Abstract
Background
Usage of intraoperative indocyanine green (ICG) to assess skin flaps prior to abdominal wall closure has been shown to decrease postoperative wound-related complications. Primary outcome assessed is the utility of ICG in intraoperative decision making. Secondary outcomes analyzed are the incidence of surgical site occurrence (SSO) and hernia recurrence rates.
Methods
A retrospective study using the MedStar Georgetown University Hospital database was conducted, incorporating all consecutive patients undergoing complex incisional hernia repair from 2008 to 2018. 146 patients underwent perforator-sparing component separation (PSCST), 88 underwent flap assessment using intraoperative ICG angiography; they were then analyzed based on patient comorbidities, Ventral Hernia Working Group grade, operative factors, and complications.
Results
A total of 146 patients were analyzed with no statistical difference in patient characteristics between the SPY and no SPY group except in BMI (30.2 vs. 33.2 kg/m2, p = 0.036). The no SPY group also had higher numbers of patients undergoing concurrent panniculectomy (12 vs. 1, p < 0.001), and extensive lysis of adhesions (30 vs. 31, p = 0.048). Of the 88 patients undergoing intraoperative SPY, 37 (42%) patients had a change of intraoperative management as defined by further subcutaneous skin flap debridement. Despite this change, there was no statistical difference in incidence of SSO between SPY and no SPY (24.3% vs. 11.8%, p = 0.12), and no difference in hernia recurrence rates 5.6% (n = 5) versus 13.7% (n = 8), p = 0.09.
Conclusion
Intraoperative ICG assessment of subcutaneous skin flaps with a perforator-sparing component separation does not result in a decrease in surgical site occurrences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Incisional hernias remain a common postoperative complication after abdominal operations that can affect as many as 5–20% of patients [1,2,3]. Optimizing subsequent hernia repairs by using techniques to medialize and approximate the rectus abdomens muscles, reinforcing the abdominal wall, and minimizing wound complications all serve to decrease rates of hernia recurrence after repair [4,5,6,7]. Minimizing recurrence is key as each subsequent hernia repair after the initial repair has a higher chance of recurrence [7].
Since the 1990s, Ramirez’s technique for component separation has been widely adopted to fix challenging incisional hernias by releasing components of the anterior abdominal wall to medialize the rectus abdomens muscles without undue tension [4]. While the technique has been successful, complex incisional hernia repairs using component separation techniques can have associated wound complications as high as 57% [8]. These surgical site occurrences (SSOs) lead to weakening of the anterior abdominal wall, failure of incorporation of the mesh, and overall suboptimal results with increased hernia recurrence rates [7]. Studies have shown, however, that by using a periumbilical perforator-sparing technique while performing a component separation, wound-related complication rates decreased by 18% [9], and that rates of skin necrosis were significantly lower (p < 0.001) [6].
After challenging hernia repairs, wound complications can lead to mesh infections, hernia recurrence, reoperations, hospital readmissions, and increasing health care costs [9]. Techniques employed to prevent wound complications include intraoperative assessment of the viability of skin flaps with indocyanine green (ICG) angiography [10,11,12,13,14,15]. This technology has been widely adopted in Breast, Plastic and Microvascular, Cardiac, and Transplant surgery to evaluate tissue perfusion [16,17,18,19,20,21]. ICG is a plasma-bound intravascular compound with a half-life of 3–5 min. It is eliminated by the liver and excreted in the bile with high-safety profile that is well tolerated with a low side-effect profile [22]. Intraoperative administration of intravenous (IV) ICG and capturing of live fluorescent images for real-time assessment of blood flow can minimize skin flap necrosis, and studies have shown that usage of ICG can significantly decrease wound infection and complication rates [13,14,15,16]. ICG has also been shown to be cost-effective and can save up to $45,000 in follow-up care per patient in those developing postoperative complications [23, 24].
ICG assessment can identify patients at risk and allows for concurrent intraoperative modifications to the flap in patients undergoing abdominal wall reconstruction. In the largest retrospective comparative cohort study in patients undergoing perforator-sparing component separation hernia repairs, the effect of ICG on postoperative outcomes was analyzed. The purpose of this study is to explore the effect of ICG on intraoperative decision making and the frequency of surgical site occurrences (SSOs) in patients where ICG was utilized compared to procedures in which it was not. We hypothesize that the use of ICG will result in changes in intraoperative management of skin flaps resulting in a decrease in associated surgical site occurrences.
