Abstract
Background
This study sought to characterize soft and hard pancreatic textures radiologically and histologically, and to identify specific risks in a soft pancreas associated with postoperative pancreatic fistula (POPF) formation after pancreaticoduodenectomy (PD).
Methods
Consecutive 145 patients who underwent PD at a single institution between January 2010 and May 2013 were studied. Pancreatic consistency was intraoperatively judged as soft or hard. Pancreatic configuration was assessed using preoperative CT. Histologic components of the pancreatic stump were evaluated using a morphometric analysis. Clinicopathologic parameters were then analyzed for the risk of clinically relevant POPF.
Results
Compared with patients with a hard pancreas (n = 66), those with a soft pancreas (n = 79) had a smaller main pancreatic duct (MPD) diameter and a larger parenchymal thickness on CT, had a smaller fibrosis ratio and a larger lobular ratio histologically, and developed clinically relevant POPF more frequently (P < 0.001 for all). In patients with a soft pancreas, an MPD diameter <2 mm, a parenchymal thickness ≥10 mm, a lobular ratio <75%, and a fat ratio ≥20% were independently associated with clinically relevant POPF (P < 0.010 for all).
Conclusion
In patients with a soft pancreas, a thick parenchyma, a small MPD, and fatty infiltration were strongly associated with clinically relevant POPF after PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A soft pancreatic consistency is a well-known risk factor for postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD).1,2,3,4,5,6 Evaluation of the pancreatic texture is usually performed intraoperatively and subjectively by surgeons. In our previous study, the intraoperatively assessed pancreatic texture was significantly correlated with the elastic modulus of the resected pancreas measured objectively based on well-established physical rules, and also significantly correlated with the occurrence of POPF after PD.7
However, the intraoperative classification of pancreatic texture into just two categories, such as soft and hard, is sometimes difficult. In addition, a soft pancreatic texture is not an absolute indicator of the development of POPF after PD. Although pancreatic consistency can only be assessed intraoperatively and subjectively at the surgeon’s discretion, preoperative, objective, and quantitative evaluations of the pancreatic texture could lead to a more widely acceptable means of assessing the risk of POPF after PD. Moreover, the early and precise determination of the POPF risk would be useful for taking measures to prevent POPF, particularly for high risk patients. The aims of this study were to elucidate the radiologic and histologic findings that were correlated with differences in pancreatic texture, and to analyze the morphologic features of soft pancreas that were strongly associated with the development of clinically relevant POPF after PD.
Materials and Methods
Patients and Clinical Data Collection
The clinical courses of 145 consecutive patients who underwent PD at the National Cancer Center Hospital East between January 2010 and May 2013 were investigated. Clinicopathologic data were reviewed from the medical records. All the patients were preoperatively examined using pancreas-protocol contrast-enhanced multidetector row computed tomography (CT) as part of the diagnostic workup. PD was indicated for patients with malignancy, suspected malignancy, or a premalignant lesion. During this period, the reconstruction method for the remnant pancreas and the postoperative management protocol were standardized. The study was approved by the institutional review board of the National Cancer Center.
Surgical Techniques and Perioperative Management
Details of the surgical procedures and perioperative management protocol were described in our previous paper.8 A subtotal stomach-preserving PD9 and modified Child’s reconstruction were performed in all the cases. An end-to-side pancreaticojejunostomy with the placement of a 6Fr internal short stent through the main pancreatic duct (MPD) was performed as a two-layered anastomosis using interrupted duct-to-mucosa sutures and coverage of the entire cut surface of the pancreas with the seromuscular layer of the jejunum. The pancreatic consistency was judged by the surgeon as either soft or hard during the operation. A Jackson-Pratt type closed suction drain was placed in the vicinity of the pancreaticojejunal anastomosis, and the amylase level and cultures of the drainage fluid were evaluated on postoperative days 1, 3, and 5. Somatostatin analogues were never administered perioperatively to prevent or treat POPF. The definition of POPF was based on the classification of the International Study Group on Pancreatic Fistula (ISGPF).10 According to this classification, clinically relevant POPF was defined as grade B or C.
