Abstract
Background
Laparoscopic pancreaticoduodenectomy (LPD), an advanced minimally invasive technique, has demonstrated advantages to open pancreaticoduodenectomy (OPD). However, this complex procedure requires a relatively long training period to ensure technical proficiency. This study was therefore designed to analyze the learning curve for LPD.
Methods
From October 2010 to September 2015, 63 standard pancreaticoduodenectomy procedures were to be performed laparoscopically by a single surgeon at the Department of Pancreatic Surgery, West China Hospital, Sichuan University, China. After applying the inclusion and exclusion criteria, a total of 57 patients were included in the study. Data for all the patients, including preoperative, intraoperative, and postoperative variables, were prospectively collected and analyzed. The learning curve for LPD was evaluated using both cumulative sum (CUSUM) and risk-adjusted CUSUM (RA-CUSUM) methods. All of the variables among the learning curve phases were compared.
Results
Based on the CUSUM and the RA-CUSUM analyses, the learning curve for LPD was grouped into three phases: phase I was the initial learning period (cases 1–11), phase II represented the technical competence period (cases 12–38), and phase III was regarded as the challenging period (cases 39–57). The operative time, intraoperative blood loss, and postoperative ICU demand significantly decreased with the learning curve. More lymph nodes were collected after the initial learning period. There were no significant differences in terms of postoperative complications or the 30-day mortality among the three phases. More challenging cases were encountered in phase III.
Conclusions
According to this study, the learning curve for LPD consisted of three phases. Conservatively, to attain technical competence for performing LPD, a minimum of 40 cases are required for laparoscopic surgeons with a degree of laparoscopic experience.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreaticoduodenectomy (PD) has been regarded historically as the most complicated abdominal surgery. Over the past few decades, the surgical mortality following PD has decreased to below 6.0 % at many high-volume centers,1–3 though the morbidity remains as high as 32.5 to 62.0 %.4–6 The improved results observed with laparoscopic surgery have made this an alternative intervention to open surgery for patients with certain surgical diseases. This technique has also been extended to pancreaticoduodenectomy.
Since laparoscopic pancreaticoduodenectomy (LPD) was first introduced in 1994,7 the acceptance and popularity of this procedure has decreased due to the inherent technical limitations of laparoscopy and the requirement for advanced laparoscopic skills. However, over the past 10 years, an increasing number of studies have affirmed the safety, feasibility, and acceptable oncological outcomes of LPD and have emphasized its advantages over open pancreaticoduodenectomy (OPD) in terms of earlier oral intake, less blood loss, shorter posthospital stay, less pain, and faster recovery.8–11
Despite the potential advantages of LPD, it is well known that surgeons require a relatively long training period to become technically proficient in this challenging operation. Although two previous studies showed the learning curve for LPD with 30 and 56 cases, respectively, the results were largely based on simple statistical analyses.12,13
An in-depth analysis is needed to assess the learning curve for LPD. The medical profession adopted the cumulative sum (CUSUM) and risk-adjusted CUSUM (RA-CUSUM) methods to analyze learning curves for surgical procedures.14,15 Thus far, to the best of our knowledge, the learning curve for LPD using both the CUSUM and RA-CUSUM methods has not been evaluated in any studies.
Therefore, we conducted this prospective study to assess the learning curve using the CUSUM and RA-CUSUM methods in LPD and to compare clinicopathological and postoperative outcomes according to this learning curve.
Methods and Materials
Patient Selection
From October 2010 to September 2015, a series of 112 consecutive standard pancreaticoduodenectomy procedures were to be performed by a single surgeon at the Department of Pancreatic Surgery, West China Hospital, Sichuan University, China. Sixty-three patients were to undergo LPD, whereas the remaining 49 cases were scheduled to undergo orthodox OPD. The selection criteria for the standard LPD included: (1) body mass index (BMI) <28.0 kg/m2; (2) ampullary tumors, duodenal tumors restricted to the second part of the duodenum, lower common bile duct tumors, and pancreatic tumors at the pancreatic head; and (3) carefully confirmed resectability of the tumors based on preoperative radiology conducted by a professional radiologist and experienced surgeons. Patients with greater than 180° superior mesenteric artery encasement, any celiac abutment, unreconstructable superior mesenteric vein/portal occlusion, and aortic invasion or encasement, with a history of previous upper abdominal surgery, tumors extending to the uncinate, and with severe cardiorespiratory comorbidities, were excluded from undergoing LPD.9,16 The final decision on whether to perform an OPD or LPD was left to the discretion of the surgeon and the patients. Those undergoing a synchronous operation (n = 3), a palliative operation (n = 2), or laparoscopic total pancreatectomy (n = 1) due to intraoperative findings were excluded in the analysis of the learning curve. The detailed inclusion and exclusion criteria in the analysis of learning curve are illustrated in Fig. 1.
