Abstract
Background
Soft pancreas with small pancreatic duct is a known risk factor for postoperative pancreatic fistula (POPF). This study demonstrated the safety and feasibility of laparoscopic duct-to-mucosa pancreaticojejunostomy (PJ) and compared perioperative outcomes of laparoscopic pancreaticoduodenectomy (LPD) and open pancreaticoduodenectomy (OPD) in patients with soft pancreas and small pancreatic duct.
Methods
From January 2014 to December 2019, 183 patients underwent LPD and 91 patients underwent OPD by a single surgeon. Data on patients with soft pancreas and combined small pancreatic duct (≤ 2 mm) were retrospectively reviewed. Clinicopathologic characteristics, and perioperative outcomes were compared between LPD and OPD. We evaluated risk factors affecting clinically relevant POPF (CR-POPF). We also correlated calculated risks of POPF and CR-POPF between the two groups.
Results
We compared 62 patients in the LPD group and 34 patients in the OPD group. Perioperative outcomes showed less blood loss, shorter hospital stays, and less postoperative pain score on postoperative day (POD)#1 and #5 in LPD compared with OPD. Postoperative complications showed no differences between LPD and OPD. LPD group showed significantly reduced CR-POPF rates compared to the OPD group (LPD 11.3% vs. OPD 29.4%, p = 0.026). Multivariate analysis identified obesity (BMI ≥ 25), thick pancreas parenchyma and open surgery as independent predicting factors for CR-POPF. The LPD group showed less CR-POPF than the OPD group according to POPF risk groups. This difference was more prominent in a high-risk group.
Conclusion
With appropriate laparoscopic technique, LPD is feasible and safe and reduces CR-POPF in soft pancreas with a small pancreatic duct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Deciding to perform laparoscopic pancreaticoduodenectomy (LPD) is not easy. LPD safety and feasibility are still controversial around the world. PD can be divided into two phases: resection and reconstruction. The technical difficulty of laparoscopic procedures at each phase may vary according to the pathologic characteristics of periampullary tumors. For pancreatic head cancer, endoscopic procedures, cancer-related pancreatitis, cholangitis, and risk of portal vein involvement make laparoscopic resection more difficult and even inappropriate. A dilated bile duct and large pancreatic duct with hard pancreas make the laparoscopic reconstruction phase more suitable.
For benign and borderline malignant tumors of the pancreatic head, the laparoscopic resection phase can be more technically acceptable, as tumors are usually without cholangitis and/or pancreatitis with no risk of tumor invasion to the portal vein system. Nevertheless, surgeons may struggle with the laparoscopic reconstruction phase due to the small diameter of the bile and pancreatic ducts with soft remnant pancreas, which limit wide expansion of minimally invasive PD [1]. In general, soft pancreas, a thick parenchyma, and a small main pancreatic duct are known risk factors for postoperative pancreatic fistula (POPF) [2].
To overcome this paradox and accomplishing the goal of minimally invasive surgery, some surgeons prefer laparoscopic resection followed by manual reconstruction through a small upper midline incision for hepaticojejunostomy and pancreaticojejunostomy (PJ) [3].
In this article, we present our technique of laparoscopic duct-to-mucosa PJ for soft remnant pancreas with small pancreatic duct (≤ 2 mm). We demonstrated the safety and feasibility of our laparoscopic procedure in patients with high risk of postoperative pancreatic fistula (CR-POPF).
