Abstract
Background
Obesity is a risk factor for cholelithiasis. Besides, rapid weight loss after bariatric surgery upsurges the rate of cholelithiasis and acute cholecystitis. This study aimed to compare gallstone development frequency after LSG under ursodeoxycholic acid (UDCA) prophylaxis.
Methods
This prospective controlled study included 332 patients scheduled for LSG randomized to receive 500 mg UDCA daily for 12 months (UDCA Group) or no treatment (Control Group). Ultrasonography was done 6 and 12 months after surgery to detect gallstones. Cholecystectomy was done for complicated cases of cholelithiasis.
Results
Seventy-one patients were lost to follow-up, and 3 developed severe adverse effects of UDCA and excluded. Data are presented for 130 patients in the UDCA group and 128 in the Control group. Collectively, 11 patients (8.5%) of the UDCA group and 41 (32.0%) of the Control group developed gall stones during the first postoperative year (p < 0.001). Cholecystectomy was indicated in 3 patients (2.3%) of the UDCA group and 9 (7.0%) of the Control group (p = 0.072). On multivariate analysis, higher BMI, dyslipidemia, and lacking UDCA prophylaxis were the independent factors significantly associated with stone development. Also, stone development was associated with higher weight loss after 6 and 12 months.
Conclusion
UDCA 500 mg once daily for 12 months after LSG is effective in reducing gallstone formation at 1 year. UDCA administration reduced the frequency of cholecystectomies from 7 to 2.3%. High BMI and dyslipidemia are the independent preoperative factors significantly associated with stone development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The global obesity epidemic continues to advance and grasp more of the world population. It is currently affecting more than 2 billion people [1]. Obesity is a major risk factor for many diseases, particularly diabetes mellitus, cardiovascular disorders, and cancer [2], and the list is expanding. A Mendelian randomization study has demonstrated a causal association between high body mass index (BMI) and gallstone formation [3].
Patients with morbid obesity have a 3–5 times higher risk of gallstone formation [4, 5]. Bariatric surgery is established as an effective and sustainable method of weight loss and improvement of obesity-related diseases [6]. Despite its safety, bariatric procedures may lead to some complications. After surgery, rapid weight loss upsurges the rate of cholelithiasis and acute cholecystitis [4, 7, 8]. Over 10 years after bariatric surgery, gallstone formation was detected in 17.4% of the patients [9]. In a retrospective analysis, the incidence of cholelithiasis and symptomatic gallstones after laparoscopic sleeve gastrectomy (LSG) was 47.9% and 22.9%, respectively [10]. In another retrospective study including 1397 patients subjected to various bariatric procedures, postoperative gallstone development incidence was 8.4% after LSG [8].
Prophylactic laparoscopic cholecystectomy (LC) was suggested to prevent gallstone formation after bariatric surgery. However, this policy is a matter of controversy [11]. The procedure may be technically challenging as the gallbladder may be engulfed by the large liver [12]. The difficulty can be increased if the gallbladder is thickened, fibrosed, and adherent [13]. Worni et al. reported increased morbidity and mortality if bariatric surgery was combined by cholecystectomy [14].
Therefore, drug therapy is the recommended alternative for the prevention of gallstone formation in this situation. A meta-analysis has demonstrated that postoperative administration of ursodeoxycholic acid (UDCA) effectively reduces postoperative gallstone formation after bariatric surgery [15]. This study aimed to compare gallstone development frequency after LSG under ursodeoxycholic acid prophylaxis.
Patients and methods
This prospective controlled study included all patients (n = 332) scheduled for LSG from June 2017 to June 2019. The inclusion criteria were body mass index (BMI) > 35 kg/m2 with at least one comorbidity or BMI > 40 kg/m2 irrespective of comorbidities. Patients with preoperative gallstones and abnormal liver function tests or previous history of cholecystectomy were excluded from the study.