All the patients were thoroughly informed about the procedure, risks, and the advantages of LPD, as well as the potential for conversion to laparotomy. Written informed consent was obtained from all the patients in our study, which was approved by the ethics committee of Sichuan University.
Data Collection and Definitions
The data were prospectively collected and entered into a database for analysis and included the following preoperative, intraoperative, and postoperative variables:
-
1.
Preoperative data: age, gender, BMI, American Society of Anesthesiology (ASA) score, carcinoembryonic antigen (CEA) level, carbohydrate antigen 19–9 (CA19-9) level, total bilirubin (TB), pancreatic duct width, initial symptoms, and the presence of comorbidities;
-
2.
Intraoperative data: operative time, conversion rate, transfusion rate, estimated blood loss, and pancreas texture (soft/firm); and
-
3.
Postoperative data: necessary transport to the intensive care unit (ICU), demand for analgesia, postoperative stay, time to first passage of flatus and oral intake, final pathologic results, short-term complications, incidence of reoperation, and 30-day mortality.
The primary endpoint was operative time and this was used for the CUSUM analysis. The secondary endpoints, including conversion rate, postoperative complications, and final pathologic outcomes, were used for the RA-CUSUM analysis and for comparisons among the learning curve phases.
Operative time was defined as the duration from the first incision to final closure; conversion was defined as the requirement for laparotomy at any time during the LPD except extraction of the resected specimen. Estimated blood loss was measured by the weight of the swabs plus the blood removed during the procedure. The surgeon assessed the pancreas texture (soft/firm) according to the margin of the resected specimen. The pancreatic duct width (≤3 or >3 mm) was measured from preoperative CT/MRI imaging anteroposteriorly at the level at which the portal vein passes posterior to the pancreatic neck. Demand for analgesia indicated that postoperative patient-controlled analgesia (PCA), 250 μg fentanyl diluted to 200 mL in normal saline, was initiated when the patients were unable to tolerate postoperative pain within 24 h after the LPD. The postoperative stay was defined as the interval from the surgery to the day of discharge. For tumor cases, the final pathological results were recorded by tumor-node-metastasis (TNM) staging, the histological grade of differentiation, total number of harvested lymph nodes, resection margin status, lymph node invasion status, and mass size based on the AJCC Cancer Staging Manual, Seventh Edition.16 R0 resection indicated that no evidence of malignancy was identified at any of the resection margins, and R1 resection was defined as malignancy infiltrating at least one of the resection margins on permanent section. Short-term complications, which were stratified by the Clavien–Dindo classification of surgical complications, indicated morbidities within 30 postoperative days.17 Postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE), and postpancreatectomy hemorrhage (PPH) were defined according to the ISGPS.18–20 Reoperation was defined as a secondary operation due to severe complications within 30 days following LPD. The patients were discharged when oral intake and moderate activity were tolerated without any abnormal postoperative complications or laboratory findings.
Perioperative Surveillance and Surgical Procedures
Perioperative surveillance and surgical procedures were described in detail in our previous study.9 Despite the expertise of the surgeon (Bing Peng) in laparoscopic surgery,21 laparoscopic pancreaticoduodenectomy began with hand-assisted LPD, switched to total LPD, and gradually progressed to laparoscopic pylorus-preserving pancreaticoduodenectomy (LPPPD), considering that LPD is a challenging operation for most surgeons. This sequence itself was a reflection of the learning curve. Furthermore, our previous study indicated that no significant differences were observed among these three operation types in terms of intraoperative parameters or postoperative complications.9 Therefore, hand-assisted LPD, total LPD, and LPPPD were regarded as homogeneous LPD methods in the analysis of the learning curve.