Methods and materials
An Institutional Review Board (IRB)-approved retrospective study using the MedStar Georgetown University Hospital database was conducted. All consecutive patients undergoing complex incisional hernia repair using the perforator-sparing anterior component separation technique (PSCST) from 2008 to 2018 by a single experienced hernia surgeon were retrospectively evaluated. Patients were excluded if they had no perforators identified at the time of the operation due to prior abdominal surgery. In this group of 146 patients, we then further identified patients who underwent ICG angiography assessment of bilateral subcutaneous skin flaps and those who did not. These patients were further analyzed based on patient characteristics, original Ventral Hernia Working Group (VHWG) grade, comorbidities, operative factors, postoperative SSOs, and recurrence rates.
Operative technique
All patients received appropriate perioperative antibiotics according to the Surgical Care Improvement Project (SCIP) protocol and perioperative antithrombotic prophylaxis prior to incision. Skin preparation was performed with a chlorhexidine–alcohol-based preparation (ChloraPrep, CareFusion Inc, Leawood, KS), and subsequently covered with an antimicrobial incise drape (Ioban™, 3 M, St. Paul, MN). They then underwent a standard midline laparotomy incision with prior scar excision including excess skin and subcutaneous tissue. A lysis of adhesions was performed if required to define fascial edges or if the patient had any prior complaints of bowel obstruction. In cases where extensive lysis of adhesions was performed, the bowel was freed from adhesions from the ligament of Treitz to the terminal ileum. Then bilateral subcutaneous flaps were elevated from the anterior fascia past the linea semilunaris. An anterior component separation was then performed 1 cm lateral to the semilunaris line, while careful to preserve perforating vessels to the rectus abdomens muscle complex (Fig. 1). At this time, the size of the defect was measured and an appropriately sized mesh either synthetic or biologic was selected at the surgeon’s discretion based on the patient’s comorbidities, risk factors, and VHWG grade. Types of mesh used included light-weight polypropylene mesh or uncrosslinked porcine acellular dermal matrix. Mesh was predominately positioned in an intraperitoneal fashion (underlay). If there was insufficient omentum to cover the bowel, in order to provide a barrier between the mesh and the bowel, the mesh was then placed in a retrorectus fashion (sublay). Mesh was cut and fitted for a 5-cm overlap of mesh past either fascial edge and then the mesh was secured with #1 PDS circumferentially. The fascial defect was then approximated with #1 PDS sutures in an interrupted figure of eight fashion over the mesh repair with complete re-approximation of the rectus abdomens muscles. In a majority of patients, a subfascial Blake drain was placed prior to closure of fascia. If the patient had redundant pannus, a concomitant transverse panniculectomy was also performed by the Plastic surgery team. If the hernia was incarcerated within the pannus, the panniculectomy occurred prior to hernia repair, if the hernia did not involve the pannus then the panniculectomy occurred after the hernia repair.
All patients undergoing PSCST from the year 2011 and onward had intraoperative ICG administered with no discrimination. For the patients who underwent ICG assessment, each patient received 4 ml (2.5 mg/ml) IV injection of ICG followed by 10 ml saline flush. The SPY Elite system (LifeCell Corporation, Branchburg, NJ, USA) was then placed over bilateral skin flaps and vascularity and viability was assessed with visual perfusion in real time. Areas that were determined to have minimal vascularity that did not appear perfused were marked and then further debrided (Fig. 2). Only one patient who had undergone a panniculectomy had ICG assessment and it was performed after the panniculectomy. Skin flaps were typically not assessed a second time with ICG after debridement. Subcutaneous drains were then placed under the flaps and then soft tissue was then closed in layers with absorbable suture and the skin either stapled or closed with 4–0 Monocryl subcuticular sutures. A negative pressure therapy incisional vacuum was employed in a subset of patients felt to be at high risk for SSO.