Materials and methods
Patient selection
From January 2014 to December 2019, 274 consecutive patients underwent open or laparoscopic PD for periampullary tumors by a single surgeon in our institution. In our institution, selection criteria for LPD are: good general condition capable of enduring prolonged pneumoperitoneum, absence of severe obesity (BMI ≥ 30), no anticipated complex vascular resection and reconstruction, benign, low-grade malignant tumor or periampullary cancer and resectable pancreatic cancer with a clear resection plane between the tumor and vascular interfaces. Among the 274 patients, we retrospectively reviewed data on patients who underwent LPD or OPD with soft pancreas with small pancreatic duct (≤ 2 mm). We evaluated the texture of pancreas and duct diameter intra-operatively by the surgeon after transection of pancreatic neck and recorded in operation record. The pancreatic texture and duct size in our data were based on the operation record. The thickness of pancreas parenchyma was obtained by subtracting the duct diameter from the antero-posterior diameter at the pancreas neck level on preoperative CT scan. Patients who underwent neoadjuvant therapy or combined resection including vascular resection were excluded to reduce selection bias. Patients who underwent a laparoscopic approach followed by open conversion were included in a open PD group as reconstruction was performed using an open approach. A flow diagram of patient selection is in Fig. 1.
Patient selection. Of a total of 274 patients, 183 underwent LPD and 91 underwent OPD. After inclusion and exclusion criteria, analysis was of 96 patients with soft remnant pancreas with small pancreatic duct (≤ 2 mm). Among 96 patients, 62 patients were LPD group and 34 patients were OPD group. LPD: laparoscopic pancreaticoduodenectomy, OPD: open pancreaticoduodenectomy
Clinicopathological characteristics and perioperative outcomes
We compared clinicopathological characteristics and perioperative outcomes between the LPD and OPD groups. American Society of Anesthesiologist physical status classification (ASA) was used to assess physical status and comorbidity of patients prior to operation [4].
In addition, we calculated the fistula risk score (FRS) [5] and the alternative fistula risk score (aFRS) [6] to compare the potential risk of fistula formation of each group. The formulas used for the FRS and aFRS are as below.
The FRS formula
Fistula risk score for prediction of clinically relevant pancreatic fistula after pancreatoduodenectomy (Model III)
Risk factor | Parameter | Points* |
|---|---|---|
Grand texture | Firm | 0 |
Soft | 2 | |
Pathology | Pancreatic adenocarcinoma or pancreatitis | 0 |
Ampullary, duodenal, cystic, islet cell | 1 | |
Pancreatic duct diameter, mm | ≥ 5 | 0 |
4 | 1 | |
3 | 2 | |
4 | 3 | |
≤ 1 | 4 | |
Intraoperative blood loss, mL | ≤ 400 | 0 |
401–700 | 1 | |
701–1000 | 2 | |
> 1000 | 3 |
The FRS scoring system consists of four parameters and is calculated by summation of scores in each parameters. They assessed 2 points in soft pancreas and 0 points in hard pancreas. The total score ranges from 0 to 10 and four risk strata can be assigned: negligible risk (0 points), low risk (1 to 2 points), intermediate risk (3 to 6 points), and high risk (7 to 10 points).
The aFRS formula
with P = probability, texture 1 = soft, and 0 if not soft, PD size = pancreatic duct size in mm (truncated at 5).
The aFRS probability is calculated by the formula above. The three risk groups can be identified based on the risk distribution: low (0 to 5%), intermediate (> 5% to 20%), and high (≥ 20%) risk of POPF. In this formula, 1 points is assessed to texture in the soft pancreas and 0 points is assessed in otherwise. The aFRS can be calculated automatically in online site “https://www.evidencio.com/models/show/621”.
We evaluated potential correlations among FSR, aFSR, and clinically relevant POPF (CR-POPF) according to a laparoscopic or open approach. CR-POPF refers to grade B or C POPF based on the 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula [7]. Numerical rating scale (NRS) pain intensity scores on postoperative days 1, 3, 5 were also reviewed and ranged from 0 (no pain at all) to 10 (worst pain possible). Complications were graded according to Clavien-Dindo classification and grouped into Grade I/II for minor complications and Grade III/IV for major complications [8]. We also evaluated risk factors affecting CR-POPF in univariate and multivariate logistic regression models. The obesity mentioned in this study was based on “The Asia–Pacific perspective: redefining obesity and its treatment” [9] published by WHO (World Health Organization), and BMI 25 or higher was defined as obesity and BMI 30 or higher as severe obesity. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (4-2017-1249).
