Abstract
Background
Minimally invasive resection of rectal cancer is controversial due to concerns of the oncologic efficacy and the difficulties of a laparoscopic total mesorectal excision (TME).
Methods
Using the National Cancer Database (NCDB), for the period 2010–2015, perioperative outcomes and overall survival (OS) in patients with rectal cancer who underwent laparoscopic or robotic low anterior resection (LLAR or RLAR) were compared to open LAR (OLAR) after propensity score matching.
Results
26,047 patients underwent LAR: 4062 (16%) RLAR, 9236 (35%) LLAR, and 12,749 (49%) OLAR. Patient and clinical tumor characteristics were similar between groups after matching. The conversion rates among patients undergoing LLAR and RLAR were 15% and 8%, respectively. In matched OLAR and LLAR patients, longitudinal and circumferential resection margins (CRM) were positive in 5.4% and 3.2% (p < 0.001) and 5.5% and 4.1% (p < 0.001); length of stay was 6 and 5 days, (p < 0.001); readmission was required in 6.5% and 7.0% (p = 0.112); OS at 1, 3, and 5 years were 95.5%, 83.7%, and 72.0% and 95.9%, 86.3%, and 76.4%, respectively (p < 0.001). In matched OLAR and RLAR patients, longitudinal and CRM were positive in 5.4% and 3.2% (p < 0.001) and 5.5% and 3.9% (p < 0.001); length of stay was 6 and 5 days (p < 0.001); readmission was required in 6.1% and 7.9%, (p = 0.010); and OS at 1, 3, and 5 years were 96.2%, 86.5%, and 77.1% and 97.5%, 89.4%, and 79.7%, respectively (p = 0.001).
Conclusions
In this national sample of propensity matched patients with rectal cancer who underwent open, laparoscopic, or robotic sphincter-saving rectal resection, only small differences in terms of resection margin status, length of stay, readmission, and overall survival were revealed. With acknowledgement of the limitations introduced by selection bias, our data indicate that each of the evaluated operative techniques results in acceptable outcomes for patients with rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is the third most common cancer diagnosed in both men and women in the United States. An estimated 44,180 new cases of rectal cancer will be diagnosed in 2019 [1]. Several randomized controlled trials have demonstrated that laparoscopic resection of colon cancer is safe, results in less post-operative pain, enhances earlier recovery, and is associated with equivalent long-term oncologic outcomes as compared with traditional open colectomy [2,3,4,5]. Minimally invasive resection for rectal cancer is more controversial due to concerns of the oncologic efficacy and compromised long-term outcomes.
The MRC CLASICC trial was the first randomized controlled trial that compared laparoscopic and open resection of rectal cancer. Although patients undergoing laparoscopic low anterior resection demonstrated increased positive circumferential radial margin (CRM) rates compared to open resection [6], there was no difference in disease-free survival (DFS) or overall survival (OS) between groups at a median follow-up of 63 months [7]. Similarly, both the Australasian Laparoscopic Cancer of the Rectum Trial (ALaCaRT) [8] and the American College of Surgeons Oncology Group (ACOSOG) Z6051 trial [9] were unable to establish noninferiority of laparoscopic compared with open surgery on pathological outcomes, but subsequent long-term analysis of both trials demonstrated equivalent 3-year DFS and OS [10, 11]. The COLOR II [12, 13] and COREAN trials [14, 15] demonstrated similar safety, resection margins, completeness of the resection, and 3-year DFS and OS in patients undergoing laparoscopic and open surgery.
Robotic surgery has the potential to overcome some of the limitations of laparoscopic surgery including a 3-dimensional depth of field and articulating instruments that may facilitate a difficult total mesorectal excision (TME) deep in the pelvis. In the ROLARR randomized clinical trial, patients undergoing robotic surgery demonstrated no difference in conversion rates, intraoperative and post-operative complications, plane of surgery, or 30-day mortality as compared to patients randomized to laparoscopic surgery [16]. In a systematic review and meta-analysis of five randomized controlled trials, robotic surgery was associated with lower conversion rates, longer operative times, similar perioperative mortality, and equivalent rates of CRM involvement compared to laparoscopic surgery [17].