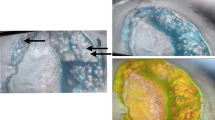
ICG Analysis of Subcutaneous Skin Flaps. A Perforator-sparing islands seen lighting up bilaterally (arrows) with well-perfused anterior rectus sheath flaps. B Area of skin with hypoperfusion concerning for potential wound necrosis. C ICG analysis of hypoperfused areas in comparison to areas of 100% perfusion. D Color analysis of perfusion and relative skin hypoperfusion
Postoperative surveillance
All patients were monitored postoperatively after hospital discharge in the office for evidence of SSO, defined as any complications relating to the surgical incision including surgical site infection, seroma, hematoma, and dehiscence. Superficial surgical site infection (SSSI) was defined as superficial cellulitis or infection of the skin only, while deep surgical site infection (DSSI) was defined as any infection just above the fascial layer or involving the fascia, but not intra-abdominal in nature. Patients were diagnosed in the office either based on physical exam or on computed tomography imaging demonstrating any of the above abnormalities. Median follow-up for patients who did not receive ICG was 94.4 months, and those that did receive ICG was 34.7 months (p < 0.001).
Data analysis
Chi-squared tests were used to compare categorical variables, student t tests were used to compare normally distributed continuous variables, and Wilcoxon rank-sum tests for non-parametric continuous variables. Parametric continuous variables are reported as means (± SD) while non-parametric continuous variables are reported as medians (interquartile range). We anticipated a surgical site infection rate in the range of those previously published after component separation of approximately 40%. A meta-analysis of an endoscopic approach to a perforator-sparing component separation saw an approximately 20% decrease in surgical site occurrences [25]. Extrapolating this information to our patient cohort where an alternate technique was used in a component separation with mesh, a total of 240 patients, 120 in each cohort, would be needed to have an 80% power to detect a 20% difference in surgical site occurrence.
Multivariable analysis was used to identify factors associated with higher complication risk. Factors included in the final model were selected based on statistical significance in the univariate model as well as a priori knowledge of predictors of complications after ventral hernia repair. Final models were adjusted for age, BMI, diabetes mellitus, gender, ASA, smoking status, diverticulitis, recurrent ventral hernia, VHWG, operative time, mesh type and size, concurrent panniculectomy, and need for extensive lysis of adhesion. A two-tailed p value of < 0.05 was considered statistically significant. Confidence intervals are reported with the Louis and Zeger method. Statistical analysis was performed using Stata 14 (StataCorp, College Station, Texas). This study was reviewed by the Georgetown University Institutional Review Board with an informed consent waiver granted.
Results
Patient demographics
From 2008 to 2018, a total of 146 patients underwent incisional hernia repair using the perforator-sparing anterior component separation technique. Eighty-eight (60.3%) patients undergoing this repair had intraoperative ICG assessment of their subcutaneous skin flaps, while the other 58 (39.7%) did not. Of these patients, 80 (54.8%) had undergone prior incisional hernia repairs and had a hernia recurrence.
A summary of patient characteristics is contained in table number one. Both cohorts were similar in age (p = 0.37). Patients with use of intraoperative ICG were more likely to be female when compared to those where ICG was not used (60% vs. 47%, p = 0.03). Additionally, the median BMI (kg/m2) in the ICG group was significantly lower than patients in the non-ICG group [30.3 (27.3,35.4) vs. 33.2 (29.2, 41.1), p = 0.03]. However, both cohorts had similar rates of patients with obesity (p = 0.11). The VHWG classification and level of wound contamination were similar in both cohorts as demonstrated in Table 1. Specifically, a majority of both cohorts were a grade II VHWG classification, with 53 patients in the ICG cohort (60%) compared to 36 patients (62%) in the non-ICG cohort (p = 0.69). While, a majority of both cohorts were clean cases with 75 patients (85%) in the ICG cohort compared to 45 patients (78%) in the non-ICG cohort (p = 0.12). Overall median length of hospital stay was similar in both groups (ICG: 6 days vs. non-ICG: 6 days, p = 0.44).
Operative details
Median operative time was 191 min (IQR 159–236), with median estimated blood loss of 100 ml (IQR 100–200). One hundred and three patients (70.5%) underwent mesh placement with a porcine acellular dermal matrix (PADM) while the remaining 43 patients (29.5%) underwent a polypropylene-based synthetic mesh placement. 142 patients underwent repair where the mesh was placed in an intraperitoneal, or underlay fashion (97.3%), while the remaining four patients (2.7%) underwent retrorectus mesh placement (sublay). Only 4 patients (2.7%) underwent a bridged repair (inlay). Median mesh size placed was 300.0 cm2 (IQR: 220.0-375.0). 61 patients (41.8%) underwent extensive lysis of adhesions, 3 (2%) had enterotomies that were repaired, and small bowel resections were performed on 8 patients (5.5%). Of all these patients, 13 (8.9%) underwent concomitant panniculectomy at the time of the component separation, and 28 (19.2%) had negative wound therapy applied to the incision (Table 2). Of the 88 patients that underwent ICG, 37 (42.0%) patients had intraoperative change in decision-making based on ICG assessment of skin flaps. In these cases, further debridement of the subcutaneous skin flaps was then performed prior to skin closure.