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 23 (SPSS, Chicago, IL, USA). For each quantitative variable, the Shapiro–Wilk test was used as a test of normality. Values are expressed as means and standard deviations or medians and ranges, when appropriate. Categorical variables were compared using the Chi-Square test and reported as number (n) and percentage (%). Continuous variables were compared using the independent t test or the Mann–Whitney test, as appropriate. The p-value for statistical significance was set at 0.05. In evaluation of risk factors affecting CR-POPF, we used univariate binomial logistic regression models, and variables whose p-value is under 0.100 in univariate analysis were used in multivariate analysis.
Technique of laparoscopic duct-to-mucosa pancreaticojejunostomy
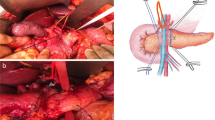
Our institutional technique for LPD is published [10]. Laparoscopic PJ with duct-to-mucosa technique is prepared by pulling up and approximating the proximal jejunum through the colonic mesentery. First, posterior interrupted sutures are placed between the pancreatic posterior and seromuscular layer of jejunum. A small jejunotomy is made after completion of the posterior interrupted suture of PJ (Fig. 2A). We start duct-to-mucosa anastomosis by tagging a stitch at 6 o’clock of the pancreatic duct for safe anastomosis. The suture material of this stitch differs from others for easy distinction when manipulating sutures under a laparoscopic environment (Fig. 2B). Needle mounting in blunt angle fashion is crucial for placing lateral side stitches of duct-to-mucosa anastomosis (Fig. 2C). After placing both lateral stitches, the stitch at 6 o’clock is used to complete the posterior 6 o’clock part of the duct-to-mucosa anastomosis. After tying the 9 o’clock side suture, the short pancreatic stent is inserted into the anastomosis site. Before tying up 3 o’clock side suture, an additional 12 o’clock side suture of the duct-to-mucosa anastomosis is placed (Fig. 2D). This sequence allows appropriate space for the 12 o’clock side duct-to-mucosa anastomosis. After tying the 12 o’clock side suture, the 3 o’clock side suture is tied to complete the duct-to-mucosa anastomosis. In the final step, serial interrupted anterior sutures are placed for completion of the laparoscopic PJ with duct-to-mucosa anastomosis. The reason for inserting a catheter is for guidance for easier anterior duct anastomosis rather than POPF prevention. If the pancreatic duct cannot be canalized, the anastomosis is conducted without insertion of catheter. The smallest catheter is a lumen of 1 mm, and most of the cases are canalized without problem when it is divided from the pancreatic neck. However, if cannulation does not work, anastomosis may be performed after widening using a dilator, and there may be cases where the pancreatic duct needs to be resected again in consideration of the possibility that it was sealed up during the transection process. Other than that, we can consider dunking pancreaticojejunostomy or transgastric pancreaticogastrostomy. Our laparoscopic duct-to-mucosa pancreaticojejunostomy technique is demonstrated in Video, Supplemental Digital Content 1.
Technique of laparoscopic duct-to-mucosa pancreaticojejunostomy. A placement of posterior interrupted sutures between the pancreatic posterior part and seromuscular layer of jejunum followed by jejunotomy. B Tagging of a stitch at 6 o’clock on the pancreatic duct with different suture material for duct-to-mucosa anastomosis. C Needle mounting in blunt angle fashion. D Additional 12 o’clock side suture of duct-to-mucosa anastomosis following placement of PJ stent
Results
Of a total of 274 patients, 183 underwent LPD and 91 underwent OPD. Intraoperative open conversion during LPD was noted in 22 patients (12.0%). After inclusion and exclusion criteria, analysis was of 96 patients with soft remnant pancreas with small pancreatic duct (≤ 2 mm).