The aim of this study is to compare perioperative and OS in patients with resectable rectal cancer undergoing robotic (RLAR) or laparoscopic low anterior resection (LLAR) to those patients undergoing open low anterior resection (OLAR) in the National Cancer Database (NCDB).
Materials and methods
Data sources and patient selection
Using the National Cancer Data Base (NCDB), we performed a retrospective cohort study of all patients with rectal adenocarcinoma between 2010 and 2015. The NCDB is a nationwide oncology outcomes database for more than 1500 Commission on Cancer-accredited facilities in the United States and Puerto Rico. Approximately 70% of all newly diagnosed cancer cases in the US are captured and reported to NCDB. This study used data that were de-identified and was exempt from Colorado Multiple Institutional Review Board (COMIRB).
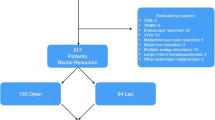
A total of 290,015 patients with rectal cancer were identified in the NCDB. Only patients with histology codes 8140, 8210, 8211, 8261, 8263, 8480, 8480, 8481, clinical category T1–T4, any N, M0 rectal adenocarcinoma undergoing low anterior resection (procedure codes 30–40) were included in the initial query. Patients were excluded if any baseline patient or tumor characteristics were missing. Patients were categorized by surgical approach as open (OLAR), laparoscopic (LLAR), or robotic (RLAR) (Fig. 1).
Baseline Characteristics
Patient demographics including age (< 50, 50–69, ≥ 70 years), gender (male, female), race (Black, Caucasian, Other), insurance status (not insured, private insurance, Medicaid/Medicare/Other Gov’t, unknown), income quartile (< $38,000, $38,000–$47,999, $48,000–$62,999, ≥ $63,000), education (> 93%, 79.0–93%, < 79.0% achieving high school diploma), living location (metro, urban, rural), Charlson–Deyo score (0, 1, ≥ 2), year of diagnosis (2010–2014), facility type (community cancer program, comprehensive community cancer program, academic cancer program, integrated network cancer program, unknown), clinical T-category (T1–T4), clinical N-category (node negative, node positive), and neoadjuvant radiation (no, yes) were collected. Program volume status was defined according to the total number of surgeries performed for rectal cancer at each cancer program per year: < 5, 6–15, 16–35, and ≥ 35.
Pathologic and perioperative outcomes
Conversion to an open procedure was recorded in patients undergoing robotic or laparoscopic surgery. Pathologic outcomes including tumor size (< 2, 2.0–3.9, 4.0–5.9, > 5.9 cm, unknown), grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, unknown), lymphovascular invasion (no, yes, unknown), longitudinal margins (negative, positive, unknown), pathologic T-category (T0, HGD, T1, T1, T2, T3, T4, unknown), N-category (node negative, node positive, unknown), number of lymph nodes examined, and rates of positive CRM were recorded. A positive CRM was defined as any tumor involving the CRM. Perioperative outcomes including length of stay, readmission, 30-day mortality, and 90-day mortality were recorded. Additionally, long-term outcomes including follow-up time, adjuvant chemotherapy, and overall survival (1-, 3-, 5-year) were recorded. Patients were followed until the time of death or until last follow-up, whichever came first.
Statistical analysis
All analyses were performed using STATA, version 15.0 (StataCorp, College Station, Texas). An intention-to-treat analysis was performed. Patients undergoing RLAR or LLAR were matched to those undergoing OLAR using pscore/psmatch2. A 1:1 matched sample was created by matching patients on the logit of the propensity score using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score. Covariates in the model included age, gender, race, insurance, income, education, living location, Charlson–Deyo score, year of diagnosis, annual volume, facility type, clinical T- and N-category, and neoadjuvant radiation. Balance between the groups was assessed using standardized differences. An absolute standardized mean difference (SMD) greater than 0.1 was considered an indicator for substantial imbalances between the 2 exposure groups, whereas an absolute SMD less than 0.1 was considered a good balance.
