Abstract
Background
Cholelithiasis is a common complication after bariatric surgery. Pure restrictive procedures such as sleeve gastrectomy and gastric banding theoretically should result in less gallstone formation because the food continues to follow the normal gastrointestinal transit, maintaining the enteric–endocrine reflex intact. To the authors’ knowledge, the literature has no studies that analyze the incidence of gallstone formation after sleeve gastrectomy. This study aimed to compare the rates of symptomatic gallstones between laparoscopic Roux-en-Y gastric bypass (RYGBP) and sleeve gastrectomy (SG).
Methods
A retrospective chart review of patients who underwent laparoscopic RYGBP and SG between 2004 and 2006 was performed. The patients with previous cholecystectomy, known gallstones with or without concomitant cholecystectomy, and previous weight-reduction operations were excluded from the analysis. The outcome measures were the numbers of patients who had experienced symptomatic and complicated gallstones. Using Cox regression analysis, comparisons was made between the patients with laparoscopic RYGBP (group A) and those with laparoscopic SG (group B).
Results
Groups A excluded 174 (26%) of 670 patients, and group B excluded 27 (34.2%) of 79 patients. The patients in group A had a significantly higher preoperative body mass index (BMI) than those in group B. Additionally, more group A than group B patients had a BMI exceeding 45 and more than a 25% loss of original weight. No significant difference in the development of symptomatic (8.7% vs. 3.8%; p = 0.296) or complicated (1.8% vs. 1.9%; p = 0.956) gallstones was noted between the two groups
Conclusions
There was no significant difference in symptomatic or complicated gallstone disease between the patients treated with laparoscopic SG and those treated with laparoscopic RYGBP. Routine prophylactic cholecystectomy should not be recommended for weight reduction during laparoscopic SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholelithiasis is not uncommon after weight-reduction surgery. In published series using regular ultrasound surveillance after gastric bypass, the rate of asymptomatic gallstone formation ranged from 30% to 52.8% within 6 to 12 months after the operation [1–3]. In addition, 7% to 16% of the patients experienced symptomatic gallstones [1, 3–5].
This phenomenon was observed and reported in the early 1990s among patients with rapid weight loss [6–8]. It was postulated that during rapid weight loss, cholesterol is mobilized from tissue stores and excreted into the bile, resulting in an increased bile cholesterol saturation index. Others found that increased gallbladder secretion of mucin and calcium and an increased presence of prostaglandins and arachidonic acid in bile contributed significantly to gallstone formation [9–12].
Information regarding gallstone formation after purely restrictive procedures for weight reduction is scarce. Kiewiet et al. [13], in their study of Dutch patients with morbid obesity, found a rate of 26.5% for asymptomatic gallstones after gastric banding. In another study by O’Brien and Dixon [14], symptomatic gallstones developed in patients at a rate of 6.8% after gastric banding, and none of them experienced complications before cholecystectomy.
Sleeve gastrectomy is a purely restrictive procedure that has attracted attention in recent years. However, the question of postoperative gallstone formation after this procedure has not been addressed in the literature.
The rates for both asymptomatic and symptomatic gallstone formation seemed to be higher after gastric bypass. One possible reason is that the duodenal exclusion after gastric bypass results in decreased gallbladder motility secondary to reduced reflex secretion of cholecystokinin. Another reason is the damage to hepatic branches of the left vagus when the lesser curve of the stomach is divided. Yet, studies on early hormonal changes after gastric bypass have shown no significant change in cholecystokinin levels before or after meals [15, 16]. In addition, the chance of damaging the hepatic branch has been low if division was made below the second descending branch of the left gastric artery.
To reduce the incidence and complications of gallstones after weight-reduction surgery, policies of routine prophylactic cholecystecystomy [17], use of intraoperative ultrasound for gallstone detection and concomitant cholecystectomy [3, 18], postoperative use of ursodeoxycholic acid [19, 20], regular ultrasound surveillance for asymptomatic gallstone [1, 3, 19, 20], or their combinations have been suggested. However, these are not practiced at our institution.
