No. 7), along the common hepatic artery (No. 8a), around the celiac artery (No. 9), and along the proximal splenic artery (No. 11p) were dissected to complete D1+ dissection.
After completing lymph node dissection, the esophagus was transected with a linear stapler. A 4-cm mini-laparotomy was performed in the umbilicus to remove the stomach. The clips on the distal side of lesion were palpated, and the stomach was transected perpendicularly to its long axis at about 2–3 cm from the clips.
Esophagogastrostomy
Creating a “double flap” on a remnant stomach (Video 1)
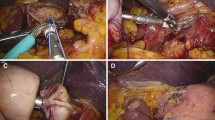
After removal of the tumor, “double door” seromuscular flaps were created at the anterior wall of the remnant stomach. The dimensions of the “window” were 2.5 cm × 3.5 cm (width × height) (Fig. 2a). The superior end of the mucosal “window” was positioned 3 cm below the top of the remnant stomach. Using an electric cautery, the submucosal layer was carefully detached exposing the mucosa, which resulted in creation of the seromuscular flaps (Fig. 2b).
Creation of “double door” seromuscular flaps on the anterior wall of the remnant stomach. The dimensions of the “window” are 2.5 cm × 3.5 cm (width × height) and the superior end of mucosal “window” is positioned 3 cm below the top of the remnant stomach (a). The seromuscular flaps were created after dissection of the submucosal layer (b)
Anastomosis (Video 2)
At first, the remnant stomach was pulled up to the esophageal hiatus to be positioned posteriorly to the esophagus. The superior end of mucosal “window” was fixed to the esophagus at 4 cm above the cut end using four stitches. Second, the staple line of resected esophagus was removed to open the esophageal orifice following which; inferior end of the mucosal “window” was opened. Third, continuous suturing was performed between the posterior wall of the esophagus and superior opening of mucosa on the remnant stomach using V-Loc™ 180 (Covidien Japan Co., Ltd., Tokyo, Japan) (Fig. 3a). For the suturing, we used a CLICKline® KOH macro needle holder with axial handle in length of 33 cm (26173KAL) (Karl Storz Co. Ltd., Tuttlingen, Germany) and a CLICKline® KELLY Dissecting and Grasping forceps, long, Maryland in length of 36 cm (Karl Storz Co. Ltd., Tuttlingen, Germany) in right and left hands, respectively. Subsequently, interrupted sutures were placed between the anterior wall of esophageal mucosa and the inferior opening of mucosa on the remnant stomach using 3-0 PDS® II (Ethicon Japan Co., Ltd., Tokyo, Japan). Continuous suturing was performed between muscular layer of the esophagus and seromuscular layer of the stomach using V-Loc™ 180 (Fig. 3b). Finally, the inferior ends on both sides of the seromuscular flaps were fixed at a position 1 cm below the inferior end of the mucosal “window”. Both side ends of the lower third of flaps were sutured upwards. This allowed the anastomosis to be completely covered by flaps. Next, both ends of the flaps were separately sutured to the esophagus to reduce tension between the flaps (Fig. 3c). Pyloroplasty was not added in any of the cases.
Esophagogastrostomy using the “open-door” technique. Continuous suturing between posterior wall of the esophagus and superior opening of the mucosa on the remnant stomach using V-Loc™ 180 (Covidien Japan Co., Ltd., Tokyo, Japan) (a). Continuous suturing between the muscular layer of esophagus and the seromuscular layer of stomach using V-Loc™ 180 (b). Completion of esophagogastrostomy using the “open-door” technique (c)
Postoperative Management
An upper gastrointestinal swallow test was performed on postoperative day 3. Water intake began on postoperative day 2 and oral feeding on postoperative day 3. A proton pump inhibitor (PPI) was administered for 3 months after surgery. The first eight patients underwent 24-h impedance-pH monitoring using ambulatory multichannel intraluminal impedance and a pH monitoring system (Sleuth; Sandhill Scientific, Inc., Highland Ranch, CO, USA) and high resolution manometry using Starlet HRM system (Star Medical, Inc, Tokyo, Japan) at 3 months after the surgery. A DeMeester score9 which is a global measure of esophageal acid exposure was calculated to quantify gastroesophageal reflux after this operation. Administration of PPI was terminated at 2 weeks before the tests. The PPI administration was terminated if no abnormal gastroesophageal acid reflux was detected.