The patients were randomly divided into two groups. The UDCA group received 500 mg ursodeoxycholic acid capsules once daily for 12 months postoperatively. The Control group received the routine follow-up protocol during the first postoperative year. Patient allocation to either arm of the study was done with simple randomization using closed envelopes.
The following data were collected: patients’ characteristics, including age, sex, preoperative weight and BMI, and weight loss after 6 and 12 months. Dyslipidemia was defined as Serum total Cholesterol over 200 mg/dL, TG over 150 mg/dL, LDL-C levels over 130 mg/dL, or HDL-C levels under 40 mg/dL. Ultrasonography (US) for detecting gall stones was done before surgery and 6 and 12 months after surgery for all patients. Our expert radiology and gastroenterology members reviewed ultrasounds. If gallstones were detected at 6 months, the patients were subjected to cholecystectomy if they had biliary colic or developed complications induced by gall stones (acute cholecystitis or obstructive jaundice). Other patients with silent gall stones continued the follow-up for another 6 months.
Surgical technique
A 12-mm optical trocar is placed under direct vision or after insufflation by veres needle, approximately 15 cm below the xiphoid and 3 cm to the left of midline. A 30-degree angled laparoscope is placed through the port into the peritoneal cavity, and two working ports are inserted to the right and the left of the camera port. Next, a 5-mm trocar port is placed below the xiphoid process for liver retraction, and another 5 mm port is placed in the left anterior axillary line for assistance. The pylorus of the stomach is then identified, and the greater curve of the stomach is elevated. An ultrasonic scalpel or LigaSure™ is then used to enter the lesser sac via division of the greater omentum. The stomach’s greater curvature is then dissected free from the omentum and the short gastric blood vessels using the laparoscopic ultrasonic scalpel or LigaSure™. The dissection is started 4 to 6 cm from the pylorus and proceeds to the Angle of His. An endoscopic linear cutting stapler is used to serially staple and transect the stomach staying just to the left and lateral to bougie size 36 French. The staple line is concurrently evaluated for bleeding, and further endoclips were used if necessary. No buttress suture was used for the staple line. We close the fascia of the left flank port site with an absorbable suture by suture passer to prevent bowel herniation but do not close the fascia defects at the remaining port sites.
The primary outcome measure was the frequency of gallstones detected on ultrasonography. The secondary outcome measures were factors associated with gallstone formation after surgery.
Statistical methods
Statistical analysis was done using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, comparison between two groups was made using independent sample t test. Multivariate analysis was done using logistic regression method for the significant factors affecting gallstone formation on univariate analysis. Odds ratio (OR) with 95% confidence interval (CI) were used for risk estimation. A p value < 0.05 was considered significant.
Results
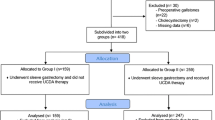
Out of the 332 patients, 71 were lost to follow-up, 33 of the UDCA group and 38 in the control group. Besides, three patients of the UDCA group developed severe adverse effects in the form of allergy and severe gastrointestinal (GI) symptoms and, consequently, were excluded from the study (Fig. 1). The results are displayed for the remaining 258 patients. Patients of the UDCA group (n = 130) were significantly older than the Control group (n = 128, p < 0.001).
Also, the UDCA group’s weight was significantly lower than that of the control group (p = 0.040), but the two groups had a comparable BMI (p = 0.377). Other baseline characteristics are shown in Table 1. After surgery, no mortalities were recorded. Four patients had bleeding, and two developed gastric leaks; both had stenting, and one mandated re-exploration.
Six and 12 months after surgery, the percentage of excess weight loss (%EWL) was significantly higher in the Control group. A significantly higher proportion of the Control group developed gall stones after 6 months (p < 0.001). Three patients of those who developed gallstones in the UDCA group and 9 of the Control group (33.3% and 25.7%, respectively, p = 0.687) were subjected to cholecystectomy (Fig. 2). Few more patients developed gall stones after 12 months in the two groups with no significant difference (p = 0.145). Collectively, 11 patients (8.5%) of the UDCA group and 41 (32.0%) of the Control group developed gall stones during the first postoperative year (p < 0.001). Cholecystectomy was indicated in 3 patients (2.3%) of the UDCA group and 9 (7.0%) of the Control group (p = 0.072) (Table 2).