In this study, we aimed to study and compare the prevalence of symptomatic gallstones and different rates for them between patients who had laparoscopic gastric bypass and those who had sleeve gastrectomy.
Methods
This study was approved by our institution review board (IRB). A retrospective review of a prospectively collected database including all the patients treated with laparoscopic Roux-en-Y gastric bypass (LRYGB) and sleeve gastrectomy between January 2004 and June 2006 was conducted. Patients with previous cholecystectomy, known gallstones with or without concomitant cholecystectomy, or previous weight-reduction surgery were excluded from the study.
All our patients considered suitable for sleeve gastrectomy were counseled in our outpatient clinic concerning the options of laparoscopic adjustable gastric banding (LAGB), LRYGB, and sleeve gastrectomy, and the respective risks of surgery were explained. All sleeve gastrectomies were done under IRB approval, which specifically explains of the lack of long-term data.
Routine preoperative gallbladder ultrasound was performed for all patients to rule out gallstones or sludge. Those with positive findings on ultrasound were counseled for concomitant laparoscopic cholecystectomy. Neither routine intraoperative ultrasound nor postoperative prescription of ursodeoxycholic acid was practiced in our management protocol.
Patients were followed up regularly in the outpatient clinic. Those with symptoms suggestive of cholelithiasis or its complications had further imaging with ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT) scan of the abdomen.
The patients were divided into two groups for comparison. Group A included the patients who had laparoscopic Roux-en-Y gastric bypass, and group B included the patients who underwent laparoscopic sleeve gastrectomy. Important baseline characteristics such as age, gender, preoperative body mass index (BMI), BMI exceeding 45 kg/m2, weight loss exceeding 25% of the original weight, and the presence of diabetes mellitus and hyperlipidemia were compared between the two groups.
The primary outcome measure was the formation of symptomatic gallstones or sludge with or without complications, and the secondary outcome measure was the development of cholelithiasis complications. Positive findings according to the ultrasound, CT scan, or MRI reports were recorded. Symptoms were considered attributable to gallstones or sludge if not clinically explainable by other causes. Patients who had complications such as acute cholecystitis, deranged liver function, acute cholangitis, or biliary pancreatitis at first presentation also were considered as symptomatic in the analysis.
Statistical analysis
Data were collected using Excel for Windows, and analysis was performed with the Scientific Package of the Social Sciences version 15.0 (SPSS, Chicago, IL, USA). The chi-square test or Fisher’s exact test was used for nominal variables as appropriate. The t-test was used to compare continuous variables. Cox regression was used as a multivariable analysis to adjust for a difference in the baseline characteristics between the two groups. A p value less than 0.05 was considered significant.
Results
Between January 2004 and June 2006, 670 laparoscopic Roux-en-Y gastric bypasses and 79 laparoscopic sleeve gastrectomies were performed at our institution. After exclusions from the study, 496 group A patients and 52 group B patients were included in the analysis (Table 1).
Preoperative gallbladder disease was present in 169 group A patients (25.3%). Of these patients, 115 (17.2%) had undergone a previous cholecystectomy, and 54 (8.1%) patients had asymptomatic gallstones or sludge identified by routine preoperative ultrasound. A higher rate (32.9%) of preoperative gallbladder diseases was found in group B. In this group, 17 patients (21.5%) had previous cholecystectomy and gallstones, and 9 patients (11.4%) had sludge identified by ultrasound.
The baseline characteristics of the patients in groups A and B are shown in Table 2. The mean age was 42.9 years (range, 17–81 years) for group A, and 370 (74.6%) of the patients were women, whereas it was 40.5 years (range, 12–71 years) for group B, and 40 (76.9%) of the patients were women. The median follow-up period was 27 months (range, 12–42 months) for group A and 17 months (range, 12–30 months) for group B.
