Abstract
Background
Duodenal switch is a choice of conversion when patients fail to lose or regain weight after Roux-en-Y gastric bypass (RYGB). This study aims to evaluate the effectiveness and safety of duodenal switch as a secondary operation for patients who presented with insufficient weight loss or weight regain after a RYGB.
Methods
A retrospective chart review was performed on 15 patients who underwent a conversion of RYGB to single anastomosis duodeno-ileal bypass with sleeve (SADI-S) or biliopancreatic diversion with duodenal switch (BPD-DS) due to weight regain between December 31, 2013 and October 31, 2018. For the body mass index (BMI) analysis, the multilevel model for change was used.
Results
Of 15 patients, 10 underwent a conversion to SADI-S, and 5 underwent a conversion to BPD-DS. Also, 7 patients underwent the conversion in two-stages, while 8 did as single-stage. One patient had a duodenal stump leak after SADI-S, and another patient had a sleeve leak after BPD-DS. One patient underwent a reoperation to increase the common channel 20 months after the conversion to BPD-DS due to malnutrition. There was no mortality. Mean percentage of total weight loss (TWL) was 18.4% at 6 months, 25.0% at 12 months, 26.4% at 18 months, and 25.7% at 24 months after the conversion. The rate of decrease in BMI was slower in SADI-S patients than in BPD-DS patients (p < 0.01), adjusting for preoperative BMI.
Conclusion
Conversions of RYGB to SADI-S and BPD-DS can provide significant additional weight loss. However, complications and malnutrition can develop after the conversion, and further research is needed for evaluating safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Roux-en-Y gastric bypass (RYGB) is one of the most common bariatric procedures today due to its effectiveness and remission in comorbidities associated with obesity [1]. Nevertheless, some patients do not reach and maintain weight loss goals and undergo a reoperation after a RYGB [2]. Weight regain, weight loss failure, or percentage of excess weight loss (EWL) of less than 50% is reported in 15–35% of RYGB patients [3,4,5]. Many patients in this category undergo a secondary bariatric operation for further weight loss. Long-limb RYGB, salvage banding, and duodenal switch are all possible revisional procedures if the patient proves to be a viable candidate [6, 7]. The overall goal of these procedures is to limit portion intake and absorption [8]. These procedures come with complications and risks, making some more effective and safer than others. Operations such as long-limb RYGB result in further weight loss but have high rates of long-term nutritional complications that may require the use of supplemental nutrition or even additional operations [8, 9]. Salvage banding can be used as a revisional procedure that controls the size of the pouch and stoma using a band. Although this procedure does not affect absorption, erosion, band slipping, and band failure are not uncommon and require further operations [10]. Duodenal switch is another procedure that has shown more significant weight loss than any other bariatric procedure at a 5-year follow-up [1]. As a secondary operation, it has the potential to be successful in long-term weight loss; but there is limited data on long-term complications [11,12,13]. This study aims to evaluate the effectiveness and safety of single anastomosis duodeno-ileal bypass with sleeve (SADI-S—defined as an investigational procedure by American Society for Metabolic and Bariatric Surgery [ASMBS] [14]) and biliopancreatic diversion with duodenal switch (BPD-DS) as secondary operations for patients who presented with insufficient weight loss or weight regain after a RYGB.
Methods
After institutional review board (IRB) approval and following the Health Insurance Portability and Accountability Act guidelines, the authors performed a retrospective chart review of a prospectively maintained database of 15 patients who underwent a laparoscopic conversion of RYGB to SADI-S or BPD-DS due to weight regain between December 31, 2013 and October 31, 2018. SADI-S procedure data were prospectively collected under the Orlando Health IRB oversight [14].
Conversions of RYGB to SADI-S and BPD-DS were performed by two surgeons when patients presented with less than 50% of the percentage of excess weight loss (EWL) after RYGB. Both procedures were extensively explained to the patients by the surgeons including possible complications and the investigational state of SADI-S [14]; the patients ultimately decided on the procedure of choice. Depending on the difficulty and the length of operative time of converting RYGB to sleeve gastrectomy, some patients underwent the conversion in two-stages, while others did in a single-stage. Both surgeons performed both conversion procedures.
