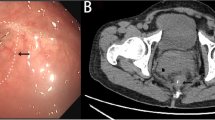
Abstract
Objective
To propose a method for the resection of the rectal anastomotic stenosis and anal reconstruction based on the transanal endoscopic technique through a transanal and transabdominal combined endoscopic resection, and to verify its clinical effectiveness.
Methods
Thirty-eight patients with anastomotic stenosis were admitted to the Sixth Affiliated Hospital, Sun Yat-sen University, China, from January 2016 to September 2019. Patients were divided into an experimental group (17 patients) and a control group (21 patients) subjected to the removal of the intestinal stenosis followed by anal reconstruction, they underwent transanal and transabdominal endoscopic surgery and traditional transabdominal surgery, respectively. Data on intraoperative blood loss, operation time, postoperative recovery, and prognosis were collected.
Results
(1) The median intraoperative blood loss was approximately 100 ml, without conversion to laparotomy during the surgery and intraoperative complications. The safety of the surgical operation was improved. (2) The operation time was shortened compared to previous reports, and the median operative time was 193 min. The average time of transanal endoscopic dissociation to the retroperitoneal fold was 76 min. (3) Laparoscopic assistance was carried out on 14 of the17 patients, and the incision was reduced. (4) The short-term curative effect was quite satisfactory, without permanent stoma. The average time to recover food intake after the surgery was 1.5 days. The average ambulation time was 3 days. Within 30 days after the surgery, one case suffered anastomotic leakage and then underwent refunctioning stoma through a second surgery. One patient suffered from intestinal obstruction, and the condition was improved through a conservative treatment. One case experienced delayed abdominal wound healing.
Conclusion
The transanal and transabdominal endoscopic resection of the rectal anastomotic stenosis and anal reconstruction reduced the difficulty of the surgery, improved its safety, shortened the operation time, decreased the operative complications, and enabled patients to recover well after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is a common malignant tumor of the digestive tract [1, 2]. Currently, the treatment for advanced rectal cancer is still based on surgery [1, 3,4,5]. Although the stapler improves the safety of the anastomosis for rectal cancer, the operative complications are inevitable [6, 7]. Anastomotic stenosis is one of the common complications after rectal cancer surgery, especially when the cancer involved the low and middle rectal area [8, 9], with an incidence in the range of 3%–30% [9, 10]. According to some studies, a rectal lumen diameter < 20 mm is considered as anastomotic stenosis [11]. Other studies suggested that the anastomotic stoma with the diameter of less than 1/3 of the original diameter of the intestinal cavity is considered as anastomotic stenosis [12]. Anastomotic stenosis not only causes symptoms of intestinal obstruction such as abdominal distension and abdominal pain, but also affect patients’ quality of life and their long-term survival [13,14,15,16].
Anastomotic stenosis is classified into membranous stenosis and tubular stenosis according to the degree of stenosis. In case of membranous stenosis, satisfactory results can be achieved through anal dilatation [17] while endoscopic balloon dilatation is the preferred treatment for tubular stenosis. Endoscopic incision and surgical incision of the scar tissue can also result in certain effects [18,19,20]. However, balloon dilation requires repeated expansion to achieve a long-term effectiveness, accompanied with the risk of perforation, pelvic infection, and bleeding [21, 22]. In addition, the effect of the balloon dilatation or incision was poor especially when the location of the anastomosis is low with severe scar and long stenosis [23]. In this case, nearly 30% of the patients needed a new anastomosis for reconstruction [10]. The main factors responsible for the formation of anastomotic stenosis include anastomotic leakage, anastomotic ischemia, chemoradiotherapy, use of stapler, pelvic infection, and low-grade anastomosis [24, 25]. Therefore, during the stenosis resection and reconstruction, the anatomical space is not clear, and complications such as ureteral injury and presacral venous bleeding may easily occur [8, 26]. Because of the difficulty of the surgery, some patients have to accept permanent stoma [9, 13].