Statistical Analysis
For quantitative data, the results were expressed as the mean ± standard deviation; median with interquartile range (IQR) was used for skewed quantitative data. For categorical data, the results were expressed as the number and percentage of cases. All statistical analyses, including one-way analysis of variance among groups (ANOVA), Kruskal–Wallis test followed by all pairwise multiple comparisons, χ2 test, Fisher’s exact test, and multivariate logistic regression with the backward stepwise method and likelihood ratio, were performed using the SPSS statistical software package (version 19.0, SPSS Inc., Chicago, IL, USA). The level for rejection of the null hypothesis was set at a P value of <0.05. Additionally, P < 0.0167 was considered statistically significant after Bonferroni correction for the post hoc analysis.
In this study, we analyzed the learning curve for LPD using the CUSUM and RA-CUSM methods.
CUSUM Method
The CUSUM technique is a graphical method that detects data trends, which is not discernible in other approaches, by calculating the sequential difference between the raw data and the mean value. In this study, the cases were first ordered chronologically from the first to the last case, and the CUSUM of the operation time (CUSUMOT) was defined as CUSUMOT = ∑ n i = 1 (x i − μ), where x i is an individual operation time, and μ is the mean overall operation time. The learning curve regarding the operation time was represented intuitively and determined by plotting the outcomes in the CUSUM curve.
RA-CUSUM Method
Risk factors associated with surgical failure might confound the outcomes from the CUSUM method. The RA-CUSUM method, an extension of CUSUM, was applied to further assess the learning curve in this study. The relevant parameters were selected to assess the failure of LPD: conversion to OPD, postoperative complications (Clavien ≥ III), and 30-day death related to LPD. In addition, LPD failure was defined as the occurrence of one of these three events.
To analyze the risk factors associated with LPD failure, all preoperative and intraoperative data and the final pathological outcomes except the three parameters mentioned above were examined by univariate analysis. When the P values were less than 0.10 on univariate analysis, the data were considered for multivariate logistic regression to calculate the probability of LPD failure, with a P value set at less than 0.05.
RA-CUSUM was defined as RA-CUSUM \( {\displaystyle {\sum}_{i=1}^n\left({x}_i-\tau \right)}+{\left(-1\right)}^{x_i}{P}_i \), where x i = 1 indicates the presence of LPD failure; otherwise, x i = 0; τ represents the observed event rate, and P i is the expected rate of LPD failure in each case, which was calculated from the logistic regression model.
Thus, every included case was plotted from left to right on the horizontal axis, and the RA-CUSUM line shifted downward for LPD success and upward for LPD failure.
Results
Basic Characteristics of All Patients
The mean age of the 57 enrolled patients (33 men and 24 women) was 61.3 ± 11.0 years. Seventeen (29.8 %) and 40 (70.2 %) patients were classified as ASA II and III, respectively. Jaundice and epigastric pain were the predominant initial symptoms. The median operative time was 480.0 min (IQR 453.0–540.0 min), and the median estimated intraoperative blood loss was 200.0 mL (IQR 150.0–400.0 mL). Conversion to OPD occurred in five (8.8 %) cases. All the patients underwent R0 resection. Based on the Clavien–Dindo classification, there were 22 (38.6 %) cases of grades I–II and 7 (12.3 %) cases of grades III–IV postoperative complications. The 30-day mortality following LPD was zero.
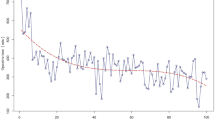
Learning Curve Analysis (Figs. 2 and 3)
The learning curve was first assessed by the CUSUM method. Based on the CUSUMOT graph (Fig. 2), there were two peak points, which were observed at the 11th and 31st cases. Therefore, three phases can be initially differentiated on the graph: phase I, cases 1–11; phase II, cases 12–31; and phase III, cases 32–57. Although the CUSUMOT graph stabilized after 11 cases and had a tendency to descend after the 31st case, the decreased operative time itself did not signify competence in and success of LPD. Therefore, the learning curve was further evaluated by the RA-CUSUM method. The three aforementioned parameters were considered to assess the probability of LPD failure. According to the RA-CUSUM graph (Fig. 3), the valley point at the 38th case represented the minimized surgical failure, and this point could be considered as the point at which competence in LPD was achieved. Combining the results from the CUSUM and the RA-CUSUM methods, the learning curve for LPD was finally determined and this consisted of three phases. Phase I represented the initial learning curve, which spanned 11 cases (cases 1–11); phase II (cases 12–38) represented increased competence in LPD; and phase III (cases 39–57) began after 38 cases and represented mastery and the challenging period. During the same period, 20 (64.5 %), 23 (46.0 %), and 6 (24.0 %) OPD procedures were performed in the three phases, respectively, and a higher percentage of patients offered LPD indicates through the learning curve that we are taking on more challenging cases (Fig. 4).