Schematic Understanding of Pancreatic Configuration
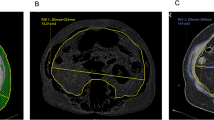
The configuration of the pancreatic stump was evaluated as described in our previous papers.8,11 The pancreatic stump was recognized as an ellipse, while the MPD was recognized as a circle (Fig. 1). Parameters including “stump thickness” and “MPD diameter” were measured using preoperative CT images obtained with a 2-mm slice at the pancreatic resection site, which was determined by comparison with postoperative CT images or by referring to the positional relationship with the adjacent vessels (e.g., right edge, middle, or left edge of the superior mesenteric vein) as mentioned in the operative notes (Fig. 2). “Parenchymal thickness” was defined as the difference between the stump thickness and the MPD diameter. All the CT scans were postoperatively reviewed in a retrospective manner by an experienced pancreatic radiologist (T.K.) who was blinded to the clinical data to ensure their usability for quantitative, anthropometric measurements. The measurements were performed by a single investigator (M.S.). This investigator was instructed and monitored closely by the pancreatic radiologist (T.K.) in the techniques required to obtain quantitative, anthropometric measurements from CT scans.
Assessment of pancreatic thickness and main pancreatic duct (MPD) diameter using preoperative pancreas-protocol multidetector contrast-enhanced CT images. Pancreatic thickness and MPD diameter were measured at the site of resection (in the middle of the superior mesenteric vein, in this case) on an axial CT image. Asterisk, superior mesenteric vein; number sign, superior mesenteric artery
Histologic Evaluation
The histologic evaluation was performed as described in our previous paper.7,12 Formalin-fixed, paraffin-embedded specimens obtained from a pancreatic stump were cut into 3-μm-thick serial sections. The sections were stained with hematoxylin and eosin (HE) to assess the areas of the entire cut surface and the MPD, and azan-Mallory (azan) staining was used to assess the degree of fibrosis. The slides were photographed using a NanoZoomer Digital Pathology virtual slide viewer (Hamamatsu Photonics, Hamamatsu, Japan) and were subjected to a morphometric analysis. The morphometric analysis was performed as described previously,7,12 and the details of the procedure are outlined in the legends of Figs. 3 and 4 in this paper. One investigator (M.S.) performed all the histologic analyses under the supervision of an experienced pathologist (M.K.).
Histologic evaluation and morphometric analysis of the pancreatic stump in case 1. The patient was a 30-year-old male who had undergone pancreaticoduodenectomy for a solid pseudopapillary neoplasm. This patient did not develop a clinically relevant postoperative pancreatic fistula. a Loupe image of a hematoxylin and eosin (HE)-stained slide. The arrow indicates a magnified view of the main pancreatic duct (MPD). The outer circumference of the entire cut surface (red line) and the inner circumference of the MPD lumen (blue line) were automatically outlined, and the corresponding areas were calculated using a tracing algorithm (WinROOF software, version 6.5; Mitani Corporation, Tokyo, Japan). The area of the entire cut surface (within the red line) was 269.9 mm2 and the MPD area (within the blue line) was 0.169 mm2. The MPD ratio was 0.063% when calculated as the percentage area of the MPD relative to the entire cut surface. b The HE-positive area was determined as the visualized area stained with HE using a color-detecting algorithm of the software and is represented as bright green in this image. The area of fat was defined as the area of the entire cut surface minus the MPD area and the HE-positive area. The fat ratio was 5.1% when calculated as the percentage area of the fat relative to the entire cut surface. c Loup image showing azan-Mallory (azan) staining, which was used to evaluate the degree of fibrosis. d The area of fibrosis was defined as the visualized area stained with aniline blue using the color-detecting algorithm of the software and is represented as bright green in this image. The fibrosis ratio was 2.7% when calculated as the percentage area of fibrosis relative to the entire cut surface. The lobular area was defined as the HE-positive area minus the MPD area and the fibrosis area. The lobular ratio was 92.1% when calculated as the percentage area of the lobules relative to the entire cut surface