Transanal total mesorectal excision (taTME) is performed using a transanal approach for total mesorectal excision, which is used to preserve the anal sphincter during low rectal cancer surgery. taTME technology shows significant advantages: Firstly, it allows an accurate separation of the distal intestine with the stenosis and the performance of the intestinal anastomosis under transanal endoscopy or direct vision, thus reducing the difficulty in the reconstruction of the digestive tract. Secondly, the gap between the stenosis lesion and the surrounding normal tissues can be precisely detected through the magnifying effect of the transanal endoscopy to reduce intraoperative complications such as bleeding and perforation. Finally, the specimen is removed from the anus without abdominal incision, thus reducing trauma. Therefore, taTME technology is useful to dissociate rectal lesions from the distal end thanks to the advantage of transanal view, so as to realize the separation of distal anastomotic stenosis. The team successfully completed 568 cases of taTME surgery, achieving good clinical results. According to taTME technology, it is hypothesized that transanal and transabdominal combined endoscopic surgery (as we previously named it as transanal endoscopic surgery (TAES) [27]) can be used to solve the technical problems of rectal anastomosis stenosis.
Therefore, this work proposed a transanal and transabdominal endoscopic surgery based on taTME technology on 17 patients who underwent rectal stenosis resection and anal reconstruction, and the effectiveness was evaluated.
Materials and methods
Patient selection and groups
The study protocol was reviewed and approved by the Institutional Review Board of the Sixth Affiliated Hospital, Sun Yat-sen University, China (2014ZSLYEC-013). Written informed consent was obtained from patients prior to the enrollment. Data on the intraoperative blood loss, operation time, postoperative recovery, and prognosis were collected.
A total of 38 cases with inflammatory scar hyperplasia caused by anastomotic leakage (Table 1) from January 2016 to September 2019 were considered in this study according to our inclusion and exclusion criteria. They were then divided into an experimental group (17 patients) and control group (21 patients). The patients were subjected to the transanal and transabdominal endoscopic surgery.
In terms of previous operation history, 14 patients in the experimental group underwent Dixon operation. The average length of the anastomotic stenosis was 2.9 cm, and the diameter of the anastomotic stenosis was approximately 4 mm. Three cases underwent endoscopic anastomotic incision, and 12 cases underwent anal dilatation and local irrigation.
The past medical history of the control group revealed the presence of 3 cases of congenital megacolon, 13 cases of laparoscopic Dixon for rectal cancer, two cases of laparoscopic sigmoid colon cancer and three cases of open radical Dixon operation for rectal cancer. Nine patients underwent endoscopic balloon dilatation, three patients underwent transanal incision, two patients had endoscopic incision, and 1 case was subjected to stent placement. The median distance between rectal stenosis and anal verge was 7 cm (3–12 cm), the median stenosis length was 13 mm (8–50 mm), and the median stenosis diameter was 6mm (3.5–10 mm). The patients were subjected to the laparotomy or laparoscopy surgery.
Inclusion and exclusion criteria
The inclusion criteria were the following: (1) The length of anastomotic stenosis was more than 5mm, and colonoscopy could not pass through the anastomosis. (2) Anal digital examination, defecography, magnetic resonance imaging (MR) and CT suggesting the presence of anastomotic stenosis. (3) Previous treatments such as anal dilatation, balloon dilatation, and narrow incision that showed poor effectiveness.
Surgical technique
Abdominal cavity exploration: laparoscopy or laparotomy could be used to explore the abdominal cavity to separate the intestinal adhesions. According to the stenosis lesion in the rectum, the proximal colon was separated. If tumor recurrence was observed, blood vessels were isolated according to the principle of radical tumor resection. If inflammatory stenosis was found, then the adhesion between the mesentery and the surrounding area was removed. Attention should be paid to the protection of the marginal arch vessels. Free proximal colon was dissected towards splenic flexure and if the intestine was not long enough, the splenic flexion was isolated.