Comparison of Patient Characteristics Among the Three Consecutive Phases (Table 1)
No significant differences among the three phases were detected with regard to most preoperative characteristics. There was an increased tendency for the presentation of comorbidities with the learning curve, suggesting that more challenging cases were handled in the later learning curve period.
Comparison of Perioperative Outcomes Among the Three Consecutive Phases (Table 2)
The operation types were significantly different among the three phases (P < 0.001). There was an inclination to perform more LPPPDs after the initial learning period [phase I vs. phase II, 0 vs. 17 (63.0 %), P < 0.0167; phase I vs. phase III, 0 vs. 16 (84.2 %), P < 0.001]. However, the distribution of operation types between phases II and III was comparable (P = 0.596). The operation time tended to decrease with the accumulation of experience, and this time stabilized after the initial learning period. Compared with phase I, the intraoperative blood loss was significantly lower in phase III [phase I vs. phase III, 400.0 mL (IQR 300.0–500.0 mL) vs. 150.0 mL (IQR 100.0–300.0 mL), P < 0.0167]. Compared with phase II, more patients in phase I required ICU admission for further care [phase I vs. phase II, 9 (81.8 %) vs. 7 (25.9 %), P < 0.0167]. There were significant differences regarding the length of postoperative stay among the three phases (P < 0.05); however, interphase comparisons showed no significant differences. All other comparisons without significant differences among the three phases are detailed in Table 2.
Comparison of Postoperative Complications Among the Three Consecutive Phases (Table 3)
Grades I–II postoperative complications showed a tendency to decline with increasing experience (P < 0.05), but this trend was not statistically significant when making interphase comparisons. Grade B POPF was the most common grade I–II postoperative complication, occurring in eight (14.0 %) patients; the next was grade B DGE, which occurred in four (7.0 %) patients. There were no significant differences in severe postoperative complications (grade ≥III) among all the learning phases. All the patients with grade ≥III postoperative complications required surgical or endoscopic interventions. The 30-day mortality was zero in each phase. Individual complications are listed in detail in Table 3.
Pathologic Outcomes (Table 4)
Analysis of the final pathologic outcomes showed that there was no significant difference with regard to the pathologic diagnosis distribution among all of the learning phases (P = 0.140). However, LPD was performed in more patients with pancreatic ductal adenocarcinoma in phase III than in phase I [phase I vs. phase III, 0 vs. 7 (36.8 %)]. Because the staging parameters for different malignancies vary, TNM staging and tumor differentiation were confirmed for individual cases according to the AJCC Cancer Staging Manual, Seventh Edition and were listed in detail instead of being compared among the three phases. Tumor size was significantly different among the three phases (P < 0.05). Although no significant difference was observed regarding the tumor size according to interphase comparisons, there was a tendency for tumor size to increase from phase I to phase III (phase I vs. phase II vs. phase III, 1.6 ± 0.9 cm vs. 2.5 ± 1.1 cm vs. 2.6 ± 1.3 cm). More lymph nodes were collected after the initial learning period. R0 resection was performed in all the patients in each phase. All other pathologic information is provided in Table 4.