Histologic evaluation and morphometric analysis of the pancreatic stump in case 2. The patient was a 31-year-old female who had undergone pancreaticoduodenectomy for a solid pseudopapillary neoplasm. This patient developed a grade B postoperative pancreatic fistula. a Loupe image of a hematoxylin and eosin-stained slide. The arrow indicates a magnified view of the main pancreatic duct (MPD). The area of the entire cut surface (within the red line) was 474.3 mm2 and the MPD area (within the blue line) was 0.263 mm2. The MPD ratio was 0.055%. b The fat ratio was 55.8%. c Loupe image showing azan-Mallory staining. d The fibrosis ratio was 3.2%, and the lobular ratio was 41.0%
Statistical Analysis
First, between patients who were intraoperatively judged as having a soft pancreas vs. a hard pancreas, the background, preoperative CT findings (pancreatic measurement based on a schematic understanding of the pancreatic configuration), intraoperative data, histologic findings of the pancreatic stump, and occurrence of POPF were compared. Categorical variables were evaluated using chi-square test and are presented as numbers and percentages, whereas continuous variables were evaluated using Mann–Whitney U test and are presented as the median and standard deviation.
Next, in patients with a soft pancreas, univariate and multivariate risk analyses for clinically relevant POPF (grade B/C) were performed using a logistic regression analysis. Parameters that were found to be significant in univariate analysis were included in multivariate analysis. To avoid considering parameters that varied with each other (confounding parameters), the correlations between the parameters were evaluated using Spearman’s correlation coefficient r. Receiver operating characteristic curves were used to set cutoff values for the continuous variables. All the P values were based on two-sided statistical tests and the significance level was set at 0.05. All the statistical analyses were performed using SPSS Statistics software (version 19.0; SPSS, Chicago, IL, USA).
Results
Differences Between Soft Pancreas and Hard Pancreas
Among the 145 patients who underwent PD, 79 patients (54.5%) were intraoperatively judged as having a soft pancreas and 66 patients (45.5%) were judged as having a hard pancreas. Table 1 shows the differences in the clinicopathologic parameters between the patients with a soft pancreas and those with a hard pancreas. Patients with a soft pancreas were younger than those with a hard pancreas (67 ± 11 vs. 70 ± 9 years, P = 0.047). Patients with a soft pancreas had a lower incidence of diabetes (17.7 vs. 30.4%, P = 0.011) and a lower incidence of a pathological diagnosis of pancreatic ductal adenocarcinoma (21.5 vs. 70.9%, P < 0.001). In terms of the preoperative CT findings, patients with a soft pancreas had a smaller MPD diameter (2.2 ± 1.9 vs. 5.8 ± 3.3 mm, P < 0.001) and a larger parenchymal thickness (9.2 ± 4.2 vs. 6.2 ± 2.8 mm, P < 0.001). No differences in intraoperative findings, such as operation time and estimated blood loss, were seen between the two groups. Regarding the histologic findings, patients with a soft pancreas had a larger area of the entire cut surface (226.6 ± 114.6 vs. 196.0 ± 70.5 mm2, P < 0.005), a smaller fibrosis ratio (3.0 ± 4.2 vs. 13.1 ± 10.9%, P < 0.001), and a larger lobular ratio (77.5 ± 14.1 vs. 56.5 ± 17.4%, P < 0.001). The fat ratios of the two groups were statistically similar (17.8 ± 13.8 vs. 22.3 ± 14.4%, P = 0.120).
Grade A POPF occurred in 26 patients (32.9%) with a soft pancreas and in 4 patients (5.1%) with a hard pancreas (P < 0.001). Twenty-seven patients (18.6%) out of a total of 145 patients developed clinically relevant POPF (grade B or C). Grade B POPF occurred in 24 patients (30.4%) with a soft pancreas and in 2 patients (2.5%) with a hard pancreas (P < 0.001). Only one patient with a soft pancreas developed grade C POPF. None of the patients died of surgical complications in this series.