Table 3 shows a comparison between patients who developed gall stones and others who did not, regarding possible preoperative factors. Gallstone developers were significantly younger (p = 0.025), having higher weight (p = 0.003) and BMI (p < 0.001), and having dyslipidemia (p < 0.001). On multivariate analysis, higher BMI, dyslipidemia, and lacking UDCA prophylaxis were the independent factors significantly associated with stone development (Table 4). Also, stone development was associated with higher weight loss after 6 and 12 months.
Discussion
This study demonstrated that prophylactic use of UDCA in a daily dose of 500 mg for 12 months after LSG significantly reduced the frequency of gallstone formation to 8.5% compared to 32.0% in the Control group. Using UDCA decreased the number of cholecystectomies from 7 to 2.3%. Gallstone formation was associated with younger age, heavier weight, higher BMI, dyslipidemia, and higher weight loss after 6 and 12 months. On multivariate analysis, higher BMI, dyslipidemia, and lacking UDCA prophylaxis were the independent factors significantly associated with stone development.
Gallstone formation is a complex problem in morbidly obese patients. Obesity—per se—is a risk factor for developing gallstones. At the same time, rapid weight loss after bariatric surgery is a known cause of gallstone formation. Gallbladder stones include cholesterol stones and black pigment stones [16]. Cholesterol stones frequently appear after rapid weight loss due to gallbladder hypomotility and cholesterol hypersaturation of bile resulting from reduced bile salt secretion [17]. Also, dividing the hepatic branch of the vagus nerve causes gallstone formation [18].
At present, there is no consensus about gallstone prevention after bariatric procedures. Prophylactic concomitant cholecystectomy was a common practice in the early era of open bariatric surgery. Now, with the development of laparoscopic surgery, most surgeons refrained from this routine [19]. Drug therapy is now recognized as the prophylactic method of choice to prevent gallstone formation.
UDCA for 6 months is recommended to prevent gallstone formation after Roux-en-Y gastric bypass (RYGB) [20, 21]. UDCA is a natural bile acid that constitutes about 5% of the human bile acid pool [22]. It has choleretic, anti-inflammatory, and cytoprotective properties [23]. Oral administration of the drug is used for gallstone dissolution since the 1970s [24]. Dissolution is above 50% in cases of small non-calcified stones, at a dose of 10 mg/kg/day for 12 months [25]. UDCA therapy reduces cholesterol intestinal absorption and secretion into bile [26].
The preventive use of UDCA after SG is not proven yet. Few studied have addressed the role of UDCA in this respect. The present study is the largest prospective study investigating the prophylactic effect of UDCA after LSG. One previous randomized study reported about 30% overall gallstone formation rate. The authors randomized 75 patients to receive a 600 mg daily dose of UDCA or no treatment. UDCA reduced incidence of gallstones after 6 months (p = 0.032), but after 1 year (p = 0.553) [27]. Another study compared the effect of UDCA 500 mg once daily for 6 months in patients subjected to RYGB and SG. It proved effective in both procedures after 1 year [9]. A retrospective Egyptian study compared those who received UCDA for 6 months after LSG with those who did not. Gallstone formation was found in 5% of untreated patients and none of those under UCDA [28]. Another study reported similar conclusions [29]. A more recent study found that 23% of the patients developed cholelithiasis after LSG. UDCA prophylaxis with 500 mg daily reduced the frequency to 10.5% compared to 37.5% in untreated patients [30]. A single prospective randomized study confirmed the effectiveness of UDCA in preventing gallstone formation up to 12 months postoperatively [31].