Patients were followed up at our office clinic at 1, 3, 6, 12, and 18 months postoperatively and bi-annually after that. Follow-up visits included weight measurement, clinical history and examination, and laboratory tests for blood glucose as well as nutrition deficiency. Comorbidity resolution followed the standardized outcome reporting published by the ASMBS [15]. Remission of hypertension was defined as blood pressure below 120/80 mmHg without medication. Remission of diabetes was defined as fasting glucose level below 125 mg/dL and HbA1c < 6.5% in the absence of antidiabetic medications. Remission of sleep apnea (subjective) was self-discontinued use of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) based on improved symptoms. Remission of GERD (subjective) was absence of symptoms without medication.
Surgical technique
The procedure is begun by taking down any adhesions around the pouch, remnant, and gastrojejunostomy, taking care to release any adhesions between the pouch and the remnant fully. The pouch is then transected about 0.5 cm proximal to the gastrojejunostomy. The pouch is then brought to the stomach remnant at the level of the antrum for the gastrogastric anastomosis. This anastomosis is performed hand sewn in two layers. Enterotomies are then made in the pouch and the remnant. A through-and-through anastomosis is then performed with a Vicryl suture. A 40 French bougie-sized tube is then passed through the anastomosis to the antrum to ensure patency. The anterior seromuscular layer is then performed. The next step in the procedure is to perform the sleeve gastrectomy. The greater omentum is separated from the greater curvature, as well as the lesser omentum from the lesser curvature close to the tube, with the vessel sealer in the standard fashion. The remnant stomach is then transected with a linear stapler just to the left of the gastrogastric anastomosis to create the sleeve. The pouch can also be trimmed with additional staple loads if needed due to dilation. The Roux-limb is left in place for possible feeding later on. Next, the proximal duodenum, the distal antrum, and the pylorus are dissected inferiorly and superiorly until a window is created around the duodenum about an inch from the pylorus. A Penrose drain is placed around the pylorus for retraction, and the duodenum is then transected using a linear stapler. The previously marked loop of the ileum is then brought to the duodenum, and the duodeno-ileal anastomosis is performed. An enterotomy is made in the duodenum and the ileum, and a through-and-through layer anastomosis is done with a running Vicryl suture. If needed, an anterior seromuscular layer is also performed (Fig. 1).
For BPD-DS, a 250 cm common channel and a 125-cm alimentary limb are created. For SADI-S, the length of the common channel is 250 cm. A more detailed description of the surgical technique for conversion of RYGB to SADI-S is published elsewhere [16].
Statistical analysis
All data for age and body mass index (BMI) are demonstrated as mean ± standard deviation unless otherwise noted. Two-tailed Student’s t test, χ2 test, and Fisher’s exact test were utilized as appropriate. For weight analysis, due to the small number of cases and the number of patients lost to follow-up, comparison among different types of conversion was not possible using cross-sectional analytic method. Therefore, a multilevel model for change was used to evaluate the changes in BMI, since this method is possible to analyze the changes with missing follow-up values using predictive values using follow-up data less than 6 months. Simple error covariance structure was used for the model. All tests of hypotheses were two sided and conducted at a 0.05 level of significance. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 15 patients underwent a conversion of RYGB to SADI-S or BPD-DS during the study period due to insufficient weight loss or weight regain. Of these, 10 underwent a conversion to SADI-S and 5 underwent a conversion to BPD-DS. Also, 7 patients underwent the conversion in two stages, while 8 did as single stage. Thirteen patients (86.7%) were females, the mean age and BMI of the patients were 42.8 years and 48.1 kg/m2, respectively, at the time of conversion (second stage if the patient underwent the conversion in two stages). Among those with available data, the mean period from primary RYGB to the conversion procedure was 125.6 months. Characteristics of the patients are shown in Table 1.