Risk Analysis for Clinically Relevant POPF After PD in Patients with a Soft Pancreas
Since clinically relevant POPF occurred predominantly in patients with a soft pancreas, a risk analysis for clinically relevant POPF was performed for the 79 patients with a soft pancreas. As shown in Table 2, significant risk factors for clinically relevant POPF after PD were as follows: body mass index (risk ratio [RR], 1.179; P = 0.028), MPD diameter (RR, 0.459; P = 0.007), parenchymal thickness (RR, 1.170; P = 0.013), fibrosis ratio (RR, 0.861; P = 0.047), lobular ratio (RR, 0.958; P = 0.019), and fat ratio (RR, 1.061; P = 0.003).
Then, correlations between the parameters were evaluated. Among the above-mentioned significant parameters, a strong correlation was observed between the lobular ratio and the fat ratio (r = −0.914). These parameters were considered to have a confounding influence on each other.
Cutoff Values for Significant Parameters
To understand the clinical benchmarks for each continuous variable showing significance in univariate analysis (Table 2), the cutoff values were determined according to the areas under the receiver operating characteristic curves to assess the relationships between each parameter and the incidence of clinically relevant POPF (Table 3). The cutoff values were set as follows: 25 kg/m2 for body mass index, 2 mm for MPD diameter, 10 mm for parenchymal thickness, 2% for fibrosis ratio, 75% for lobular ratio, and 20% for fat ratio.
Multivariate Risk Analysis for Clinically Relevant POPF After PD Using Cutoff Values
A body mass index ≥25 kg/m2, an MPD diameter <2 mm, a parenchymal thickness ≥10 mm, a fibrosis ratio ≥2%, a lobular ratio <75%, and a fat ratio ≥20% were considered in multivariate analysis. Since a lobular ratio and a fat ratio were considered to have a confounding influence on each other, they were included separately in different multivariate analysis models (Table 4). In model 1, a body mass index ≥25 kg/m2, an MPD diameter <2 mm, a parenchymal thickness ≥10 mm, a fibrosis ratio ≥2%, and a lobular ratio <75% were included. The independent risk factors for clinically relevant POPF were an MPD diameter <2 mm (RR, 14.251; P < 0.001), a parenchymal thickness ≥10 mm (RR, 7.824; P = 0.007), and a lobular ratio <75% (RR, 10.946; P = 0.003). In model 2, a fat ratio ≥20% was included, replacing a lobular ratio <75%. Independent risk factors for clinically relevant POPF were an MPD diameter <2 mm (RR, 9.897; P = 0.001), a parenchymal thickness ≥10 mm (RR, 6.079; P = 0.010), and a fat ratio ≥20% (RR, 4.561; P = 0.026).
Discussion
A soft pancreatic texture has been accepted as a significant risk factor for POPF after PD,1,2,3,4,5,6,7 since it is usually associated with a small MPD (making the pancreaticojejunostomy stitches technically difficult to perform) and abundant lobules that produce an abundance of pancreatic juice and that may disrupt the pancreaticojejunostomy. The occurrence of clinically relevant POPF after PD, as determined according to the ISGPF criteria, is reportedly 11–37% for soft pancreas and 1–6% for hard pancreas.2,3,4,5,6 These rates are consistent with those observed in the present study (31.6 and 2.5%, respectively). Because the occurrence of clinically relevant POPF in patients with a hard pancreas was rare and almost negligible, the clinicopathologic findings that were associated with the development of clinically relevant POPF after PD were evaluated in patients with a soft pancreas.