Patient pathologic and perioperative outcomes were compared in each treatment regimen. A Chi square test was used for categorical variables, and a Student’s t test or Wilcoxon rank-sum (Mann–Whitney) test were used for continuous variables. The Kaplan–Meier method was used to generate survival curves and they were compared using the log-rank test. A multivariable Cox proportional hazard model was applied to estimate hazard ratios (HR) of predictors of mortality. All variables with a p value of 0.10 or less on univariable analysis were utilized on multivariable analysis. All comparisons were 2-sided and statistical significance was defined as a p < 0.05.
Results
All patients
Among 26,047 patients, 4062 (16%) patients underwent RLAR, 9236 (35%) underwent LLAR, and 12,749 (49%) underwent OLAR. The median age of all patients was 62 years (IQR 53–71) and 61% of patients were male (n = 15,935). Six percent (n = 1650) of patients were treated at a community cancer program, 41% (n = 10,620) at a comprehensive community cancer program, 38% (n = 9830) at an academic cancer program, and 15% (n = 3947) at an integrated network cancer program. A clinical T1 tumor was seen in 15% (n = 3866) of patients, T2 in 18% (n = 4693), T3 in 63% (n = 16,407), and T4 in 4% (n = 1081). Nodal disease was clinically positive in 39% (n = 10,089) of patients and 63% (n = 16,455) received neoadjuvant radiation.
Laparoscopic (LLAR) versus open low anterior resection (OLAR)
Among the 21,985 unmatched patients, 9236 (42%) patients underwent LLAR and 12,749 (58%) underwent OLAR. Patients in the laparoscopic group were more likely to have a higher income, graduate with a high school degree, live in a metropolitan area, be treated at a high-volume center, be treated later in the study period, have a lower clinical T-category, and less likely to receive neoadjuvant radiation (all ASD > 0.1). There was no difference in age, gender, race, insurance status, Charlson–Deyo score, facility type, and clinical N-category (all ASD < 0.1). Based on the propensity model, 8663 patients undergoing LLAR were matched to 8663 patients undergoing OLAR. After matching, there were no statistically significant differences between the two propensity-matched groups with respect to age, gender, race, insurance status, income, education, living location, Charlson–Deyo score, year of diagnosis, annual volume, facility type, clinical T- and N-category, and receipt of neoadjuvant radiation (all ASD < 0.1) (Table 1).
On surgical pathology, tumors in the LLAR group were more likely to have a lower T-category (p < 0.001), but there was no difference in tumor size, grade, lymphovascular invasion, number of lymph nodes examined, or lymph node positivity (all p > 0.05). Patients in the LLAR group were less likely to have a positive longitudinal margin (3.8% vs. 5.2%) or a positive CRM (4.1% vs. 5.3%) (both p < 0.001). The conversion rate among patients undergoing LLAR was 14.7% (n = 1276). Median length of hospital stay was shorter in the LLAR group (5 vs. 6 days; p < 0.001). There was no difference in readmission rates, 30-day mortality, 90-day mortality, or receipt of adjuvant chemotherapy between groups (all p > 0.05) (Table 2).