Transanal group
Purse strings suture was placed 0.5–1 cm from the stricture ring to tightly occlude the rectal lumen. If the stenosis was located at a lower position and below the dentate line, it was necessary to perform a circular incision on the intestinal tube before the suture. After closing the intestinal cavity, if it is close to anal canal and the transanal single hole port cannot be placed, a circular incision was performed on the whole layer of the intestinal wall at 0.5 cm from the bottom of the purse suture. The port was implanted when the intestine wall was dissociated to 4 cm from the anal border. Pneumoperitoneum was established by Airseal constant pressure pneumoperitoneum. The pressure was set at 15 mmHg.
In the transanal group, the dissociation was performed under the endoscope. Firstly, the correct anatomical space was found by dissociation lateral side. The gap between the front and the posterior wall of the vagina or the prostate should be carefully distinguished when the front side was separated. As regard the back wall dissociation, the damage on the presacral vessels should be avoided, since it could cause bleeding.
The transanal group and the transabdominal group were joined and then the specimens were dragged out through the anus. According to the distance from the distal intestine to the anal border, the stapler anastomosis or manual anastomosis was adopted. As regard the manual anastomosis, the muscularis layer of the intestinal wall was discontinuously fixed by 2-0 VICRYL, and then the whole layer of the intestinal wall was sutured continuously with 3-0 barbed wire. If the patient was at high risk during anastomosis, the Bacon surgery was considered, and the intestine was pulled out through the anal canal to about 4 cm from the anal border. The final anastomosis was performed according to the Bacon technique at 2 weeks.
Statistical analysis
All statistical analyses were conducted with SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Descriptive outcomes were reported as median with range and Mann–Whitney U test was used for intergroup variation. Categorical variables were compared using the Chi-square test. Fischer’s exact test was used for the comparison of the causes of stenosis, previous treatments and anastomosis method between the two groups. Level of significance was set at P values < 0.05.
Results
General information
As shown in Table 1, no significant difference was found in the age, sex composition ratio, previous operation methods, stenosis length and treatment methods (P > 0.05), while significant differences was observed in the distance between the stenosis and anal verge and the diameter of stenosis (P < 0.05) between the two groups.
Comparison of intraoperative data
Among the 17 patients in the experimental group, 14 underwent laparoscopic Dixon for rectal cancer, and 3 underwent partial rectal resection for benign rectal tumors. Seven of the 17 patients received preoperative radiotherapy. All the 17 patients underwent transanal and transabdominal endoscopic surgery (Table 2). The laparoscopic surgery was performed on 14 patients, while three patients underwent laparotomy due to many previous surgeries. The median operative time was 193 min (110–457 min). The median time to perform the transanal operation was 76 min. However, in the Case 6, the operative time was 457 v because of the combination with the right colon cancer. Median intraoperative blood loss was 100 ml. No complications occurred in any patient and no blood transfusion was required.
The operation time in the experimental group dramatically shortened (P < 0.001), while the intraoperative blood loss markedly decreased compared to the control group (P < 0.05). In addition, the choice to perform a laparoscopy procedure was markedly increased in the experimental group compared to the control group (14/17 vs 7/21, P < 0.001). Notably, four cases were converted to laparotomy due to the difficulty of laparoscopic surgery in the control group, while no similar cases were observed in the experimental group (P < 0.05). No serious intraoperative complications occurred in both groups. No significant difference was found in the digestive tract reconstruction between the two groups (P = 0.282) (Table 2).
Comparison of postoperative data
In the experimental group, the average time to recover for food intake after the surgery was approximately 1.5 days, and the average walking recovery time after the surgery was approximately 3 days. After surgery, one case suffered anastomotic leakage and the ostomy operation was performed once more. One case suffered incomplete intestinal obstruction, and the condition was improved after conservative treatment. One case suffered wound infection and delayed healing after laparotomy. The median follow-up time was approximately 10 months. Thirteen out of 17 patients underwent a successful stoma closure. Defecography showed that the anastomosis was unobstructed. No local anastomotic recurrence was found until the follow-up date.