As shown in Table 1, the differences in the clinicopathologic findings between patients with a soft pancreas and those with a hard pancreas were distinct. On preoperative CT images, patients with a soft pancreas had a smaller MPD diameter and a larger parenchymal thickness than those with a hard pancreas. A histologic evaluation showed a larger area of the entire cut surface, a smaller fibrosis ratio, and a larger lobular ratio for patients with a soft pancreas, compared with those with a hard pancreas. The fat ratio was not significantly different. Among the patients with a soft pancreas, an MPD diameter <2 mm, a parenchymal thickness ≥10 mm, a lobular ratio <75%, and a fat ratio ≥20% were independently associated with clinically relevant POPF after PD (Table 4).
Interestingly, the present study demonstrated the presence of two histologic patterns in a soft pancreas: preserved lobules without significant fatty infiltration and decreased lobules with significant fatty infiltration. A pancreas with either of these histologic patterns is likely to feel soft; however, the latter pattern appeared to be associated with a significantly greater risk of clinically relevant POPF after PD. Pancreatic parenchyma with the latter feature might be more friable and easier to be disrupted during the placement of the pancreaticojejunostomy stitches than pancreatic parenchyma with the former feature. Fatty degeneration and a decrease in the lobules were considered as processes that could progress synchronously in patients with a soft pancreas and that would elevate the risk of POPF.
“Fatty pancreas” is reportedly associated with a high risk of POPF. Mathur et al. demonstrated a negative correlation between pancreatic fat and fibrosis.13 They concluded that patients with increased fat and decreased fibrosis had a higher risk of POPF after PD. Rosso et al. showed a positive correlation between pancreatic fat and body mass index but did not observe any correlation between pancreatic fat and fibrosis, and they concluded that fatty infiltration >10% was a risk factor for POPF after PD.14 Gaujoux et al. showed that an increased body mass index, fatty pancreas, and the absence of fibrosis were associated with a risk of POPF after PD.15 While all these studies included both soft and hard pancreas tissues, hard pancreas is actually a distinct entity, exhibiting significant fibrosis, and is associated with a minimal risk of POPF. Also, none of these studies used a detailed morphometric analysis with quantification of the pancreatic components, and the relationship between fatty infiltration and the lobular volume was not investigated.
The present study focused on the patients with a soft pancreas and demonstrated that a pancreas with an MPD diameter <2 mm or a parenchymal thickness ≥10 mm on preoperative CT images was predictively associated with the occurrence of clinically relevant POPF after PD. Given the significant differences in these parameters between soft and hard pancreases, these predictive values are likely to be preserved even without the assessment of pancreatic texture during surgery. It should be noted that a pancreas with a thin parenchyma and an enlarged MPD did not have a high risk of POPF, even though the “pancreatic stump” was thick. This type of pancreatic configuration might represent atrophic pancreatic parenchyma with a decreased exocrine function: features of a hard pancreas. Measurement of the pancreatic configuration and assessment of the POPF risk before surgery was shown to be feasible using preoperative CT at the estimated site of the pancreatic resection.
Interestingly, the detailed morphometric analysis performed in this study also showed that a pancreas with decreased lobules (<75%) or significant fatty infiltration (≥20%) was also significantly associated with a greater risk of clinically relevant POPF after PD, independently from the preoperative CT findings. Because the risk of POPF should ideally be evaluated before surgery, any pancreas with these histologic features should also have been interpreted using preoperative imaging modalities. Lee et al. reported that a decrease in the relative signal intensity between in-phase and opposed-phase images obtained using magnetic resonance imaging was correlated with pancreatic fatty infiltration and the occurrence of POPF (grade A, B, or C) after PD.16 Wong et al. identified a fatty component threshold of 10.4% in the pancreas as being indicative of “fatty pancreas” in the general population, using magnetic resonance imaging with 3D technique.17 Failure to evaluate fatty infiltration in the pancreas using these dedicated imaging modalities accounts for one of the limitations of the current study.
Another important limitation of this study was the retrospective nature of this investigation. Our data should be validated in other studies. Moreover, the pancreatic consistency was assessed intraoperatively as just either soft or hard. However, a soft pancreas with decreased lobules and increased fatty infiltration might feel differently from one with preserved lobules and less fat. More precise intraoperative assessments of the pancreatic consistency during operation might be warranted.