Weight loss
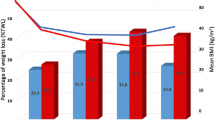
Mean percentage of total weight loss (TWL) was 18.4 ± 5.8% (range 11.0–29.6) at 6 months, 25.0 ± 10.0% (range 14.6–42.1) at 12 months, 26.4 ± 11.1% (range 14.9–43.8) at 18 months, and 25.7 ± 9.0% (range 17.4–36.5) at 24 months after the conversion (Table 2). Percentage of excess BMI loss (EBMIL) was 48.5 ± 30.3% (range 25.2–113.1) at 6 months, 63.0 ± 39.0% (range 28.9–124.8) at 12 months, 58.2 ± 33.7% (range 35.0–116.7) at 18 months, and 57.7 ± 27.9% (range 36.7–97.3) at 24 months after the conversion.
The multilevel model for change analysis of BMI suggests that preoperative BMI is the most significant indicator for initial status and the rate of change in BMI (Table S1 in the Supplementary Appendix). The difference in mean preoperative BMI was not significant between SADI-S and BPD-DS patients (p = 0.16). Adjusting for preoperative BMI, type of procedure was significantly associated with the rate of change in BMI. The rate of decrease was 0.39 slower in SADI-S patients than in BPD-DS patients (p < 0.01) (Fig. 2).
Comorbidities
A total of 6 patients presented with hypertension at the time of conversion. Of these patients, follow-up data were available for 5 patients, and 2 patients (40.0%) had a remission of hypertension at their last follow-up. A total of 5 patients presented with reflux symptoms at the time of conversion. Of these patients, follow-up data were available for 3 patients, and 1 patient (33.3%) had a complete resolution of reflux symptoms at follow-up. One patient who did not complain of reflux symptoms at conversion developed reflux symptoms after the conversion.
A total of 13 patients presented with type 2 diabetes at the time of the procedure. Of these patients, follow-up data were available for 7 patients, and all seven patients (100%) had remission of their diabetes. A total of 2 patients had sleep apnea at the time of conversion, and follow-up data were available for 1 patient. One patient still reported having sleep apnea. A total of 2 patients had hyperlipidemia at the time of conversion, and follow-up data were not available for these patients.
Laboratory data
The laboratory data included patients who were eligible for at least 6 months of follow-up, and therefore only 7 patients were included at baseline.
The mean hemoglobin dropped postoperatively and was below normal level for most patients during follow-up (Table 3). The mean protein and calcium levels also dropped immediately after the operation but increased at 2-year follow-up. The mean levels of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) were in the normal range for all follow-up.
Regarding the fat-soluble vitamins, majority of patients had vitamin A and E levels in the normal range (Table 4). However, several patients showed low levels of vitamin D and Ferritin during the follow-up. Few patients also reported elevated parathyroid hormone (PTH).
Complications
One patient (4.8%) had a duodenal stump leak 2 days following a single-stage conversion to SADI-S. The patient underwent laparoscopic drainage of bile and wash out and insertion of duodenostomy tube drainage. Another patient had a sleeve leak 2 days after the first stage of two-stage conversion to BPD-DS. She underwent oversewing of the leakage. Both patients were discharged under stable condition.
One patient developed malnutrition and dysphagia 15 months after conversion to a BPD-DS. She underwent a reoperation to increase the common channel 20 months after the conversion due to persistent malnutrition and diarrhea.
Discussion
The gathered data suggest that duodenal switch as a secondary procedure for failed RYGB led to successful mid-term weight loss and lowering of BMI. The risk of early postoperative complications in this secondary procedure was not low (13.3%) but acceptable.