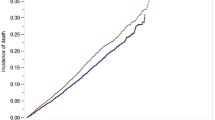
The median follow-up was 37 months (IQR 21–56) in the OLAR group and 39 months in the LLAR group (p < 0.001). LLAR was associated with a small increase in OS compared to OLAR: 1-year (95.9% vs. 95.5%), 3-year (86.3% vs. 83.7%), and 5-year (76.4% vs. 72.0%) (Table 2, Fig. 2). After adjusting for patient and tumor-related characteristics, LLAR remained associated with an improved OS compared to OLAR (HR 0.85; 95% CI 0.80–0.91). Other factors independently associated with OS on multivariable analysis were age, gender, race, insurance, income, Charlson–Deyo score, annual volume, facility type, tumor size and grade, lymphovascular invasion, margin status, and pathological T- and N-category (Table 3).
Robotic (RLAR) versus open LAR (OLAR)
Among the 16,811 unmatched patients, 4062 (24%) patients underwent RLAR and 12,749 (76%) underwent OLAR. Patients in the RLAR group were more likely to be younger, have private insurance, graduate with a high school degree, have a higher income, and to receive neoadjuvant radiation. Additionally, patients in the RLAR group were more likely to be treated later in the study period and to have surgery at an academic cancer program performing > 35 rectal resections per year (all ASD > 0.1). There was no difference in gender, race, living location, Charlson–Deyo score, and clinical T- and N-category (all ASD < 0.1). Based on the propensity model, 3944 patients undergoing RLAR were matched to 3944 patients undergoing OLAR. After matching, there were no statistically significant differences between the two propensity-matched groups with respect to age, gender, race, insurance status, income, education, living location, Charlson–Deyo score, year of diagnosis, annual volume, facility type, clinical T- and N-category, and receipt of neoadjuvant radiation (all ASD < 0.1) (Table 4).
On surgical pathology, tumors in the RLAR group were more likely to have a lower T- and N-category, but there was no difference in tumor size, grade or lymphovascular invasion between groups (both p > 0.05). Patients in the RLAR group were less likely to have a positive longitudinal margin (3.2% vs. 5.4%; p < 0.001) or a positive CRM (3.9% vs. 5.5%; p = 0.001). The conversion rate among patients undergoing robotic surgery was 8% (n = 302). Although median length of hospital stay (5 vs. 6 days; p < 0.001) was shorter in the RLAR group, readmission rates were higher in the RLAR group compared to the OLAR group (8% vs. 6%) (p = 0.010). Thirty-day mortality was similar between groups, but 90-day mortality was significantly higher in the OLAR group (2% vs. 1%; p = 0.027). There was no difference in the percentage of patients receiving adjuvant chemotherapy between groups (p = 0.669) (Table 5).
The median follow-up in the RLAR group was 32 (IQR 20–47) months compared to 31 (IQR 19–47) months in the OLAR group (p = 0.022). RLAR was associated with a small increase in OS compared to OLAR: 1-year (97.5% vs. 96.2%), 3-year (89.4% vs. 86.5%), and 5-year (79.7% vs. 77.1%) OS (p = 0.001) (Table 5, Fig. 3). After adjusting for patient and tumor-related characteristics, RLAR remained associated with an improved OS compared to OLAR (HR 0.84, 95% CI 0.75–0.95). Other factors independently associated with OS on multivariable analysis were age, gender, insurance, income, Charlson–Deyo score, annual surgical volume, neoadjuvant radiation, tumor size and grade, margin status, and pathological T- and N-category (Table 6).
Discussion
A total mesorectal excision (TME) is the standard of care for the surgical treatment of rectal cancer. Preserving the integrity of the mesorectal fascial envelope is associated with a negative CRM, minimizing the risk of pelvic recurrence and improving long-term outcomes [18,19,20,21]. Based on the results of prior randomized controlled trials, minimally invasive resection for rectal cancer currently is controversial due to concerns of the oncologic efficacy and inadequate TME.