The inclusion of a prophylactic measure to prevent cholelithiasis after LSG is a matter of controversy based on the low incidence of 6.0%–7.5% reported in some studies [32,33,34,35]. However, other studies reported incidences that are high enough to justify prophylaxis [10, 30, 36]. Out of the 258 patients in the current study, 20% developed cholelithiasis at 1 year. More importantly, complicated cases necessitating cholecystectomy represented 23.5% of stone developers. We studied preoperative factors that may help patient selection to receive prophylactic treatment. Higher preoperative BMI and dyslipidemia independently predicted stone development. Coupaye et al. found that only preoperative BMI > 50 kg/m2 was associated with developing gallstones [29]. It has been stated that traditional risk factors for gall bladder disease may not be predictive of post-bariatric cholelithiasis [37, 38]. Nevertheless, rapid weight loss has been reported as the most important risk factor for gallstone formation after SG [28, 34, 38, 39]. In the current study, higher weight loss at six but not 12 months was an independent predictor of stone formation.
Another question is how long UDCA should be administered. Most studies prescribe the drug for 6 months [9, 27, 28, 30, 31]. However, this period may not be sufficient based on the results of previous studies. It has been shown that the peak incidence of symptomatic gallstones was at 16 months after surgery [37, 40, 41]. Therefore, in this study, we extended the treatment period to 12 months. This approach may overcome the problem of decreased compliance due to the daily use of the drug over a long time. We did not face a significant problem of incompliance because the patients used to follow a regimen of administration of vitamin supplements for 12 months after surgery. The addition of UDCA tablets did not constitute a burden for the patients and did not result in major incompliance.
Novelty of the study
Although, there are some previous studies addressing the topic of UDCA use after bariatric Surgery, yet, up to our knowledge, the current research seems to be the first one, or at least one of the first researches, that focused on LSG as the most commonly performed bariatric surgery in many centers worldwide.
Furthermore, among the strengths of this study are the large number of included patients with a good follow-up period. This point may make our study fairly unique as the first prospective randomized design addressing this topic. Therefore, the current study adds more and more strength to the available evidence on whether UDCA after LSG is needed or not.
Moreover, a new insight into the advantages of UDCA use after LSG with minimal side effects may trigger the bariatric surgeons to use it routinely especially in patients with high BMI and co-exisiting dyslipidemia. Yet, these suggestions should be advocated in future studies.
One of the limitations of this study includes lack of data regarding the size of gall stones. Another liming factor is the absence of standardization of postoperative eating habits which could be one of the contributing factors that trigger gall stone formation.
In conclusion, this randomized trial showed that UDCA 500 mg once daily for 12 months after LSG is effective in reducing gallstone formation for at least 1 year. The incidence of gallstone formation after LSG in this study was 20%. Prophylactic use of UDCA reduced the incidence from 32 to 8.5%. Also, UDCA administration reduced the number of cholecystectomies from 7 to 2.3%. High BMI and dyslipidemia are the independent preoperative factors significantly associated with stone development.
References
Afshin A et al (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377:13–27
Scherer PE, Hill JA (2016) Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res 118:1703–1705
Stender S, Nordestgaard BG, Tybjaerg-Hansen A (2013) Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology 58:2133–2141
Iglézias Brandão de Oliveira C, Adami Chaim E, da Silva BB. Impact of rapid weight reduction on risk of cholelithiasis after bariatric surgery. Obes Surg. 2003;13:625–8.