Discussion
Two decades after the first reported case, laparoscopic pancreaticoduodenectomy remains limited to several expert centers.7 Some inspiring results from recently published studies presented the obvious advantages and superiority of LPD over OPD, mainly in terms of earlier oral intake, less blood loss, shorter posthospital stay, less pain, and quicker recovery. However, the intrinsic limitations of laparoscopic surgery, including reduced freedom of movement, 2D view, reduced precision, and poor ergonomics, contribute to the requirement of a long training period to become technically proficient in LPD. Although the learning curves for some major laparoscopic procedures have been well established using the CUSUM and/or RA-CUSUM methods, no studies have been conducted to determine the learning curve for laparoscopic pancreaticoduodenectomy using both of these methods.22–24
Although two previous publications assessed the learning curve for LPD (Table 5), the results were limited by simply splitting chronological cases into predefined segments. Kuroki et al. reported a learning curve of 10 cases among 30 consecutive patients, indicating that operative time and blood loss significantly decreased after surmounting the learning curve.13 Speicher et al. drew the same conclusion, suggesting that 10 to 50 cases might represent the learning curve for LPD.12 In the present study, the learning curve for LPD was determined using both the CUSUM and RA-CUSUM methods. The three phases of the learning curve were defined as the initial period, the competence period, and the challenging period. When determining the learning curve, we considered not only the operative time but also the failure of LPD because laparoscopic surgical completion needed technical mastery to achieve surgical outcomes (including lower conversion rate, lower complication rate, lower surgical mortality, and oncologic safety), as well as a quicker operative time.15,25–27 In this study, the RA-CUSUM method was applied to evaluate the parameters affecting surgical outcomes. The minimum surgical failure was observed by the 38th case, as seen in Fig. 3, and the 38th case was located after the plateau (case 11 to case 31), as seen in Fig. 2, indicating that although the learning curve had been overcome by the 31st case in terms of operative time, the probability of LPD failure did not reach the lowest point until case 38. Therefore, case 38 was regarded as the point at which to achieve competence in LPD in this study. However, it is notable that after the valley point, the RA-CUSUM graph showed increased surgical failure, which can be explained by the shift toward more technically demanding procedures and high-risk patients in the later period of the learning curve. As shown in Table 1, more patients with preoperative comorbidities (particularly diabetes) were included after case 38, which makes the patients more susceptible to suffering major postoperative complications. As shown in Table 4, more patients with pancreatic ductal adenocarcinoma (seven cases in total) were included in phase III, and two of them were converted to OPD due to bleeding from the portal vein. Both these patients contributed to the decreased surgical success in the later period. Similar findings have also been reported in previous studies.15,24,27
While some earlier studies have shown data that support the feasibility and advantages of implementing LPD,10,11 the long operative time of LPD remains a topic of debate. Dokmak et al. indicated that surgical time for LPD was still longer than for OPD, even in the late learning period.28 Although the relationship between long operative time and postoperative outcomes is unclear, it can be harmful, and this was recently reported with colonic laparoscopic surgery.29 In this study, the operative time decreased significantly with the learning curve, but it appeared that the operative time itself was not associated with the postoperative outcomes. Additional studies are needed to corroborate this result.
The conversion rate is commonly regarded as an important criterion to evaluate the quality of laparoscopic surgery. The rate of laparoscopic conversion to OPD varies among studies. According to a recently published systematic review, the overall conversion rate was 9.1 % (range, 0 to 40.0 %).30 In this study, the overall conversion rate was 8.8 %, and the conversions were principally due to bleeding resulting from adhesion of the tumor to the portal vein (n = 3, 5.3 %), pneumoperitoneum intolerance (n = 1, 1.8 %), or severe intra-abdominal adhesion (n = 1, 1.8 %). Previous studies have reported a trend of decreasing conversion rate with accumulation of experience31,32; however, in this study, the opposite was observed, which might be interpreted as being due to pancreaticoduodenectomy itself being a major operation, the increased freedom of surgeons to select patients, and the increased willingness of surgeons to handle challenging cases after achieving competence in LPD. Therefore, it is strongly believed that the conversion rate is heavily dependent on patient selection and operative policy rather than on technical skill and experience. Additionally, a conversion rate of zero should not be regarded as a realistic goal during the learning curve for LPD.
Various studies have shown a decreased incidence of postoperative complications with increasing laparoscopic experience33; however, this result was not demonstrated in the current study. In contract, more severe postoperative complications (grade ≥III) were encountered in phase III, although there was no significant difference among the three phases. This paradox can be attributed to the rising proportion of more challenging and high-risk cases as the surgeon gains experience and confidence. In addition, the observed length of the postoperative stay was longer in phase III than in phases I and II, and this result can be interpreted as being the result of more severe postoperative complications in phase III and the surgeon’s intention for an additional and closer postoperative observation for patients with severe complications.