Clinicopathological characteristics
Comparison of clinicopathological characteristics of the LPD and OPD groups is in Table 1. LPD was performed more frequently in younger patients with better physical status (age: LPD 57.5 years vs. OPD 63.7 years, p = 0.008; ASA class 1: LPD 8 (12.9%) vs. OPD 1 (2.9%), class 2: LPD 37 (59.7%) vs. OPD 16 (47.1%), class 3: LPD 17 (27.4%) vs. OPD 17 (50.0%), p = 0.047) and more often in patients with benign tumors and smaller tumor size (tumor size LPD 2.3 ± 1.3 vs. OPD 3.0 ± 1.7, p = 0.018). The thickness of pancreas parenchyma did not differ between two groups (LPD: 12.4 ± 3.6 vs OPD: 13.2 ± 3.8, p = 0.330). The pathology of the OPD group was predominantly cancer, mainly common bile duct cancer (19 cases, 55.9%) with only 1 benign tumor case (pancreatic neuroendocrine tumor). In the LPD group, the most common pathologic result was also common bile duct cancer (14 cases, 22.6%), but 25 cases (40.3%) of benign and low-grade malignant tumors were included, a much larger, significantly different number than in the OPD group (p = 0.010).
Perioperative outcomes and complications
Comparative analysis for perioperative outcomes and complications between LPD and OPD are in Table 2. No significant difference was seen in operation time between the two groups (461.3 min vs. 449.1 min, p = 0.539). However, the LPD group showed significantly superior results to the OPD group with less blood loss (LPD 239.5 ml vs. OPD 492.8 ml, p = 0.005), shorter length of hospital stays (LPD 15.7 days vs. OPD 23.7 days, p = 0.010), and lower NRS pain intensity score on POD#1 (LPD 4.3 vs. OPD 5.6, p = 0.003), POD#5 (LPD 2.8 vs. OPD 4.0, p = 0.002).
No difference was seen in postoperative complications between the two groups according to Clavien-Dindo classification (grade I-II: LPD 21 cases, 33.9% vs. OPD 15 cases, 44.1%; grade III-IV: LPD 2 cases, 3.2% vs. OPD 2 cases, 5.9%; p = 0.446). Grade I and II complications were mainly antibiotics use due to intra-abdominal surgical site infection, wound infection, delayed gastric emptying and transfusion. Grade III-IV complications of LPD were PTBD insertion due to obstructive cholangitis with afferent loop obstruction(GradeIIIa) and EGD hemostasis followed by ICU care due to duodenojejunostomy site ulcer bleeding (Grade IVa). Grade III-IV complications of OPD were pigtail insertion on POD#10 due to pancreaticojejunostomy site leakage with fluid collection and common hepatic artery embolization followed by ICU care due to arterial active bleeding. No postoperative mortality occurred in either group. For CR-POPF (grade B and C), 11.3% (7 of 62 patients) in the LPD group and 29.4% (10 of 34 patients) in the OPD group had CR-POPF, showing that the OPD group had significantly more association with CR-POPF than the LPD group (p = 0.026).
Risk factors of CR-POPF
The superiority of LPD over OPD for CR-POPF was also seen in univariate and multivariate analyses of clinical factors to predict CR-POPF. In univariate analysis, the OPD group had higher risk of POPF than the LPD group with odds ratio 3.274 (1.114–9.624, p = 0.031). Patients with obesity (BMI ≥ 25) showed higher risk of CR-POPF with odds ratio 2.915 (0.998–8.514, p = 0.050) than BMI less than 25. The thickness of pancreas parenchyma was also significant risk factor with odds ratio of 1.216 (1.046–1.413, p = 0.011). Multivariate analysis showed similar results, with open surgery, obesity and the thick parenchyma of pancreas as risk factors for CR-POPF. (OR: 5.334, p = 0.012; OR: 5.099, p = 0.015; OR: 1.228, p = 0.018, respectively) (Table 3).