The patients in group A had a significantly higher preoperative BMI than the patients in group B. Group A also had significantly more patients with a BMI exceeding 45 kg/m2 and a postoperative weight loss exceeding 25% of the original weight.
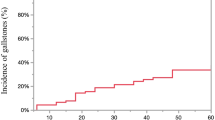
Symptomatic gallstones or sludge had developed in 43 group A patients (8.7%) compared with 2 group B patients (3.8%) (Table 3). The symptomatic gallstones developed in the 43 group A patients a median of 7 months (range, 1–39 months; mean, 10.6 ± 9.6) months) after surgery, but in the 2 group B patients respectively 3 and 8 months after surgery. The mean weight loss at which the symptoms developed was 38.3% ± 9.5% in group A and 35.2% in group B. Despite a more than twofold increase in the incidence of symptomatic gallbladder disease in group A, this difference did not reach statistical significance (p = 0.296). Cox regression analysis, with adjustment for all the potential differences in the covariates, also showed no significant difference in symptomatic cholelithiasis between the two groups.
No significant difference in the incidence of complicated gallstones was found between the two groups: group A (1.8%) versus group B (1.9%) (p = 0.956). Four patients in group A experienced acute cholecystitis. Two of these patients had obstructive jaundice without acute cholangitis, and three experienced biliary pancreatitis. On the other hand, one group B patient had acute cholecystitis at presentation.
Discussion
Laparoscopic cholecystectomy in the morbidly obese may be associated with increased operative difficulty and morbidity compared with nonobese patients [21]. However, the role of prophylactic cholecystectomy at the time of bariatric surgery remains controversial. The fact that pathologic evidence of gallbladder diseases has been found in more than 75% of routinely resected specimens supports those who advocate prophylactic cholecystectomy [17, 22]. On the other hand, the fact that only 7% to 16% of patients will develop symptomatic gallstones [1, 3, 4] and that less than 10% of patients with negative ultrasound exams require subsequent cholecystectomy [23, 24] does not support performing a prophylactic cholecystectomy.
In this series, symptomatic gallstones or sludge was noted to develop in 8.7% of patients after laparoscopic Roux-en-Y gastric bypass and 3.8% of patients after sleeve gastrectomy. Dhabuwala et al. [4] reported a higher rate (11.8 %) for symptomatic gallstone formation among patients after gastric bypass. In another study of gastric bypass patients by Scott et al. [20], intraoperative ultrasound with concomitant cholecystectomy, if positive, and postoperative prescription of ursodeoxycholic acid when ultrasound was negative were used, but a similar rate of 10.1% for symptomatic gallstones was detected after a mean follow-up period of 10 months. Another randomized study by Miller et al. [19] using postoperative ursodeoxycholic acid showed a cholecystectomy rate of 4.7% in 2 years after vertical banded gastroplasty and adjustable gastric banding. These are clear indicators that no definite effective measure exists to prevent postoperative cholelithiasis.
In this study, 195 (26%) of 749 patients had preoperative evidence of gallbladder disease shown either by a history of cholecystectomy or cholelithiasis detected on routine preoperative transabdominal ultrasound. Again, this was similar to other studies [7, 9, 10].
Risk factors for gallstone formation such as female gender and increasing age are well known to surgeons. The Nurses’ Health Study showed a sevenfold increased risk of gallstone formation for women with a BMI exceeding 45 kg/m2 [11], whereas more than a 24% loss of original body weight was found to be a significant risk factor for gallstone formation [25, 26]. These two factors were therefore included also in our baseline characteristics comparisons.
Theoretically, cholelithiasis should be more common after gastric bypass than after a purely restrictive procedure such as sleeve gastrectomy. However, studies on early hormonal effects after gastric bypass have shown no significant change in cholecystokinin level before or after meals [15, 16]. In addition, factors that promote cholelithiasis such as reduction in gallbladder emptying, increased gallbladder residual volume, and decreased refilling have been demonstrated after gastric banding [12].