Given that pancreaticoduodenectomy is often performed in patients with malignancy, it is necessary and important to explore whether the oncological outcomes might be compromised during the initial training period due to a surgeon’s lack of expertise. Although in the present study, the numbers of harvested lymph nodes in phase I were fewer than those in phases II and III, the median number of lymph nodes collected in phase I was 11, which approached the recommended range (11–17 lymph nodes) for the minimum number of collected lymph nodes necessary to provide optimal staging and to serve as an indicator of quality.34,35 In addition, the rate of R0 resection was achieved in all patients in the three phases, suggesting that the short-term oncological outcomes can be acceptable, even in the early learning curve period. However, whether the learning curve for LPD will influence the long-term oncological outcomes is still unclear and cannot be determined based on this study.
One could argue that the types of surgery used in the current study, including hand-assisted, total LPD and LPPPD, were heterogeneous, which may confound our results. In our opinion, homogeneous types of operation might show similar results compared with heterogeneous ones. There are several reasons to support this opinion. First, the most essential, challenging, and time-consuming aspects of LPD are the dissection of the tumor from the portal vein and pancreatojejunostomy, both of which were performed in the same manner regardless of the surgical types used in this study. For this aspect, the different surgical types had little influence on operative time and surgical performance. Second, in our previous study, no significant differences were observed regarding the intraoperative and postoperative outcomes among the three different surgical types.9 Third, the shift itself from hand-assisted to LPPPD reflected the training process of LPD, and it is radical for a surgeon to directly perform LPPPD. Overall, we think that the learning curves for homogeneous LPD and heterogeneous LPD may not differ.
It should be emphasized that before commencing LPD, this surgeon (Bing Peng) had performed over 30 open pancreaticoduodenectomy procedures, and this complicated procedure was completely mastered by the open approach. The surgeon also performed over 300 laparoscopic splenectomy procedures, 4 laparoscopic spleen-preserving distal pancreatectomy operations, and 17 consecutive laparoscopic cholecystectomy and splenectomy procedures before and during the period when LPD was performed. These skill sets likely enhanced the ability of the surgeon to finish LPD successfully.21,36,37 Therefore, the results from this study might not expand to surgeons lacking a similar level of laparoscopic skills.
It can be argued that this study had limitations, including the small number of patients, data being from a single center, the heterogeneous operation types, the heterogeneous tumor types, and the absence of long-term results. Thus, more studies from multiple centers are needed to support our conclusions.
In summary, based on this study, the learning curve for LPD consisted of three phases. The first 11 cases represented the initial learning curve period, the next 27 cases represented the technical competence period, and the 38th case was the turning point after which more challenging cases were encountered. The learning curve was associated with decreased operative time, blood loss, and postoperative ICU demand. Conservatively, to attain technical competence in LPD, a minimum of 40 cases is required for laparoscopic surgeons with a certain degree of laparoscopic experience.
References
de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, Porte RJ, Gouma DJ, Busch OR, Molenaar IQ, Dutch Pancreatic Cancer G: Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg 2012, 99: 404–10.
Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V: Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg 2008, 95: 357–62.
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA: Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997, 226: 248–57; discussion 57–60.
Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, Kim SG, Kim SW: A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 2014, 259: 656–64.
Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY, Pawlik TM: Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012, 16: 1727–35.
Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, Friess H, Buchler MW: Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg 2005, 92: 547–56.
Gagner M, Pomp A: Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994, 8: 408–10.
Tran TB, Dua MM, Worhunsky DJ, Poultsides GA, Norton JA, Visser BC: The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc 2015. doi: 10.1007/s00464-015-4444-y
Wang M, Zhang H, Wu Z, Zhang Z, Peng B: Laparoscopic pancreaticoduodenectomy: single-surgeon experience. Surg Endosc 2015, 29: 3783–94.
Croome KP, Farnell MB, Que FG, Reid-Lombardo K, Truty MJ, Nagorney DM, Kendrick ML: Total Laparoscopic Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma: Oncologic Advantages Over Open Approaches? Ann Surg 2014, 260: 633–40.
Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ: Matched Case–control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015, 262: 146–55.
Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG, 3rd, Clary BM, Pappas TN, Tyler DS, Perez A: Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014, 21: 4014–9.
Kuroki T, Kitasato A, Adachi T, Tanaka T, Hirabaru M, Matsushima H, Soyama A, Hidaka M, Takatsuki M, Eguchi S: Learning Curve for Laparoscopic Pancreaticoduodenectomy: A Single Surgeon's Experience with Consecutive Patients. Hepatogastroenterology 2014, 61: 838–41.
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM: Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011, 25: 855–60.
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK: Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 2014, 28: 2821–31.
American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M: The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009, 250: 187–96.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M: Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005, 138: 8–13.
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW: Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142: 761–8.
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW: Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142: 20–5.
Wang X, Li Y, Crook N, Peng B, Niu T: Laparoscopic splenectomy: a surgeon's experience of 302 patients with analysis of postoperative complications. Surg Endosc 2013, 27: 3564–71.
Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B: Learning curve for laparoscopic major hepatectomy. Br J Surg 2015, 102: 796–804.
Okrainec A, Ferri LE, Feldman LS, Fried GM: Defining the learning curve in laparoscopic paraesophageal hernia repair: a CUSUM analysis. Surg Endosc 2011, 25: 1083–7.
Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, Mokart D, Houvenaeghel G, Giovannini M, Delpero JR: The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 2010, 251: 249–53.
Song MH, Kuwabara F, Ito T: Risk-adjusted mortality rate cumulative sum analysis based on the Japan SCORE represents a learning curve in mitral valve reparative surgery. Surg Today 2014, 44: 1253–7.
Kim HJ, Choi GS, Park JS, Park SY: Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon's experience. Dis Colon Rectum 2014, 57: 1066–74.
Son G-M, Kim J-G, Lee J-C, Suh Y-J, Cho H-M, Lee Y-S, Lee I-K, Chun C-S: Multidimensional Analysis of the Learning Curve for Laparoscopic Rectal Cancer Surgery. Journal of Laparoendoscopic & Advanced Surgical Techniques 2010, 20: 609–17.
Dokmak S, Fteriche FS, Aussilhou B, Bensafta Y, Levy P, Ruszniewski P, Belghiti J, Sauvanet A: Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 2015, 220: 831–8.
Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP: Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum 2014, 57: 616–22.
Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S, Mosca F: Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015, 29: 9–23.
Marusch F, Gastinger I, Schneider C, Scheidbach H, Konradt J, Bruch HP, Kohler L, Barlehner E, Kockerling F, Laparoscopic Colorectal Surgery Study G: Experience as a factor influencing the indications for laparoscopic colorectal surgery and the results. Surg Endosc 2001, 15: 116–20.
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW: Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005, 242: 83–91.
Larach SW, Patankar SK, Ferrara A, Williamson PR, Perozo SE, Lord AS: Complications of laparoscopic colorectal surgery. Analysis and comparison of early vs. latter experience. Dis Colon Rectum 1997, 40: 592–6.
Valsangkar NP, Bush DM, Michaelson JS, Ferrone CR, Wargo JA, Lillemoe KD, Fernandez-del Castillo C, Warshaw AL, Thayer SP: N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013, 17: 257–66.
Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, Donohue J, Nagorney D, Farnell M, Sarr M: Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg 2012, 16: 920–6.
Cai Y, Peng B, Mai G, Ke N, Liu X: Laparoscopic distal pancreatectomy for solid-pseudopapillary tumor of the pancreas. Surg Laparosc Endosc Percutan Tech 2015, 25: e8-e10.
Wang MJ, Li JL, Zhou J, Wu Z, Peng B: Consecutive laparoscopic gallbladder and spleen resections in cirrhotic patients. World J Gastroenterol 2014, 20: 546–54.
Acknowledgments
The West China Hospital, Sichuan University funded this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure Statement
The authors have no competing financial interests to report.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Additional information
Zhaoda Zhang and Bing Peng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, M., Meng, L., Cai, Y. et al. Learning Curve for Laparoscopic Pancreaticoduodenectomy: a CUSUM Analysis. J Gastrointest Surg 20, 924–935 (2016). https://doi.org/10.1007/s11605-016-3105-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3105-3