Conversions to BPD-DS or SADI-S are both viable options for RYGB patients with weight loss failure [7]. A study conducted by Halawani et al. [12] showed that conversion of failed RYGB to BPD-DS in 9 cases resulted in a mean EWL of 56.4% at 2 years. Parikh et al. [17] reported that 12 patients undergoing BPD-DS after a failure of RYGB had a mean EWL of 62.7% at a mean follow-up of 11 months. Parikh et al. [17] additionally reported that all obesity-related comorbidities including hypertension and diabetes resolved after the conversion in their 8 patients. Surve et al. [18] showed that after conversion of RYGB to SADI-S and BPD-DS, the mean EWL was 56.0% and 56.4% at 18 and 24 months, respectively. In our study, both BPD-DS and SADI-S were performed on RYGB patients with failure of weight loss. Our results were similar to what had been reported for conversions to various types of duodenal switch and showed EBMIL of 48.5%, 63.0%, 58.2%, and 57.7% at 6, 12, 18, and 24 months, respectively. We observed an excellent rate of comorbidity resolution as well; 7 patients with diabetes and follow-up information all had remission of their diabetes.
Surve et al. [18] compared the weight loss results of 9 patients undergoing conversions to a BPD-DS to that of 23 patients undergoing conversions to SADI-S; and concluded that the mean EWL at 12 and 24 months after the conversion were similar for both procedures. However, it is unclear if they had a sufficient number of patients in each group at each follow-up point to detect a significant difference (type II error), since only 18 and 11 patients reported weight 12 and 24 months after the conversion, respectively. The number of patients in this study was also not large enough to detect a significant difference in weight loss between the two procedures at single time point. However, we identified that the rate of weight loss was significantly faster after conversion to BPD-DS than conversion to SADI-S. We are cautious in interpreting this as BPD-DS being superior to SADI-S; after the rate of weight loss slows down, sustainability of weight loss would be more important in the long term. Of note, the mean period from RYGB to conversion was longer among BPD-DS patients than SADI-S patients. Longer period from RYGB to conversion did not necessarily mean they sustained their weight loss for a longer time; numerous factors, including insurance coverage, were taken into consideration when a patient underwent a conversion [19].
Revisions and conversions are associated with higher risks of developing complications [7]. Because duodenal switch procedures require longer operative time and are technically more challenging than other bariatric procedures, utilizing them as a secondary procedure may have been hesitated by some surgeons [20]. Parikh et al. [17] reported that 4 out of 12 patients undergoing the conversion developed strictures at the gastogastrostomy, and one of the 4 required a surgical revision. Two other patients also underwent reoperation for different reasons. Surve et al. [18] also reported that 15.6% of their patients required a readmission within 30 days after the conversion. In this study, we also observed that 13.3% (n = 2) of our patients underwent a reoperation within 30 days. Although we were not able to show statistical differences, one complication occurred after one-stage SADI-S and another occurred after two-stage BPD-DS, possibly indicating that the number of stages and type of procedures were not associated with the rate of complication. Nevertheless, complications were manageable, and these patients were discharged in a stable condition.
This study is limited due to the retrospective nature of the study and small sample size. Many patients also did not have sufficient follow-up information. Thus, we were not able to draw conclusions about the long-term complications regarding SADI-S and BPD-DS as revisional procedures. However, all follow-up information was utilized by modeling a longitudinal analysis, and we were able to present a predicted trend for weight loss after the conversion for each procedure. A prospective randomized study on RYGB conversions with a larger sample size and longer follow-up would provide more strength to our results.
Conclusions
Conversions of RYGB to SADI-S and BPD-DS can provide significant additional weight loss. Our limited evidence suggests that conversion to a BPD-DS may result in faster weight loss than conversion to a SADI-S. However, our initial experience showed perioperative complications which may need a refinement of the technique or modification of the limb lengths. Malnutrition can develop after the conversion, and further research is needed for evaluating safety.