Surgical site occurrences
A total of 27 (18.5%) patients had surgical site occurrences reported in the postoperative period and is broken down as follows: 3 (2.1%) hematomas, 12 (8.2%) seromas, 14 (9.6%) surgical site infections, 1 (0.68%) fascial dehiscence (Table 3), and 9 (6.2%) requiring operative debridement (two patients had SSO in two categories: infected hematoma, and infection with fascial dehiscence). No patients had any intra-abdominal infections. One hematoma required intervention due to infection and was drained by interventional radiology (IR), the rest were managed expectantly. 2 (1.4%) of the seromas required intraoperative drainage due to size and discomfort. Upon further analysis, there were 6 (4.1%) superficial surgical site infections (SSSIs), and 8 (5.5%) deep surgical site infections (DSSIs). All 6 SSSIs were treated with oral antibiotics, none required intravenous antibiotics or debridement (Clavien–Dindo Class II). Of the 8 DSSIs, 6 required both IV antibiotics and debridement in the operating room (Clavien–Dindo Class IIIb), with no patients requiring mesh explantation. Two of the DSSI patients required IV antibiotics only (Clavien–Dindo Class II). The only patient who had a fascial dehiscence originally had excision of a large abdominal wall desmoid tumor requiring a large 12-cm bridged repair with PADM. Postoperatively this patient developed a DSSI requiring IV antibiotics and debridement of the fascia and abdominal wall leading to an acquired fascial dehiscence. One patient required OR debridement of skin flaps caused by pressure necrosis of a JP drain under an abdominal binder, but was not counted as a SSO. Patients that had PADM placement was associated with a lower occurrence of surgical site complication (17.5% vs. 20.9%, p = 0.62). The rate of surgical site occurrence was increased in those with negative pressure wound therapy applied at the conclusion of the case (21.4% vs. 17.8%, p = 0.66).
Effect of intraoperative ICG angiography
Of the 88 patients undergoing intraoperative ICG, 37 (42%) patients had a change of intraoperative management as defined by further subcutaneous skin flap debridement based on ICG perfusion results. When breaking down the incidence of SSOs and the need for return to the OR for further debridement, we see that there is no significant difference in SSO in patients who underwent ICG (17%) versus no ICG (21%), p = 0.58 (Table 3). Furthermore, there was not a statistically significant difference in surgical site occurrence in patients with use of intraoperative ICG with a change in intraoperative management compared to those without (24.3% vs. 11.8%, p = 0.12). Of those undergoing intraoperative ICG, patients with intraoperative changes to management had similar returns to the OR for wound debridement when compared to those without ICG-driven intraoperative changes (2.7% vs. 7.8%, p = 0.3).
Hernia recurrence
Thirteen (8.9%) patients had recurrent hernias on postoperative follow-up and 10 (6.8%) patients went on to have a subsequent hernia repair. Of the patients who recurred, 7 (53.8%) had already had a previous hernia repair. When comparing patients who underwent ICG versus no ICG, we find that the recurrence rates were lower in the ICG group at 5.6% (n = 5) versus 13.7% (n = 8), p = 0.09, but not significantly so (Fig. 3). Median follow-up time was 49.9 months (IQR 27.8–86.9 months).
Multivariable analysis
After adjustment for patient and procedure-specific factors, on multivariable analysis (Table 4), we found that surgical site occurrences were similar in patient undergoing intraoperative ICG angiography when compared to those without ICG use (OR: 0.260.812.50, p = 0.71). Increased body mass index was associated with increased occurrence of surgical site infections. Specifically, every 1 kg/m2 increase in BMI was associated with a 12% increase in surgical site occurrence (aOR: 1.031.121.22, p = 0.01). Patients who smoked (OR: 0.342.4818.05, p = 0.37), and those who had a prior hernia repair were also at higher risk (OR: 0.581.725.09, p = 0.33) for SSO, though not statistically significantly so. PADM use was associated with a 75% reduction in surgical site occurrences when compared to those with synthetic mesh in this cohort (aOR: 0.070.250.94, p = 0.04).