To our knowledge, no studies have compared symptomatic cholelithiasis after gastric bypass and sleeve gastrectomy. In our study, we focused on symptomatic and complicated cholelithiasis as the outcome measures because they are of more clinical significance. In addition, routine postoperative ultrasound is not part of the follow-up protocol at our institution. Due to the limited number of patients and events and the assumption that the two procedures work under similar mechanisms, we did not attempt to compare the outcomes between patients who underwent adjustable gastric banding and those who had sleeve gastrectomy. In this study, although the patients with gastric bypass were heavier and had more significant weight loss than those with sleeve gastrectomy, no significant difference was noted in the rate for symptomatic and complicated cholelithiasis between the two groups of patients. Cox regression analysis also showed similar findings. However, because our sample size was limited, the study may not have been adequately powered to note a small difference. The rate of complicated cholelithiasis was low (1.8% vs. 1.9%), with no significant difference between the two groups.
Our study findings had two important implications. First, because symptomatic cholelithiasis showed no increase compared with gastric bypass and because the rate of complicated gallbladder disease was low after sleeve gastrectomy; routine prophylactic cholecystectomy is not indicated for these patients. Second, from an etiologic point of view, the traditional belief that hormonal changes and left vagal hepatic branch denervation after gastric bypass result in gallstone formation may no longer hold true because patients who had food going through the usual gastrointestinal transit without duodenal exclusion did not have a lower risk for symptomatic gallstone formation. The speed and the degree of weight loss after surgery may be solely related to symptomatic cholelithiasis rather than the choice of the procedure itself. Further studies are necessary to address this important issue so that recommendations regarding selective rather than routine ultrasound for gallstone surveillance after weight-reduction surgery can be made according to the progress of weight loss.
The current study had several limitations. First, it was a retrospective study of consecutive patients without adequate matching between the two groups in terms of the number and baseline characteristics of patients. A report with data from the International Bariatric Surgery Registry showed an increasing trend favoring combined restrictive–malaborptive procedures from 33% to 94% over a recent 18-year period (1987–2004) [27]. A matched study was difficult because many important variables were different between the two studied groups. Moreover, in actual practice, the selective criteria for the respective operations were different.
Second, although the false-negative rate of ultrasound for gallstones in severely obese patients was found to be less than 2% in experienced hands [28, 29], we have not yet validated its accuracy for patients with a different BMI value at our institution. Because our patients in the gastric bypass group were significantly heavier than those who underwent a restrictive procedure, significant bias may have occurred in the exclusion of cases for analysis in the former group.
Finally, the length of the follow-up period was significantly different between the two groups of patients (median, 27 vs. 17 months). However, the effect of this difference on our outcomes may not have been profound because gallstones developed in most of our patients within 12 months after surgery.
Conclusions
Cholelithiasis was common in our patients before weight-reduction surgery. The rate for symptomatic gallstones after surgery was not higher, even without a policy of intraoperative ultrasound followed by concomitant cholecystectomy, postoperative ursodeoxycholic acid, and regular ultrasound surveillance. No significant difference in symptomatic and complicated gallstone disease was found between the patients who underwent laparoscopic gastric bypass and those who had sleeve gastrectomy. Routine prophylactic cholecystectomy should not be recommended for the latter group of patients.