In the postoperative pathological report, no significant difference in the length of the specimen was found between the two groups. In the process of postoperative recovery, the recovery time of the anal exhaust in the test group was significantly shorter than that in the control group (P < 0.01). No significant difference was observed between the two groups in postoperative ambulation time, postoperative hospital stay, incidence of complications within 30 days and stoma close rate (P > 0.05) (Table 3).
Discussion
Resection of the rectal anastomotic stenosis and anal reconstruction are two difficult aspects of the colorectal surgery [28, 29], since presacral hemorrhage may easily occur due to the unclear anatomic level. Transanal minimally invasive surgery was used for local anastomotic stenosis [30, 31]. Transanal minimally invasive surgery combined with laparoscopy is applied for low rectal anastomosis and reconstruction of the ileum anal canal [32]. Based on these previous studies, a transanal and transabdominal combined endoscopic surgery based on taTME technology was proposed. The enlargement function of the transanal endoscope allowed a clear detection of the anatomical location of the stenosis, to facilitate the removal of the distal rectum lesion, and reduce the difficulty in reconstructing the digestive tract. The results showed that this method could reduce the difficulty of the surgery, improve its safety, reduce complications, and improve the patients’ recovery after operation.
By the combination of anal endoscopy, the distal intestine could be accurately separated from the stenosis. The anastomosis was performed through the endoscopy or direct vision, solving the most difficult problem of dissociating the distal rectal lesions during the rectum anastomosis stenosis surgery, and reducing the difficulty in reconstructing the digestive tract. In particular, this technique presented certain advantages to patients with previous abdominal operation history. Previous reports mostly mentioned the requirement of laparotomy due to the difficulty in performing rectal anastomotic stricture surgery (Table 4). Among the 33 cases reported by Lefevre et al. [26], 27 underwent laparotomy and 3 underwent conversion to laparotomy because of severe pelvic adhesion, and other 6 underwent laparoscopic surgery. The conversion rate was 50%, and the average operative time was 279 min (133–480 min). Schlegel et al. [33] reported the surgery of 27 cases. Since 7 cases presented a higher location of the stenosis, direct resection was used to perform anastomosis and anal reconstruction. Twenty cases displayed severe pelvic fibrosis and the surgery was difficult, and therefore the Soave's procedure was conducted for most patients. Pitel et al. [34] studied 66 cases in their report; 27 underwent Soave's procedure and 9 underwent additional surgery due to postoperative complications. Westerduin et al. [35] systematically analyzed 290 cases, in which the occurrence rate of intraoperative complications, total complications and severe complications was 12.8% (32/250), 34.1% (92/270) and 14.4% (37/257), respectively. In the control group of our study, seven patients underwent laparoscopic surgery, but four of them were converted to laparotomy because of the high risk of presacral hemorrhage. The median operation time was 347 min. Both the conversion rate and intraoperative complication rate in the experimental group were 0%. The median operation time in the experimental group was significantly shorter than that in the control group. The average transanal operation time was 73 min. Three cases underwent laparotomy because of multi abdominal surgery history. However, the transanal part could still be completed under the endoscope, and the average transanal operation time was approximately 70 min.