In addition to a precise assessment of the POPF risk, safer operative techniques and perioperative management strategies, especially for high risk patients, are needed. Various anastomotic technique and pharmacological measures, such as pancreaticojejunostomy vs. pancreaticogastrostomy,18,19 duct-to-mucosa vs. invagination technique,20 Blumgart vs. Cattell Warren technique,21 use of an internal or external stent at the anastomosis,22,23 placement of an autologous graft at the anastomotic site,24 or the use of somatostatin analogues including pasireotide,25,26,27 have been studied; however, a consensus among all pancreatic surgeons regarding definitive means of preventing or minimizing POPF has not yet been established. Surgeon experience and/or socioeconomical issues also seem to be relevant. In the near future, reconstruction techniques and perioperative management should be tailored to individual patients according to their precise risk of POPF.
In conclusion, distinct differences in preoperative CT findings, morphometric data for histologic specimens, and the occurrence of clinically relevant POPF after PD were observed between patients with a soft pancreas and those with a hard pancreas. In patients with a soft pancreas, an MPD diameter <2 mm or a parenchymal thickness ≥10 mm on preoperative CT images or a lobular ratio <75% or a fat ratio ≥20% on histologic specimens was associated with the occurrence of clinically relevant POPF after PD. Assessment of pancreatic configuration on preoperative CT was shown to connote a predictive value for clinically relevant POPF. Fatty infiltration with decreased lobules in pancreas was considered a degenerative process containing risk for clinically relevant POPF.
References
Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg 2004;8:951–959.
Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008;32:419–428.
Wellner UF, Kayser G, Lapshyn H, Sick O, Makowiec F, Höppner J, Hopt UT, Keck T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB (Oxford) 2010;12:696–702.
Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, Unno M, Satoi S, Kwon AH, Hatori T, Yamamoto M, Matsumoto J, Murakami Y, Doi R, Ito M, Miyakawa S, Shinchi H, Natsugoe S, Nakagawara H, Ohta T, Takada T. Predictive risk factors for clinically-relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:601–608.
Ansorge C, Strömmer L, Andrén-Sandberg Å, Lundell L, Herrington MK, Segersvärd R. Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. Br J Surg 2012;99:1076–1082.
El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A, Abdel Wahab M, Abdallah T. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg 2013;37:1405–1418.
Sugimoto M, Takahashi S, Kojima M, Gotohda N, Kato Y, Kawano S, Ochiai A, Konishi M. What is the nature of pancreatic consistency? Assessment of the elastic modulus of the pancreas and comparison with tactile sensation, histology, and occurrence of postoperative pancreatic fistula after pancreaticoduodenectomy. Surgery 2014;156:1204–1211.
Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, Konishi M. Schematic pancreatic configuration: A risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 2013;17:1744–1751.
Hayashibe A, Kameyama M, Shinbo M, Makimoto S. The surgical procedure and clinical results of subtotal stomach preserving pancreaticoduodenectomy (SSPPD) in comparison with pylorus preserving pancreaticoduodenectomy (PPPD). J Surg Oncol 2007;95:106–109.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13.
Sugimoto M, Gotohda N, Kato Y, Takahashi S, Kinoshita T, Shibasaki H, Nomura S, Konishi M, Kaneko H. Risk factor analysis and prevention of postoperative pancreatic fistula after distal pancreatectomy with stapler use. J Hepatobiliary Pancreat Sci 2013;20:538–544.
Sugimoto M, Takahashi S, Kobayashi T, Kojima M, Gotohda N, Satake M, Ochiai A, Konishi M. Pancreatic perfusion data and post-pancreaticoduodenectomy outcomes. J Surg Res 2015;194:441–449.
Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg 2007;246:1058–1064.
Rosso E, Casnedi S, Pessaux P, Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D, Bachellier P. The role of “fatty pancreas” and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 2009;13:1845–1851.
Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148:15–23.
Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg 2010;251:932–936.