Dittrick GW, Thompson JS, Campos D, Bremers D, Sudan D (2005) Gallbladder pathology in morbid obesity. Obes Surg 15:238–242
Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J et al (2019) Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO Global Registry report 2018. Obes Surg 29:782–795
Erlinger S (2000) Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol 12:1347–1352
Tapas M, Kona Kumari L, Kiran Kumar P (2016) Prevalence of cholelithiasis and choledocholithiasis in morbidly obese South Indian patients and the further development of biliary calculus disease after sleeve gastrectomy, gastric bypass and mini gastric bypass. Obes Surg 26:2411–2417
Coupaye M, Castel B, Sami O, Tuyeras G, Msika S, Ledoux S (2015) Comparison of the incidence of cholelithiasis after sleeve gastrectomy and Roux-en-Y gastric bypass in obese patients: a prospective study. Surg Obes Relat Dis 11:779–784
Manatsathit W, Leelasinjaroen P, Al-Hamid H, Szpunar S, Hawasli A (2016) The incidence of cholelithiasis after sleeve gastrectomy and its association with weight loss: a two-centre retrospective cohort study. Int J Surg 30:13–18
Plecka Östlund M, Wenger U, Mattsson F, Ebrahim F, Botha A, Lagergren J (2012) Population-based study of the need for cholecystectomy after obesity surgery. Br J Surg 99:864–869
Nguyen NT, Hinojosa MW, Slone J, Lee J, Khiatani V, Wilson SE (2007) Laparoscopic transgastric access to the biliary tree after Roux-en-Y gastric bypass. OBES SURG 17:416–419
Hussain A, El-Hasani S (2016) Potential benefits of prophylactic cholecystectomy in patients undergoing bariatric bypass surgery. Obes Surg 26:865–865
Worni M, Guller U, Shah A, Gandhi M, Shah J, Rajgor D et al (2012) Cholecystectomy concomitant with laparoscopic gastric bypass: a trend analysis of the nationwide inpatient sample from 2001 to 2008. Obes Surg 22:220–229
Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Chatedaki C, Sioka E et al (2017) Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: an updated systematic review and meta-analysis. Obes Surg 27:3021–3030
Portincasa P, Moschetta A, Palasciano G (2006) Cholesterol gallstone disease. The Lancet 368:230–239
Tsai C-J (2006) Weight cycling and risk of gallstone disease in men. Arch Intern Med 166:2369
Yi S-Q, Ohta T, Tsuchida A, Terayama H, Naito M, Li J et al (2007) Surgical anatomy of innervation of the gallbladder in humans and Suncus murinus with special reference to morphological understanding of gallstone formation after gastrectomy. World J Gastroenterol 13:2066–2071
Tustumi F, Bernardo WM, Santo MA, Cecconello I (2018) Cholecystectomy in patients submitted to bariatric procedure: a systematic review and meta-analysis. Obes Surg 28:3312–3320
Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon M et al (2013) Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 21:S1-27
Obésité : prise en charge chirurgicale chez l’adulte [Internet]. Haute Autorité de Santé. https://www.has-sante.fr/jcms/c_765529/fr/obesite-prise-en-charge-chirurgicale-chez-l-adulte. Accessed 7 Jun 2021
Machado FHF, de Castro HF, de Babadopulos RF, Rocha HAL, da Costa M, de Rocha JL et al (2019) Ursodeoxycholic acid in the prevention of gallstones in patients subjected to Roux-en-Y gastric bypass1. Acta Cir Bras 34(1):e20190010000009
Paumgartner G, Beuers U (2004) Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis 8(67–81):vi
Lee JM, Hyun JJ, Choi IY, Yeom SK, Kim SY, Jung SW et al (2015) Comparison on response and dissolution rates between ursodeoxycholic acid alone or in combination with chenodeoxycholic acid for gallstone dissolution according to stone density on CT scan: strobe compliant observation study. Medicine (Baltimore) 94(50):e2037