Through the combination of transabdominal procedure with anorectal endoscopy, it is easy to find the anatomical space to remove and repair the fistula tissue. The presacral hemorrhage complication was decreased, and the safety of the surgery was improved. Intraoperative presacral hemorrhage is one of the severer complications in the resection of rectal anastomotic stenosis and anal reconstruction [17, 36]. Genser et al. [37] studied 50 cases and 12 of them (24%) received intraoperative blood transfusion, with an average blood transfusion of 2.5 units (1–7). The incidence of intraoperative complications was 12%. Among them, 5 cases suffered from bladder injury due to an unclear anatomical position and one case underwent splenectomy due to spleen injury. Lefevre et al. [26] reported 33 cases and the incidence of perioperative complications was 54.5%, among which 24 cases (72%) belonged to grade Dindo II and below, seven cases (21%) to Dindo III, and 6% cases to Dindo IV (6%). The incidence of anastomotic leakage was 12% (4/33), and that of pelvic abscess was 18% (6/33). The incidence of postoperative intestinal obstruction was 12% (4/33), and the incidence of a second surgery was 21% (7/33). Among the 66 cases reported by Pitel et al. [34], the incidence of perioperative complications was 32.3%. In this study, the median intraoperative blood loss in the experimental group was significantly lower than that in the control group. No intraoperative presacral hemorrhage was observed, or blood transfusion was required in the experimental group. The average blood loss was approximately 100 ml, and the safety of the surgery was greatly improved. Among them, five cases suffered rectal fistula and two suffered combined rectovaginal fistula. These problems were cause by the anterior wall of the rectum adjacent to the posterior wall of the prostate, seminal vesicle, bladder or the vagina, and the anastomotic leakage was often combined with rectovaginal fistula and rectovesical fistula. In a previous treatment of anastomotic stricture with rectal or rectovaginal fistula, it was difficult to find the precise location for the removal through abdominal operation. However, through the transanal endoscopic technique, the fistula was successfully removed to complete the repair.
The combination of transanal endoscopy with laparoscopy avoided permanent stoma, and therefore presented a good short-term effect on rectal anastomotic stenosis. According to Westerduin et al. [38], the anastomotic leakage was as high as 41% at 14 months after coloanal anastomosis. During the 27-month follow-up, only 66% of patients reconstructed the intestinal continuity, 24% underwent colonic permanent stoma and 10% retained ileostomy. Remzi et al. [39] investigated 67 cases who underwent staging anastomosis with a success rate of 75%. In this study, no significant was found difference between the two groups in the incidence of postoperative complications and postoperative stoma recovery rate. Nine cases and 5 cases in the experimental group were treated with stapler anastomosis and manual anastomosis, respectively. With only one case suffering anastomotic leakage, the other patients recovered well. Based on Bacon technique, three cases were treated by transanal prolapse and staging anastomosis, and they successfully underwent resection surgery 2 weeks after surgery (Figs. 1, 2).
Surgical procedures. A Exposure by anal retractor; B Purse string suture; C Circular incision on the whole layer of the intestinal wall; D Longitudinal muscle fiber used as an important anatomical marker; E The front was close to the posterior vaginal wall; F Posterior dense scar tissue; G Manual anastomosis; H Stapler anastomosis
The feasibility and safety of this method in the treatment of anastomosis stenosis are evident. No cases were converted to laparotomy, no serious intraoperative complications occurred, and no local anastomotic recurrence was found until the follow-up date.
This study has some limitations. The number of patients was low. Furthermore, a case-matched control study could be considered when more cases are included. Although most of the patients were treated with stoma closure during the follow-up, no objective data were collected on the functional evaluation and quality of life survey.
Conclusion
In this study, a method for the resection of the rectal anastomotic stenosis and anal reconstruction was proposed based on the transanal endoscopic technique through a transanal and transabdominal combined endoscopic resection. The clinical effectiveness was demonstrated. The transanal and transabdominal endoscopic resection of the rectal anastomotic stenosis and anal reconstruction could reduce the difficulty of the surgery, improve the safety, shorten the operation time, decrease the operative complications, and enable patients to recover well after surgery.