Component analysis of calculated FRS
We evaluated FRS and aFRS to predict potential pancreatic fistula risk for the groups. The calculated FRS for both groups showed intermediate risk of pancreatic fistula. The OPD group showed significantly higher risk scores than the LPD group (LPD 6.35 vs. 6.79 OPD, p = 0.046). This difference was mainly due to intraoperative bleeding scores for the two groups (LPD 0.19 vs. 0.71 OPD, p- = 0.011). Other criteria included in FRS (gland texture, pathology, pancreatic duct diameter) showed almost identical scores (LPD 6.16 vs. OPD 6.09, p = 0.54). For aFRS not including intraoperative bleeding, the criteria also showed similar probabilities of fistula between the two groups (LPD 23.63% vs. OPD 23.13%, p = 0.674) (Table 1).
Potential correlation between FRS, aFRS, and CR-POPF
Using the FRS and aFRS scoring systems, we correlated categorized risk of POPF and CR-POPF according to laparoscopic or open approach. for FRS, 57 patients had intermediate risk and 39 had high risk. In the intermediate risk group, the incidence of CR-POPF was 15.8% (9 of 57 patients). Among these patients, the LPD group was 12.5% (5 of 40 patients) and the OPD group was 23.5% (4 of 17 patients) and CR-POPF was without significant difference (p = 0.428). In the high-risk group, overall CR-POPF was 20.5% (8 of 39 patients) including 9.1% for CR-POPF in the LPD group (2 of 22 patients) and 35.3% in the OPD group (6 of 17 patients) with a tendency toward significant difference (p = 0.059). No significant difference was seen for total CR-POPF for intermediate- and high-risk groups (p = 0.552).
For aFRS, 31 patients had intermediate risk and 65 patients had high risk. The overall incidence of CR-POPF was 3.2% (1 of 31 patients) in the intermediate risk group and 24.6% (16 of 65 patients) in the high-risk group, which was a significant difference (p = 0.010*). In an intermediate group, LPD showed no POPF (0 of 17 patients). OPD was 7.1% of CR-POPF (1 of 14 patients) without significant difference (p = 0.452). In a high-risk group, CR-POPF for LPD group was 15.6% (7 of 45 patients) and for the OPD group was 45% (9 of 20 patients) with significance (p = 0.026).
By both scoring systems, the LPD group showed less CR-POPF than the OPD group within identical risk groups. These differences in CR-POPF were more prominent in the high-risk group, especially in aFRS analysis. These results are summarized in Fig. 3.
Discussion
In our study of multivariate analysis affecting CR-POPF, obesity with BMI 25 or higher, open surgery and the thick parenchyma of pancreas were found to be significant risk factors. Obesity is also shown in previous papers to be a risk for CR-POPF due to accompanying obesity-related comorbidity or difficulty in surgical approach. The most notable result in our study is that open surgery was a significant risk factor for CR-POPF, and an LPD group had a significantly lower CR-POPF ratio than an OPD group. This finding was contrary to the prediction that laparoscopic anastomosis would not be superior to an open approach because of its technical difficulties. We focused on differences in intraoperative bleeding between the two groups. FRS for the OPD group was significantly higher than for the LPD group (LPD: 6.35 vs. OPD: 6.79, p = 0.046), mainly due to differences in intraoperative bleeding score (LPD: 0.19 vs. OPD: 0.71, p: 0.011). The sums of other criteria scores (gland texture, pathology, pancreatic duct diameter) were almost identical for the groups (LPD: 6.16 vs. OPD: 6.09, p = 0.54). This result was also seen for aFRS, which did not contain an intraoperative bleeding score that was different between the two groups (LPD: 23.63% vs. OPD: 23.13%, p = 0.674). In univariate analysis, FRS, aFRS, and intraoperative bleeding did not directly affect occurrence of POPF. The difference in bleeding can affect FRS and subsequently affect attenuation of POPF risk. This risk reduction effect of LPD was also observed in clinical correlation analysis for FRS/aFRS and CR-POPF. The LPD group showed consistently lower CR-POPF ratios than the OPD group within identical risk groups. This risk reduction effect was more prominent in a high-risk group (p = 0.026 in high-risk group for aFRS).