May GR, Sutherland LR, Shaffer EA (1993) Efficacy of bile acid therapy for gallstone dissolution: a meta-analysis of randomized trials. Aliment Pharmacol Ther 7:139–148
Lazaridis KN, Gores GJ, Lindor KD (2001) Ursodeoxycholic acid “mechanisms of action and clinical use in hepatobiliary disorders.” J Hepatol 35:134–146
Adams LB, Chang C, Pope J, Kim Y, Liu P, Yates A (2016) Randomized, prospective comparison of ursodeoxycholic acid for the prevention of gallstones after sleeve gastrectomy. Obes Surg 26:990–994
Abdallah E, Emile SH, Elfeki H, Fikry M, Abdelshafy M, Elshobaky A et al (2017) Role of ursodeoxycholic acid in the prevention of gallstone formation after laparoscopic sleeve gastrectomy. Surg Today 47:844–850
Coupaye M, Calabrese D, Sami O, Siauve N, Ledoux S (2019) Effectiveness of ursodeoxycholic acid in the prevention of cholelithiasis after sleeve gastrectomy. Obes Surg 29:2464–2469
Şen O, Türkçapar AG, Yerdel MA (2020) Cholelithiasis after sleeve gastrectomy and effectiveness of ursodeoxycholic acid treatment. J Laparoendosc Adv Surg Tech A 30:1150–1152
Nabil TM, Khalil AH, Gamal K (2019) Effect of oral ursodeoxycholic acid on cholelithiasis following laparoscopic sleeve gastrectomy for morbid obesity. Surg Obes Relat Dis 15:827–831
Sioka E, Zacharoulis D, Zachari E, Papamargaritis D, Pinaka O, Katsogridaki G et al (2014) Complicated gallstones after laparoscopic sleeve gastrectomy. J Obes 2014:468203
Moon RC, Teixeira AF, DuCoin C, Varnadore S, Jawad MA (2014) Comparison of cholecystectomy cases after Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding. Surg Obes Relat Dis 10:64–68
Tsirline VB, Keilani ZM, El Djouzi S, Phillips RC, Kuwada TS, Gersin K et al (2014) How frequently and when do patients undergo cholecystectomy after bariatric surgery? Surg Obes Relat Dis 10:313–321
Dakour Aridi H, Sultanem S, Abtar H, Safadi BY, Fawal H, Alami RS (2016) Management of gallbladder disease after sleeve gastrectomy in a selected Lebanese population. Surg Obes Relat Dis 12:1300–1304
Guzmán HM, Sepúlveda M, Rosso N, San Martin A, Guzmán F, Guzmán HC (2019) Incidence and risk factors for cholelithiasis after bariatric surgery. Obes Surg 29:2110–2114
Miller K, Hell E, Lang B, Lengauer E (2003) Gallstone formation prophylaxis after gastric restrictive procedures for weight loss. Ann Surg 238:697–702
Li VKM, Pulido N, Martinez-Suartez P, Fajnwaks P, Jin HY, Szomstein S et al (2009) Symptomatic gallstones after sleeve gastrectomy. Surg Endosc 23:2488–2492
Melmer A, Sturm W, Kuhnert B, Engl-Prosch J, Ress C, Tschoner A et al (2015) Incidence of gallstone formation and cholecystectomy 10 years after bariatric surgery. Obes Surg 25:1171–1176
Deitel M, Petrov I (1987) Incidence of symptomatic gallstones after bariatric operations. Surg Gynecol Obstet 164:549–552
Amaral JF, Thompson WR (1985) Gallbladder disease in the morbidly obese. Am J Surg 149:551–557
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Mohamed AbdAlla Salman, Ahmed Salman, Usama Shaker Mohamedm Ahmed Mahmoud Hussein, Mahmoud A. Ameen, Hitham S.E. Omar, Ahmed Elewa, Ahmed hamdy, Abd Al-Kareem Elias, Mohamed Tourky, Alaa Helal, Ahmed Abdelrahman Mahmoud, Feras Aljarad, Ahmed Moustafa, Hossam El-Din Shaaban, Ahmed Nashat, Amr Mahmoud Hussein, Tamer Omar, and Hany Balamoun have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salman, M.A., Salman, A., Mohamed, U.S. et al. Ursodeoxycholic acid for the prevention of gall stones after laparoscopic sleeve gastrectomy: a prospective controlled study. Surg Endosc 36, 6396–6402 (2022). https://doi.org/10.1007/s00464-021-08980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08980-3