In the ACOSOG Z6051 trial [9], a successful resection, defined as a complete total mesorectal excision, clear CRM (≥ 1 mm), and clear distal margins (≥ 1 mm), was achieved in 82% of patients in the laparoscopic surgery group compared to 87% in the open surgery group (p = 0.41 for noninferiority). The CRM positivity rate in the laparoscopic arm was 12.1% and 7.7% in the open arm (p = 0.11). In the ALaCaRT trial [8], a successful resection was achieved in 82% of patients in the laparoscopic group and 89% in the open group (p = 0.38 for noninferiority). Laparoscopic surgery was associated with a positive CRM rate of 7% compared to 3% for open surgery (p = 0.06). Based on the results of these studies, the authors concluded that the there is insufficient evidence for the routine use of laparoscopic surgery.
However, in the COLOR II trial [12], the completeness of the resection was not different between the laparoscopic and open surgery groups. A positive CRM was noted in 10% of patients in both the laparoscopic and open surgery groups and the median tumor distance to resection margin were not different between groups. Similarly, in the COREAN trial [14], involvement of the CRM was seen in 4% of patients in the open group and 3% in the laparoscopic group. These authors concluded that in select patients with rectal cancer treated by skilled surgeons, laparoscopic surgery results in similar safety, resection margins, and completeness of resection to that of open surgery.
While there are few studies that have directly compared open and robotic rectal cancer surgery [22,23,24], a recent systematic review and network meta-analysis indicated that different approaches to mesorectal excision, including open and robotic, resulted in largely similar outcomes including resection margin status, major morbidity, reoperation, and 5-year OS [25]. Prospective randomized controlled trials that have compared laparoscopic and robotic rectal cancer resection have also demonstrated similar outcomes for the two techniques [16, 26].
In the present study, a positive CRM occurred in ≤ 5% of patients, regardless of surgical technique. While this result compares favorably to the results of the ACOSOG, ALaCaRT, and COLOR II trials, and is in line with those of the COREAN study, differences in study design, specimen examination, and data collection make cross-study comparisons difficult. In our study, there were statistically significant differences in CRM positivity that favored laparoscopic and robotic surgical techniques. However, the differences were actually very small (4% vs. 5%) and with consideration of selection bias, the absence of randomization, and unmeasurable variables (e.g., surgeon experience), our results should not be used to support one surgical technique over another. Due to limitations of the database, we are not able to assess the impact of CRM positivity on recurrence rates and disease-free survival between groups.
Long-term follow-up studies of the prior randomized controlled trials described previously suggest that long-term oncologic outcomes are similar between laparoscopic and open approaches. In the ACOSOG Z6051 trial [11], 2-year DFS was 79.5% in the laparoscopic group and 83.2% in the open group. Local/regional and distant recurrence were 4.6% and 14.6%, respectively, in the laparoscopic group and 4.5% and 16.7%, respectively, in the open group. In the ALaCaRT trial [10], 2-year DFS and OS were 80% and 94%, respectively, in the laparoscopic group and 82% and 93%, respectively, in the open group. In the COLOR II trial [13], 3-year DFS and OS were 74.8% and 86.7%, respectively, in the laparoscopic group and 70.8% and 83.6%, respectively, in the open group. In the COREAN trial [15], 3-year DFS was 79.2% for the laparoscopic surgery group and 72.5% for the laparoscopic surgery group, with a difference that was lower than the prespecified noninferiority margin. In our study, only overall survival could be assessed and for all patients was in the range of 95–97%, 84–89%, and 72–80%, at 1, 3, and 5 years, respectively, with little measurable difference among operative techniques after adjusting for baseline patient demographics and pathologic tumor characteristics. These overall survival estimates appear to be in line with those of the randomized ALaCaRT and COLOR II trials.
The conversion rate of laparoscopic rectal cancer surgery ranges from 1% in the COREAN trial, 9% in the ALaCaRT trial, 11% in the ACOSOG Z6051 trial, 17% in the COLOR II trial, to 34% in the CLASSIC trial. Robotic surgery may facilitate a difficult dissection in the pelvis by providing superior visualization and improved range of motion with articulating instruments resulting in fewer conversions to an operation. In the ROLARR trial [16], the conversion rate was not statistically different between the laparoscopic (12%) and robotic (8%) groups, which is comparable to the conversion rate in the present study (LLAR 15%, RLAR 8%).