Sugimoto et al. [2] suggested a thick parenchyma (≥ 10 mm) and fatty infiltration as a risk factor of CR-POPF in addition to soft pancreas and small main pancreatic duct. In a total of 96 patients of our study, the average thickness of pancreas parenchyma was 12.7 mm (range 5-23 mm). Among them, 76 patients had thick pancreas parenchyma ≥ 10 mm, high risk) and 20 patients had less than 10 mm (low risk). CR-POPF occurred in 17 patients out of 76 patients in high risk group, however, none of the patients had CR-POPF in low risk group (p = 0.019). Univariate and multivariate analysis shows the thickness of pancreas parenchyma as significant risk factor of CR-POPF. Even in 76 patients with high risk parenchyma (≥ 10 mm), LPD showed superior result to OPD in CR-POPF significantly (LPD: 14.3% vs OPD: 37.0%, p = 0.023).
In the last two decades, minimally invasive surgery (MIS) has been introduced into various fields of surgery. Research had shown the superiority of MIS for postoperative complications without degrading oncologic outcomes compared to conventional surgery. As a result of these efforts, MIS such as laparoscopic surgery has replaced open surgery as a standard procedure in some fields. Articles have demonstrated the oncologic and surgical stability of minimally invasive pancreatic surgery. A Chinese group reported that robotic pancreaticoduodenectomy (RPD) is associated with decreased CR-POPF by comparing 405 OPD and 460 RPD cases in 2019 [11]. Korean researchers in 2020 showed better or at least similar early perioperative outcomes and equivalent midterm survival outcomes for MIS compared to OPD [12]. According to the literature on POPF following open PJ in remnant soft pancreas, overall POPF rate was 29.3% (range 24.6–68.6%) and CR-POPF (grade B and C) was noted to be 23.4% (range 14.7–30.4%, Table 4). Patients in this study all had high risk of POPF from small pancreatic duct with soft remnant pancreas. In our results for OPD, POPF rates were similar to previously reports and a superior outcome was noted for LPD. LPD is highly likely to become a safe and useful surgical method in the near future, as the number of low-grade tumors increases due to screening examinations.
The operating surgeon in this study conducted more than 200 laparoscopic cases until October 2020, and about 100 open cases before the first laparoscopic case in 2007. In the first laparoscopic case, resection was performed by laparoscopic approach, and anastomosis was performed through mini-laparotomy with manual reconstruction. Some authors reported learning curves of laparoscopic PPPD as 40 to 80 cases [13,14,15,16,17]. Recently reported randomized controlled trial comparing laparoscopic and open PPPD mentioned prolonged operating times and technical complexity as the discouraging factors for use of laparoscopic PPPD [18]. There should be about 80 to 100 cases of long learning curves in the true sense that can have superiority compared to open surgery. A specific guidance by expert laparoscopic surgeons and education system can reduce the learning curve [13]. Our institution has developed a porcine model for duct‐to‐mucosa PJ (Yonsei‐PJD™) [19] that enables new pancreatic surgeons to conduct effective laparoscopic PJ techniques. In laparoscopic surgery, no studies have been reported that open surgery experts can be more beneficial than beginners without significant experience. From the experience, surgical approach and techniques are different from open and laparoscopy, and it may be difficult to proceed with the concept fixed to the open procedure. However, in the case of open conversion, there is an advantage to recover with a skillful technique, which can be advantageous in terms of patient safety.