References
Villegas L, Schneider B, Provost D, Chang C, Scott D, Sims T, Hill L, Hynan L, Jones D (2004) Is routine cholecystectomy required during laparoscopic gastric bypass? Obes Surg 14:206–211
Brandao I, de Oliveira C, Adami Chaim E, da Silva BB (2003) Impact of rapid weight reduction on risk of cholelithiasis after bariatric surgery. Obes Surg 13:625–628
Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW (1991) Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol 86:1000–1005
Dhabuwala A, Cannan RJ, Stubbs RS (2000) Improvement in comorbidities following weight loss from gastric bypass surgery. Obes Surg 10:428–435
Portenier DD, Grant JP, Blackwood HS, Pryor A, McMahon RL, Demaria E (2007) Expectant management of the asymptomatic gallbladder at Roux-en-Y gastric bypass. Surg Obes Relat Dis 3:476–479
Bennion LJ, Grundy SM (1992) Risk factors for the development of cholelithiasis in man (second of two parts). N Engl J Med 307:798–800
Diehl AK (1991) Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am 20:1–19
Jorgensen T (1989) Gallstones in a Danish population: relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut 30:528–534
Mason EE (2002) Gallbladder management in obesity surgery. Obes Surg 12:222–229
Heaton KW, Braddon FEM, Mountford RA, Hughes AO, Emmett PM (1991) Symptomatic and silent gallstones in the community. Gut 32:316–320
Stampfer MJ, Maclure KM, Colditz GA, Manson JE, Willett WC (1992) Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr 55:652–658
AI-Jiffry BO, Shaffer EA, Saccone GT, Downey P, Kow L, Toouli J (2003) Changes in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obesity. Can J Gastroenterol 17:169–174
Kiewiet RM, Durian MF, van Leersum M, Hesp FLEM, van Vliet ACM (2006) Gallstone formation after weight loss following gastric banding in morbidly obese Dutch patients. Obes Surg 16:592–596
O’Brien PE, Dixon JB (2003) A rational approach to cholelithiasis in bariatric surgery. Arch Surg 138:908–912
Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E (2004) The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240:236–242
Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ (1990) Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 211:763–771
Amaral JF, Thompson WR (1985) Gallbladder disease in the morbidly obese. Am J Surg 149:551–557
Shiffman ML, Sugerman HJ, Kellum JH, Brewer WH, Moore EW (1993) Gallstones in patients with morbid obesity: relationship to body weight, weight loss, and gallbladder bile cholesterol solubility. Int J Obes Relat Metab Disord 17:153–158
Miller K, Hell E, Lang B, Lengauer E (2003) Gallstone formation prophylaxis after gastric restrictive procedured for weight loss: a randomized double-blind placebo-controlled trial. Ann Surg 238:697–702
Scott DJ, Villegas L, Sims TL (2003) Intraoperative ultrasound and prophylactic ursodiol for gallstone prevention following laparoscopic gastric bypass. Surg Endosc 17:1796–1802
Ammori BJ, Vezakis A, Davides D, Martin IG, Larvin M, McMahon MJ (2001) laparoscopic cholecystectomy in morbidly obese patients. Surg Endosc 15:1136–1139
Fobi M, Lee H, Igwe D, Felaphy B, James E, Stanczyk M, Fobi N (2002) Prophylatic cholecystectomy with gastric bypass operation: incidence of gallbladder disease. Obes Surg 12:350–353
Fakhry SM, Herbst CA, Buckwalter JA (1987) Cholecystectomy in morbidly obese patients. Am Surg 53:26–28
Jones KB Jr (1995) Simultaneous cholecystectomy: to be or not to be. Obes Surg 5:52–54
Erlinger S (2000) Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol 12:1347–1352
Yang H, Peterson GM, Roth MP, Schoenfield LJ, Marks JW (1992) Risk factors for gallstones formation during rapid loss of weight. Dig Dis Sci 37:912–918
Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M (2006) Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg 192:657–662
Mark SS, John JC, Francis HS, Marleen MM, Barbara S, William T, Gary SD (1988) Ultrasound evaluation of cholelithiasis in the morbidly obese. Abdom Imaging 13:345–346
Oria HE (1998) Pitfalls in the diagnosis of gallbladder disease in clinically severe obesity. Obes Surg 8:444–451
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, V.K.M., Pulido, N., Martinez-Suartez, P. et al. Symptomatic gallstones after sleeve gastrectomy. Surg Endosc 23, 2488–2492 (2009). https://doi.org/10.1007/s00464-009-0422-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0422-6