References
Brenner H, Kloor M (2014) Pox cP. colorectal cancer. Lancet 383(9927):1490–1502
Lai S, Huang L, Luo S, Liu Z, Dong J, Wang L, Kang L (2020) Systemic inflammatory indices predict tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncol Lett 20(3):2763–2770
Lu P, Fields AC, Vise AS, Shabat G, Irani JL, Bleday R, Goldberg JE, Melnitchouk N (2019) Anatomic distribution of colorectal adenocarcinoma in young patients. Dis Colon Rectum 62(8):920–924
Olivo R, Ratnayake S (2019) Colorectal cancer in young patients: a retrospective cohort study in a single institution. ANZ J Surg 89(7–8):905–907
Chen J, Zeng Z, Huang L, Luo S, Dong J, Zhou FH, Zhou K, Wang L, Kang L (2020) Photothermal therapy technology of metastatic colorectal cancer. Am J Transl Res 12(7):3089
Moran B (1996) Stapling instruments for intestinal anastomosis in colorectal surgery. Br J Surg 83(7):902–909
Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F (2017) Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc 31(12):5318–5326
Khan F, Shen B (2018) Endoscopic treatment of concurrent colorectal anastomotic stricture and prolapse. Endoscopy 50(09):E235–E236
Lee SY, Kim CH, Kim YJ, Kim HR (2018) Anastomotic stricture after ultralow anterior resection or intersphincteric resection for very low-lying rectal cancer. Surg Endosc 32(2):660–666
Hiranyakas A, Da Silva G, Denoya P, Shawki S, Wexner S (2013) Colorectal anastomotic stricture: is it associated with inadequate colonic mobilization? Tech Coloproctol 17(4):371–375
Graffner H, Fredlund P, Olsson S-Å, Oscarson J, Petersson B-G (1983) Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. Dis Colon Rectum 26(2):87–90
Fegiz G, Angelini L, Bezzi M (1983) Rectal cancer: restorative surgery with the EEA stapling device. Int Surg 68(1):13–18
Hughes DL, Cornish J, Morris C (2017) Functional outcome following rectal surgery—predisposing factors for low anterior resection syndrome. Int J Colorectal Dis 32(5):691–697
Bruns ER, Borstlap WA, van Duijvendijk P, van der Zaag-Loonen HJ, Buskens CJ, van Munster BC, Bemelman WA, Tanis P (2019) The association of preoperative anemia and the postoperative course and oncological outcome in patients undergoing rectal cancer surgery: a multicenter snapshot study. Dis Colon Rectum 62(7):823–831
Kraenzler A, Maggiori L, Pittet O, Alyami M, Prost à la Denise J, Panis Y (2017) Anastomotic stenosis after coloanal, colorectal and ileoanal anastomosis: what is the best management? Colorectal Dis 19(2):O90–O96
Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Leslie Bokey E (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240(2):255–259
Garcea G, Sutton C, Lloyd T, Jameson J, Scott A, Kelly M (2003) Management of benign rectal strictures. Dis Colon Rectum 46(11):1451–1460
Shimada S, Matsuda M, Uno K, Matsuzaki H, Murakami S, Ogawa M (1996) A new device for the treatment of coloproctostomic stricture after double stapling anastomoses. Ann Surgery 224(5):603
Jain D, Sandhu N, Singhal S (2017) Endoscopic electrocautery incision therapy for benign lower gastrointestinal tract anastomotic strictures. Ann Gastroenterol 30(5):473
Bravi I, Ravizza D, Fiori G, Tamayo D, Trovato C, De Roberto G, Genco C, Crosta C (2016) Endoscopic electrocautery dilation of benign anastomotic colonic strictures: a single-center experience. Surg Endosc 30(1):229–232
Artifon E, Castaño RL, Otoch J, Tchekmedyian A (2015) Endoscopic dilation of the gastrointestinal tract. Revista de gastroenterologia del Peru: organo oficial de la Sociedad de Gastroenterologia del Peru 35(1):45–61
Nguyen-Tang T, Huber O, Gervaz P, Dumonceau JM (2008) Long-term quality of life after endoscopic dilation of strictured colorectal or colocolonic anastomoses. Surg Endosc 22(7):1660–1666
Suchan K, Muldner A, Manegold B (2003) Endoscopic treatment of postoperative colorectal anastomotic strictures. Surg Endosc Other Interv Tech 17(7):1110–1113