Minimally invasive techniques in rectal cancer resection may improve perioperative recovery. Patients undergoing laparoscopic surgery in the COREAN, MRC CLASSIC, COLOR II, ALaCaRT, and ACOSOG Z6051 trials had earlier return to bowel function, but only the CLASSIC (laparoscopic 11 days, open 13 days) and COLOR II (laparoscopic 8 days, open 9 days) trials demonstrated a shorter length of hospital stay. A recent systematic review and network meta-analysis also demonstrated faster recovery with decreased morbidity with laparoscopic or robotic techniques compared to open surgery [25]. In the present study, median length of hospital stay was shorter in the LLAR and RLAR groups compared to the OLAR group (5 vs. 6 days, respectively). These are shorter than reported in the ROLARR trial (8 days in both laparoscopic and robotic groups) and may have resulted in a higher 90-day readmission rate in the RLAR group compared to the OLAR group (8% vs. 6%, respectively). Alternatively, RLAR may be associated with increased post-operative complications. However, due to limitations of the database, we cannot evaluate perioperative complications in the present study. The COREAN, MRC CLASSIC, COLOR II, ALaCaRT, and ACOSOG Z6051 trials demonstrated equivalent perioperative morbidity and mortality in the laparoscopic and open groups.
The present study does have several limitations. Although the NCDB is a large, powerful database, it does have inherent weaknesses [27]. Many potentially important health-associated factors (e.g., comorbidities, functional status, etc.) are not recorded in the NCDB. Prior studies suggest that the Charlson–Deyo comorbidities scores reported in the NCDB may be lower than those reported based on SEER-Medicare Prior claims [28, 29] and misclassification in the NCDB comorbidity ascertainment may impact survival results [30]. Because the NCDB does not include datapoints (i.e., overall state of health, physical capacity, body habitus, prior abdominal operations resulting in dense adhesions, tumor distance from the anal verge, a threatened TME plane, etc.) that may be used to determine the individualized decision for selecting LLAR or RLAR, a selection biases in the propensity matching may account for the differences in outcomes. Recurrence and complication rates are not recorded in the NCDB, so we are not able to evaluate the impact of these outcomes on overall survival. Lastly, this study cannot evaluate other factors that may influence outcomes such as multidisciplinary clinics, advanced surgical training, surgical quality measures, staffing of intensive care units, availability of diagnostic technology, access to clinical trials, and the intensity post-resection cancer surveillance.
In conclusion, in this national sample, a comparison of matched groups of patients with rectal cancer who underwent open, laparoscopic, or robotic sphincter-saving rectal resection, revealed only small differences in terms of resection margin status, length of stay, readmission, and overall survival. With acknowledgement of the meaningful limitations introduced by selection bias, our data indicate that each of the evaluated operative techniques results in acceptable outcomes for patients with rectal cancer.