The limitations of this study are mainly relatively small number of cases and the retrospective design that limits interpretation of the results. There is a probability of occurring type II error statistically due to the small sample size. Through exclusion criteria, we tried to reduce the selection bias inherent in the study design. However, clinicopathological characteristics between LPD and OPD showed significant differences in age, ASA class and pathologic diagnosis. Patients with LPD were more fit for operations in their physical status. To overcome this limitation and show the feasibility and safety of MIS in pancreatic surgery, in the near future, we hope a well-designed randomized clinical trials (RCT) comparing open and laparoscopic PJ (duct-to-mucosa) will address this issue. Some practical problems such as technical standardization, controversies about safety issues, and few available surgeons capable of this technique are challenges to accomplishing a successful RCT. Alternatively, multicenter retrospective data with propensity score matching analysis may be an option to overcome these problems.
In conclusion, LPD for remnant soft pancreas with pancreatic duct ≤ 2 mm did not have higher morbidity and even had a better outcome for CR-POPF. LPD had less postoperative pain and intraoperative blood loss and shorter hospital stays. With appropriate laparoscopic techniques, risky pancreas can also be a good candidate for LPD.
References
Kang CM, Lee SH, Chung MJ, Hwang HK, Lee WJ (2015) Laparoscopic pancreatic reconstruction technique following laparoscopic pancreaticoduodenectomy. J Hepato-Biliary Pancreat Sci 22:202–210
Sugimoto M, Takahashi S, Kojima M, Kobayashi T, Gotohda N, Konishi M (2017) In patients with a soft pancreas, a thick parenchyma, a small duct, and fatty infiltration are significant risks for pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 21:846–854
Koh FH, Kow AW (2017) Minimally invasive Whipple’s technique for laparoscopic-assisted pylorus-preserving pancreaticoduodenectomy. J Am Coll Surg 224:e1–e3
Fitz-Henry J (2011) The ASA classification and peri-operative risk. Ann R Coll Surg Engl 93:185–187
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM (2013) A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216:1–14
Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, de Pastena M, Klompmaker S, Marchegiani G, Ecker BL (2019) Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg 269:937–943
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
World Health Organization. Regional Office for the Western P (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia, Sydney
Navarro JG, Kang CM (2019) Pitfalls for laparoscopic pancreaticoduodenectomy: need for a stepwise approach. Ann Gastroenterol Surg 3:254–268
Cai J, Ramanathan R, Zenati MS, Al Abbas A, Hogg ME, Zeh HJ, Zureikat AH (2019) Robotic pancreaticoduodenectomy is associated with decreased clinically relevant pancreatic fistulas: a propensity-matched analysis. J Gastrointest Surg 24:1111–1118
Kim HS, Kim H, Kwon W, Han Y, Byun Y, Kang JS, Choi YJ, Jang JY (2020) Perioperative and oncologic outcome of robot-assisted minimally invasive (hybrid laparoscopic and robotic) pancreatoduodenectomy: based on pancreatic fistula risk score and cancer/staging matched comparison with open pancreatoduodenectomy. Surg Endosc. https://doi.org/10.1007/s00464-020-07551-2
Song KB, Kim SC, Lee W, Hwang DW, Lee JH, Kwon J, Park Y, Lee SJ, Park G (2020) Laparoscopic pancreaticoduodenectomy for periampullary tumors: lessons learned from 500 consecutive patients in a single center. Surg Endosc 34:1343–1352
Choi M, Hwang HK, Lee WJ, Kang CM (2020) Total laparoscopic pancreaticoduodenectomy in patients with periampullary tumors: a learning curve analysis. Surg Endosc. https://doi.org/10.1007/s00464-020-07684-4
Kim S, Yoon YS, Han HS, Cho JY, Choi Y, Lee B (2020) Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg Endosc. https://doi.org/10.1007/s00464-020-07724-z