Results
The patients’ characteristics and surgical outcomes are described in Tables 1 and 2, respectively. The median operation time was 333 min, while the median anastomotic time was 81 min. None of the 20 patients experienced anastomotic leakage, while two patients experienced anastomotic stricture requiring endoscopic balloon dilatation. Postoperative contrast media swallow test showed no regurgitation into the esophagus when the patients lay down or even when they were placed in the Trendelenburg position (Video 3). A pseudofornix was formed after the operation via gastric endoscopy (Fig. 4).
Of the eight patients who underwent 24-h impedance-pH monitoring at 3 months after surgery, no patient experienced heartburn, while four experienced sticky feelings at the site of anastomosis. Six patients maintained acid secretion capacity in the stomach (Table 3). A representative waveform of a patient (case 1) is shown in Fig. 5. All but one patient (case 3) had normal gastroesophageal acid reflux with the acid percent time of <1.1% and all reflux percent time of <1.4%. One patient (case 3) who had marginally abnormal, postoperative gastroesophageal reflux had a normal DeMeester score of 3.0. Six months after the surgery, no patient required antacid drugs including PPIs in outpatient clinics. The median pressure at the site of anastomosis was 20.5 mmHg (range, 13.5–36.8 mmHg) which is between the 5th and the 95th percentiles of the resting EGJ pressure of normal subjects.10
Discussion
In this article, we present safety and feasibility of performing LAPG with esophagogastrostomy using the “open-door” technique. Moreover, to the best of our knowledge, we are the first group to demonstrate satisfactory clinical outcomes along with physiological functions using this technique.
Our study shows that esophagogastrostomy using the “open-door” technique for LAPG was safe and feasible. However, the median operation time for this procedure was as long as 333 min. We have previously reported that LAPG with esophagogastrostomy using OrVil™ had a median operation time of 280 min, which is significantly shorter to the “open-door technique (P = 0.002, data not shown). If surgeons who participated in this study had been more proficient in laparoscopic suturing, operation time would not have been so long. Improving surgeon’s skill in suturing could overcome this drawback.
This procedure is not so easy for beginners in laparoscopic surgery to perform properly. In this study, all surgeries were performed by two qualified surgeons who were certified by endoscopic surgical skill qualification system conducted by Japan Society for Endoscopic Surgery.11 To become the qualified surgeon, sufficient technique for suturing and knot tying is mandatory. This surgery is not so difficult to perform by surgeons who have the same level of skills as the qualified surgeons. Indeed, we did not perform this surgery before the 20 patients.
There are some portions to be difficult to learn. First, when raising the seromuscular flaps, we might put a hole in the gastric mucosa. To avoid this, we injected normal saline into submucosal layer to raise the seromuscular flaps safely. Secondly, when performing continuous suturing between the posterior wall of the esophagus and superior opening of mucosa on the remnant stomach, it is difficult to confirm a muscular layer of the posterior wall of the esophagus. To suture the whole layer of the esophagus properly during the continuous suturing, we put one stitch at the middle of the posterior cut line of the esophagus to put esophageal mucosal and muscular layers together. That could prevent us from suturing between only the mucosal layers of the esophagus and the stomach during continuous suturing.
We performed most of the anastomosis using running sutures with V-Loc™ 180. These sutures did not require ligation, which contributed to saving the anastomotic time. On the other hand, the more one pulls the thread, the shorter the suture line becomes, which may result in the anastomotic stricture. Indeed, two patients (10%) required endoscopic balloon dilatation and four patients complained of sticky feelings at the site of anastomosis at 3 months after the surgery. Esophagogastrostomy in the proximal gastrectomy sometimes induces anastomotic strictures. We have reported that the stricture rate in esophagogastrostomy using OrVil™ is as high as 28%.5 We speculated that if the disadvantage of anastomotic stricture in LAPG could be overcome, LAPG would be a superior method compared to LATG. The “open-door” technique might make it possible for LAPG to be performed with a lesser rate of anastomotic stricture. Therefore, we believe that LAPG with esophagogastrostomy using the “open-door” technique could become the standard treatment for cT1N0 upper third gastric cancer.
In Japan, standard gastrectomy is recommended for gastric cancer staged as more advanced than cT1N0 (cStage IA).8 Standard gastrectomy involves resection of at least two thirds of the stomach, that is, distal gastrectomy or total gastrectomy, with a D2 lymph node dissection. For upper third gastric cancer, standard gastrectomy is total gastrectomy, whereas proximal gastrectomy is not recommended for gastric cancer staged as more advanced than cT1N0. Therefore, we applied this surgery only for patients with cT1N0.