Kawada K, Sakai Y (2016) Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol 22(25):5718
Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR (1997) Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Surg 185(2):105–113
Lefevre JH, Bretagnol F, Maggiori L, Ferron M, Alves A, Panis Y (2011) Redo surgery for failed colorectal or coloanal anastomosis: a valuable surgical challenge. Surgery 149(1):65–71
Kang L, Chen W-H, Luo S-L, Luo Y-X, Liu Z-H, Huang M-J, Wang J-P (2016) Transanal total mesorectal excision for rectal cancer: a preliminary report. Surg Endosc 30(6):2552–2562
Gülen M, Leventoğlu S, Ege B, Menteş BB (2016) Surgical treatment of anal stenosis with diamond flap anoplasty performed in a calibrated fashion. Dis Colon Rectum 59(3):230–235
Maggiori L, Blanche J, Harnoy Y, Ferron M, Panis Y (2015) Redo-surgery by transanal colonic pull-through for failed anastomosis associated with chronic pelvic sepsis or rectovaginal fistula. Int J Colorectal Dis 30(4):543–548
Valdes-Hernandez J, Del Rio F, Gomez-Rosado J, Cintas-Catena J, Torres C, Perez-Sanchez A, Oliva F, Capitan-Morales L (2018) TAMIS repair of a rectal stenosis not treatable by endoscopy. Techn Coloproctol 11(22):891–891
Bong JW, Lim SB (2019) Transanal minimally invasive surgery as a treatment option for a completely occluded anastomosis after low anterior resection: a new approach to severe anastomotic stenosis. Asian J Endosc Surg 12(2):175–177
Borstlap W, Harran N, Tanis P, Bemelman W (2016) Feasibility of the TAMIS technique for redo pelvic surgery. Surg Endosc 30(12):5364–5371
Schlegel RD, Dehni N, Parc R, Caplin S, Tiret E (2001) Results of reoperations in colorectal anastomotic strictures. Dis Colon Rectum 44(10):1464–1468
Pitel S, Lefèvre JH, Tiret E, Chafai N, Parc Y (2012) Redo coloanal anastomosis: a retrospective study of 66 patients. Ann Surg 256(5):806–811
Westerduin E, Klaver CE, van Geloven AA, Westerterp M, Bemelman WA, Tanis P (2018) Outcome after redo surgery for complicated colorectal and coloanal anastomosis: a systematic review. Dis Colon Rectum 61(8):988–998
Brisinda G, Vanella S, Cadeddu F, Marniga G, Mazzeo P, Brandara F, Maria GJWJoGW, (2009) Surgical treatment of anal stenosis. World J Gastroenterol 15(16):1921–1928
Genser L, Manceau G, Karoui M, Breton S, Brevart C, Rousseau G, Vaillant J-C, Hannoun L (2013) Postoperative and long-term outcomes after redo surgery for failed colorectal or coloanal anastomosis: retrospective analysis of 50 patients and review of the literature. Dis Colon Rectum 56(6):747–755
Westerduin E, Borstlap W, Musters GD, Westerterp M, van Geloven AA, Tanis PJ, Wolthuis AM, Bemelman WA, D’Hoore A (2018) Redo coloanal anastomosis for anastomotic leakage after low anterior resection for rectal cancer: an analysis of 59 cases. Colorectal Dis 20(1):35–43
Remzi F, El Gazzaz G, Kiran R, Kirat H, Fazio V (2009) Outcomes following Turnbull-Cutait abdominoperineal pull-through compared with coloanal anastomosis. Br J Surg: Incorporat Eur J Surg Swiss Surg 96(4):424–429
Acknowledgements
This study was supported by National Health and Medical Research Council (NHMRC) Grant (1158402), Natural Science Foundation of Guangdong Province (China) (2018A030313621), and Sun Yat-sen University Clinical Research 5010 Program (China) (2016005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
All authors including Drs. Shuangling Luo, Xingwei Zhang, Yujie Hou, Huanxin Hu, Jianghui Dong, Liping Wang and Liang Kang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, S., Zhang, X., Hou, Y. et al. Transanal and transabdominal combined endoscopic resection of rectal stenosis and anal reconstruction based on transanal endoscopic technique. Surg Endosc 35, 6827–6835 (2021). https://doi.org/10.1007/s00464-020-08188-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08188-x