References
AC Society (2019) Cancer facts & figures 2019. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. Accessed 30 Jan 2019
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359(9325):2224–2229. https://doi.org/10.1016/s0140-6736(02)09290-5
Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350(20):2050–2059. https://doi.org/10.1056/NEJMoa032651
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25(21):3061–3068. https://doi.org/10.1200/jco.2006.09.7758
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10(1):44–52. https://doi.org/10.1016/s1470-2045(08)70310-3
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726. https://doi.org/10.1016/s0140-6736(05)66545-2
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. https://doi.org/10.1002/bjs.8945
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314(13):1356–1363. https://doi.org/10.1001/jama.2015.12009
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314(13):1346–1355. https://doi.org/10.1001/jama.2015.10529
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J (2018) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. https://doi.org/10.1097/sla.0000000000003021
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of Stage II to III Rectal Cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269(4):589–595. https://doi.org/10.1097/sla.0000000000003002
van der Pas MH, Haglind E, Cuesta MA, Furst A, Lacy AM, Hop WC, Bonjer HJ (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14(3):210–218. https://doi.org/10.1016/s1470-2045(13)70016-0
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332. https://doi.org/10.1056/NEJMoa1414882
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11(7):637–645. https://doi.org/10.1016/s1470-2045(10)70131-5
Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15(7):767–774. https://doi.org/10.1016/s1470-2045(14)70205-0
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318(16):1569–1580. https://doi.org/10.1001/jama.2017.7219
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 267(6):1034–1046. https://doi.org/10.1097/sla.0000000000002523
Parfitt JR, Driman DK (2007) The total mesorectal excision specimen for rectal cancer: a review of its pathological assessment. J Clin Pathol 60(8):849–855. https://doi.org/10.1136/jcp.2006.043802
Faerden AE, Naimy N, Wiik P, Reiertsen O, Weyessa S, Tronnes S, Andersen SN, Bakka A (2005) Total mesorectal excision for rectal cancer: difference in outcome for low and high rectal cancer. Dis Colon Rectum 48(12):2224–2231. https://doi.org/10.1007/s10350-005-0191-9
Cecil TD, Sexton R, Moran BJ, Heald RJ (2004) Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum 47(7):1145–1149. https://doi.org/10.1007/s10350-004-0086-6(Discussion 1149–1150)
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26(2):303–312. https://doi.org/10.1200/jco.2007.12.7027
Corbellini C, Biffi R, Luca F, Chiappa A, Costa S, Bertani E, Bona S, Lombardi D, Tamayo D, Botteri E, Andreoni B (2016) Open, laparoscopic, and robotic surgery for rectal cancer: medium-term comparative outcomes from a multicenter study. Tumori 102(4):414–421. https://doi.org/10.5301/tj.5000533
Kim JC, Yu CS, Lim SB, Park IJ, Kim CW, Yoon YS (2016) Comparative analysis focusing on surgical and early oncological outcomes of open, laparoscopy-assisted, and robot-assisted approaches in rectal cancer patients. Int J Colorectal Dis 31(6):1179–1187. https://doi.org/10.1007/s00384-016-2586-6
Silva-Velazco J, Dietz DW, Stocchi L, Costedio M, Gorgun E, Kalady MF, Kessler H, Lavery IC, Remzi FH (2017) Considering value in rectal cancer surgery: an analysis of costs and outcomes based on the open, laparoscopic, and robotic approach for proctectomy. Ann Surg 265(5):960–968. https://doi.org/10.1097/sla.0000000000001815
Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R, Adamina M, Tekkis PP (2019) Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg 270(1):59–68. https://doi.org/10.1097/sla.0000000000003227
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 267(2):243–251. https://doi.org/10.1097/sla.0000000000002321
Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP (2017) Using the National Cancer Database for outcomes research: a review. JAMA Oncol 3(12):1722–1728. https://doi.org/10.1001/jamaoncol.2016.6905
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619
Lin CC, Virgo KS, Robbins AS, Jemal A, Ward EM (2016) Comparison of comorbid medical conditions in the National Cancer Database and the SEER-medicare database. Ann Surg Oncol 23(13):4139–4148. https://doi.org/10.1245/s10434-016-5508-5
Funding
This manuscript was not financially supported and has not been previously presented, published or being considered for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Drs. Brandon C. Chapman, Mark Edgcomb, Ana Gleisner, and Jou D. Vogel have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chapman, B.C., Edgcomb, M., Gleisner, A. et al. Outcomes in rectal cancer patients undergoing laparoscopic or robotic low anterior resection compared to open: a propensity-matched analysis of the NCDB (2010–2015). Surg Endosc 34, 4754–4771 (2020). https://doi.org/10.1007/s00464-019-07252-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07252-5