The “open-door” technique in open proximal gastrectomy has been performed at a few institutions in Japan. With recent advancements in laparoscopic surgery, gastrectomy has been increasingly performed laparoscopically and reported to be safe and feasible.12 However, there has been no report on postoperative physiological functions including gastroesophageal reflux. Herein, we report satisfactory physiological function after LAPG with the “open-door” technique for the first time. The reason behind prevention of the reflux may be that the seromuscular double flaps together with the new fundus (pseudofornix, Fig. 4) of remnant stomach and the residual food could shut the anastomotic orifice and work as a backflow preventer, particularly when patients lie down. We believe that this technique will prevail in institutions specialized in laparoscopic gastrectomy.
Our study has several limitations. First, this is a retrospective single-center study with short time follow-up, and number of recruited patients is as small as 20 and only 8 patients underwent 24-h impedance-pH monitoring test. Secondly, although short-term outcomes of the “open-door” technique were fair, the long-term outcomes, in particular the gastroesophageal reflux are unknown. Thirdly, this surgery was performed for the Japanese patients who had relatively small and thin physique. In western countries, where most of the patients are large and obese, the esophagus could not be brought sufficiently into the abdomen. Indeed, we were unable to bring the esophagus into the abdomen sufficiently for one relatively obese patient. For this patient, we opened esophageal hiatus widely by dissecting the left and right crus of the diaphragm and performed anastomosis at the mediastinum. Though the operation took a long time of 614 min, we were able to perform this technique successfully. Therefore, skilled surgeons could perform this technique successfully in western countries.
Conclusion
In conclusion, though esophagogastrostomy using the “open-door” technique takes a longer time, it is a safe and feasible procedure for LAPG. The degree of gastroesophageal reflux was acceptable in this technique. We believe that this anastomotic technique is promising for performing LAPG with esophagogastrostomy. Randomized controlled trials with long-term follow-up are required to confirm the superiority of this technique over others.
References
Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002;26(9):1150-1154.
Furukawa H, Hiratsuka M, Imaoka S, Ishikawa O, Kabuto T, Sasaki Y et al. Limited surgery for early gastric cancer in cardia. Ann Surg Oncol. 1998;5(4):338-341.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1-27.
Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg. 2009;209(3):344-351.
Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Shibata T et al. Potential benefits of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy for cT1 upper-third gastric cancer. Surg Endosc. 2015.
Kamikawa YKT, Kamiyama S, Satomoto K. A new procedure of esophagogastrostomy to prevent reflux following proximal gastrectomy (in Japanese). Shoukakigeka. 2001;24:1053–1060.
Nisizaki MKS, Matsumura T, Takashima H, Katoh H, Kikuchi S, Kuwada K, Kagawa S, Fujiwara T. Valvuloplastic esophagogastrostomy using double flap technique after proximal gastrectomy. Rinsho Geka. 2014;69:1464–1471.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–123.
Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62(4):325-332.
Kuribayashi S, Iwakiri K, Kawada A, Kawami N, Hoshino S, Takenouchi N et al. Variant parameter values-as defined by the Chicago Criteria-produced by ManoScan and a new system with Unisensor catheter. Neurogastroenterol Motil. 2015;27(2):188-194.
Tanigawa N, Lee SW, Kimura T, Mori T, Uyama I, Nomura E et al. The Endoscopic Surgical Skill Qualification System for gastric surgery in Japan. Asian J Endosc Surg. 2011;4(3):112-115.
Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg. 2016.
Authors’ Contributions
All authors have made substantial contributions to the design and analysis of this work as well as drafting and appraisal of the manuscript. All authors have approved the final manuscript for submission and agreed to be accountable for the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kei Hosoda, Keishi Yamashita, Hiromitsu Moriya, Hiroaki Mieno, and Masahiko Watanabe have no conflicts of interest or financial ties to disclose.
Conflicts of Interest and Sources of Funding
None declared
Electronic Supplementary Material
Below is the link to the electronic supplementary matherial
(WMV 76342 kb)
(WMV 89851 kb)
(WMV 6670 kb)
Rights and permissions
About this article
Cite this article
Hosoda, K., Yamashita, K., Moriya, H. et al. Laparoscopically Assisted Proximal Gastrectomy with Esophagogastrostomy Using a Novel “Open-Door” Technique. J Gastrointest Surg 21, 1174–1180 (2017). https://doi.org/10.1007/s11605-016-3341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3341-6