References
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H et al (2017) Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 27(9):2279–2289
Moon RC, Teixeira AF, Neto MG, Zundel N, Sander BQ, Ramos FM et al (2018) Efficacy of z in Roux-en-Y gastric bypass patients: a multi-center study. Obes Surg 28(9):2737–2744
Rawlins ML, Teel D 2nd, Hedgcorth K, Maguire JP (2011) Revision of Roux-en-Y gastric bypass to distal bypass for failed weight loss. Surg Obes Relat Dis 7(1):45–49
Nguyen D, Dip F, Huaco JA, Moon R, Ahmad H, LoMenzo E et al (2015) Outcomes of revisional treatment modalities in non-complicated Roux-en-Y gastric bypass patients with weight regain. Obes Surg 25(5):928–934
Moon RC, Teixeira AF, Jawad MA (2014) Treatment of weight regain following roux-en-Y gastric bypass: revision of pouch, creation of new gastrojejunostomy and placement of proximal pericardial patch ring. Obes Surg 24(6):829–834
Irani K, Youn HA, Ren-Fielding CJ, Fielding GA, Kurian M (2011) Midterm results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Obes Relat Dis 7(2):219–224
Tran DD, Nwokeabia ID, Purnell S, Zafar SN, Ortega G, Hughes K et al (2016) Revision of Roux-En-Y gastric bypass for weight regain: a systematic review of techniques and outcomes. Obes Surg 26(7):1627–1634
Liu S, Ren-Fielding CJ, Schwack B, Kurian M, Fielding GA (2018) Long-term results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Obes Relat Dis 14(10):1501–1506
Khoursheed MA, Al-Bader IA, Al-asfar FS, Mohammad AI, Shukkur M, Dashti HM (2011) Revision of failed bariatric procedures to Roux-en-Y gastric bypass (RYGB). Obes Surg 21(8):1157–1160
Vijgen GH, Schouten R, Bouvy ND, Greve JW (2012) Salvage banding for failed Roux-en-Y gastric bypass. Surg Obes Relat Dis 8(6):803–808
Trelles N, Gagner M (2009) Revision bariatric surgery: laparoscopic conversion of failed gastric bypass to biliopancreatic diversion with duodenal switch. Minerva Chir 64(3):277–284
Halawani HM, Bonanni F, Betancourt A, Antanavicius G (2017) Conversion of failed Roux-en-Y gastric bypass to biliopancreatic diversion with duodenal switch: outcomes of 9 case series. Surg Obes Relat Dis 13(8):1272–1277
Topart P, Becouarn G (2016) One-stage conversion of Roux-en-Y gastric bypass to a modified biliopancreatic diversion with duodenal switch using a hybrid sleeve concept. Surg Obes Relat Dis 12(9):1671–1678
Kim J, American Society for M (2016) American Society for Metabolic and Bariatric Surgery statement on single-anastomosis duodenal switch. Surg Obes Relat Dis 12(5):944–945
Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A et al (2015) Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis 11(3):489–506
Jawad MA, Nelson L, Moon RC, Teixeira AF (2017) Techniques of single-stage laparoscopic conversion of Roux-en-Y gastric bypass to single anastomosis Bilio-pancreatic diversion with Duodenal switch. Obes Surg 27(4):1109–1111
Parikh M, Pomp A, Gagner M (2007) Laparoscopic conversion of failed gastric bypass to duodenal switch: technical considerations and preliminary outcomes. Surg Obes Relat Dis 3(6):611–618
Surve A, Zaveri H, Cottam D, Belnap L, Medlin W, Cottam A (2016) Mid-term outcomes of gastric bypass weight loss failure to duodenal switch. Surg Obes Relat Dis 12(9):1663–1670
Switzer NJ, Karmali S, Gill RS, Sherman V (2016) Revisional bariatric surgery. Surg Clin North Am 96(4):827–842
Buchwald H, Oien DM (2017) Revision Roux-en-Y gastric bypass to biliopancreatic long-limb gastric bypass for inadequate weight response: case series and analysis. Obes Surg 27(9):2293–2302
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Teixeira is a consultant for Intuitive Surgical and Ethicon Endo-surgery. Dr. Jawad is a consultant for Ethicon Endo-surgery. Dr. Moon, Mr. Alkhairi, and Dr. Wier have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MOV 276167 kb)
Rights and permissions
About this article
Cite this article
Moon, R.C., Alkhairi, L., Wier, A.J. et al. Conversions of Roux-en-Y gastric bypass to duodenal switch (SADI-S and BPD-DS) for weight regain. Surg Endosc 34, 4422–4428 (2020). https://doi.org/10.1007/s00464-019-07219-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07219-6