Wong VW, Wong GL, Yeung DK, Abrigo JM, Kong AP, Chan RS, Chim AM, Shen J, Ho CS, Woo J, Chu WC, Chan HL. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol 2014;109:589–597.
Crippa S, Cirocchi R, Randolph J, Partelli S, Belfiori G, Piccioli A, Parisi A, Falconi M. Pancreaticojejunostomy is comparable to pancreaticogastrostomy after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Langenbecks Arch Surg 2016;401:427–437.
Qin H, Luo L, Zhu Z, Huang J. Pancreaticogastrostomy has advantages over pancreaticojejunostomy on pancreatic fistula after pancreaticoduodenectomy. A meta-analysis of randomized controlled trials. Int J Surg 2016;36:18–24.
Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, Hyslop T, Schmidt CM, Rosato EL, Lavu H, Nakeeb A, Pitt HA, Lillemoe KD, Yeo CJ. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg 2009;208:738–747.
Halloran CM, Platt K, Gerard A, Polydoros F, O’Reilly DA, Gomez D, Smith A, Neoptolemos JP, Soonwalla Z, Taylor M, Blazeby JM, Ghaneh P. PANasta Trial; Cattell Warren versus Blumgart techniques of panreatico-jejunostomy following pancreato-duodenectomy: Study protocol for a randomized controlled trial. Trials 2016 Jan 15;17:30.
Moriya T, Clark CJ, Kirihara Y, Kendrick ML, Reid Lombardo KM, Que FG, Farnell MB. Stenting and the rate of pancreatic fistula following pancreaticoduodenectomy. Arch Surg 2012;147:35–40.
Hong S, Wang H, Yang S, Yang K. External stent versus no stent for pancreaticojejunostomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg 2013;17:1516–1525.
Tani M, Kawai M, Hirono S, Hatori T, Imaizumi T, Nakao A, Egawa S, Asano T, Nagakawa T, Yamaue H. Use of omentum or falciform ligament does not decrease complications after pancreaticoduodenectomy: nationwide survey of the Japanese Society of Pancreatic Surgery. Surgery 2012;151:183–191.
Allen PJ, Gönen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, Carlucci KE, D’Angelica MI, DeMatteo RP, Kingham TP, Fong Y, Jarnagin WR. Pasireotide for postoperative pancreatic fistula. N Engl J Med 2014;370:2014–2022.
Ma LW, Dominguez-Rosado I, Gennarelli RL, Bach PB, Gonen M, D’Angelica MI, DeMatteo RP, Kingham TP, Brennan MF, Jarnagin WR, Allen PJ. The Cost of Postoperative Pancreatic Fistula Versus the Cost of Pasireotide: Results from a Prospective Randomized Trial. Ann Surg 2016;265:11–16.
Goyert N, Eeson G, Kagedan DJ, Behman R, Lemke M, Hallet J, Mittmann N, Law C, Karanicolas PJ, Coburn NG. Pasireotide for the Prevention of Pancreatic Fistula Following Pancreaticoduodenectomy: A Cost-effectiveness Analysis. Ann Surg 2016;265:2–10.
Author Contribution
Sugimoto designed the study and wrote the initial draft of the manuscript. Takahashi contributed to interpretation of the data and the critical revision of the manuscript for important intellectual content. All the other authors (Kojima, Kobayashi, Gotohda, and Konishi) contributed to the data collection and interpretation and critically reviewed the manuscript. All the authors have read and approved the final version of the manuscript, and have agreed to be accountable for all aspects of the study, ensuring that any questions related to the accuracy or integrity of any part of the work are answerable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugimoto, M., Takahashi, S., Kojima, M. et al. In Patients with a Soft Pancreas, a Thick Parenchyma, a Small Duct, and Fatty Infiltration Are Significant Risks for Pancreatic Fistula After Pancreaticoduodenectomy. J Gastrointest Surg 21, 846–854 (2017). https://doi.org/10.1007/s11605-017-3356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3356-7