Wang M, Meng L, Cai Y, Li Y, Wang X, Zhang Z, Peng B (2016) Learning curve for laparoscopic pancreaticoduodenectomy: a CUSUM analysis. J Gastrointest Surg 20:924–935
Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG 3rd, Clary BM, Pappas TN, Tyler DS, Perez A (2014) Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 21:4014–4019
Bhingare P, Wankhade S, Gupta B, Dakhore S (2019) A prospective randomized controlled trial comparing laparoscopic versus open Whipple’s procedure for periampullary malignancy. Int Surg J 6:679
Choi M, Kang CM (2020) Developing an in vivo porcine model of duct-to-mucosa pancreaticojejunostomy (Yonsei-PJ(DTM)). Ann Gastroenterol Surg 4:180–184
Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M (2012) Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg 99:524–531
Kurumboor P, Palaniswami KN, Pramil K, George D, Ponnambathayil S, Varma D, Aikot S (2015) Octreotide does not prevent pancreatic fistula following pancreatoduodenectomy in patients with soft pancreas and non-dilated duct: a prospective randomized controlled trial. J Gastrointest Surg 19:2038–2044
Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, Wong J (2007) External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 246:425
Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P (2010) Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 252:207–214
Pessaux P, Sauvanet A, Mariette C, Paye F, Muscari F, Cunha AS, Sastre B, Arnaud J-P (2011) External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg 253:879–885
Yamamoto T, Satoi S, Yanagimoto H, Hirooka S, Yamaki S, Ryota H, Kotsuka M, Matsui Y, Kon M (2017) Clinical effect of pancreaticojejunostomy with a long-internal stent during pancreaticoduodenectomy in patients with a main pancreatic duct of small diameter. Int J Surg 42:158–163
Rungsakulkij N, Mingphruedhi S, Tangtawee P, Krutsri C, Muangkaew P, Suragul W, Tannaphai P, Aeesoa S (2017) Risk factors for pancreatic fistula following pancreaticoduodenectomy: a retrospective study in a Thai tertiary center. World J Gastrointest Surg 9:270
Ke Z, Cui J, Hu N, Yang Z, Chen H, Hu J, Wang C, Wu H, Nie X, Xiong J (2018) Risk factors for postoperative pancreatic fistula: analysis of 170 consecutive cases of pancreaticoduodenectomy based on the updated ISGPS classification and grading system. Medicine 97:e12151
Eshmuminov D, Schneider MA, Tschuor C, Raptis DA, Kambakamba P, Muller X, Lesurtel M, Clavien P-A (2018) Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB 20:992–1003
Acknowledgments
Some part of these results were presented in the International Video Symposium-5 at the 70th General Meeting of the Japanese Society of Gastroenterological Surgery, in Hamamatsu, Japan, on July 15, 2015, and symposium 33- at the sixth Biennial Congress of the Asian-Pacific Hepato-Biliary-Pancreatic Surgery and the 29th Meeting of Japanese Society of Hepato-Biliary-Pancreatic Surgery, in Yokohama, Japan, on June 10 , 2017. The authors would like to express sincere thanks to Professor Katsuhiko Uesaka (Shizuoka Cancer Center), Satoshi Hirano (Hokkaido University), and Yoshiharu Nakamura (Nippon Medical School) for valuable comments and questions during that symposium, which were the basis for the discussion session in this manuscript. Authors also would like to express sincere thanks to Hera Kang for comprehensive narration in video materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Also, all authors Seung Soo Hong, Jae Uk Chong, Ho Kyoung Hwang, Woo Jung Lee and Chang Moo Kang, the corresponding author have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 93664 KB)
Rights and permissions
About this article
Cite this article
Hong, S.S., Chong, J.U., Hwang, H.K. et al. Laparoscopic pancreaticoduodenectomy reduces incidence of clinically relevant postoperative pancreatic fistula in soft pancreas with a smaller than 2 mm pancreatic duct. Surg Endosc 35, 7094–7103 (2021). https://doi.org/10.1007/s00464-020-08226-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08226-8