Discussion
Usage of intraoperative ICG angiography assessment has been utilized in abdominal wall reconstruction (AWR) as a tool to identify at-risk subcutaneous skin flaps and decrease subsequent skin necrosis and associated postoperative wound complications [13,14,15,16, 23]. Most previous evidence for usage of ICG angiography in AWR comes from smaller retrospective cases series and one randomized control trial. In these studies, patients undergoing flap modifications after ICG assessment had less wound-related complications than those patients in which ICG was not used. In particular, Patel et al. demonstrated that ICG usage decreased wound complications from 42 to 20% in a total of 17 patients [12]. Cho et al. in a cohort of 10 patients also found a decrease of wound infections from 57 to 33%, and intraoperative modifications required in 70% of patients undergoing ICG [13]. Wormer et al. similarly found that hypoperfusion on ICG demonstrated higher rates of wound infection (28% vs. 9,4%, p < 0.02), but that overall flap modification did not necessarily prevent wound-related complications (p = 0.99) [15].
Most studies done thus far differ from our study in two major aspects: the size of their patient population [13, 14, 23], and the surgical technique used [15, 23]. In Wormer et al. despite the randomized control trial nature of the study, a perforator-sparing technique was used infrequently, and many more patients had concurrent panniculectomy which could lend itself to decreasing ability to spare periumbilical perforators [15]. In Colavita et al. the number of patients undergoing panniculectomy was 10 (66.6%) of a total of 15 studied [23]. Due to the complexity of AWR and variety of operations performed based on appropriateness for each patient and hernia defect, there is great heterogeneity in this particular population of patients in which we are assessing with ICG angiography. In this particular study, in an attempt to homogenize patients, only those undergoing a perforator-sparing anterior component separation were examined and analyzed. Even if patients had prior hernia surgery or concomitant panniculectomy, a portion of the periumbilical perforators were preserved.
Because of the periumbilical perforator-sparing technique, in theory more blood flow to the subcutaneous skin flaps should be preserved leading to decreased rates of flap necrosis and subsequent SSOs [6, 9]. Clarke et al. demonstrated 25% compared to 0% (p < 0.001) rate of skin necrosis when using perforating sparing technique in component separation technique [6]. Similarly, Saulis et al. also demonstrated decreased wound complication rate from 20 to 2% (p < 0.05) in using the perforator-sparing technique in a retrospective 66 case series [9]. This leads us to postulate that within our own population of patients undergoing PSCST, wound complication rates should be overall minimized, and that those patients undergoing intraoperative ICG angiography should have even better optimization of their wound healing. When examining our rates of SSOs (18.5%), we find that our numbers are well within the limits of those quoted in prior studies 12.5–42% [6, 9, 15].
Interestingly, however, we did not see a significant difference in the rate of SSOs between ICG and no ICG cohorts (17% vs. 21%, p = 0.58), despite having 42% of patients undergo change in intraoperative management in the ICG group. Between the cohort of patients that underwent further operative debridement in the ICG group and those that did not, we also find no significant difference in SSOs (24.3% vs. 11.8%, p = 0.12), suggesting that perhaps intraoperative debridement of small areas of malperfusion may not have an overall impact on postoperative wound healing and complication rates. There was also no difference in return to OR rates between those that received further debridement based on ICG results in comparison to those patients that did not (2.7% vs. 7.8%, p = 0.3). Similarly, these results were also demonstrated in Wormer et al., where 95 patients in a randomized double-blinded control trial underwent ICG assessment, patients with positive ICG results who both underwent flap modifications, and those who did not had no differences in wound infection, breakdown, necrosis, reoperation, or readmission rates (p > 0.05) across all comorbidities [15]. It is unclear why interventions on select hypoperfused tissue did not appear to improve patient outcomes, other than indicating a multi-factorial process.
Although not yet demonstrated in literature pertaining to AWR, there has been a study in the plastic and reconstructive literature regarding ICG overprediction of areas of skin necrosis in mastectomies [17]. In Phillips et al., 32 patients who underwent bilateral mastectomies with intraoperative ICG and fluorescein assessment of mastectomy flaps, the areas of poor perfusion were marked out and transferred to cellulose film. Then only areas of poor perfusion determined by clinical assessment alone were then excised. Ultimately, ICG overpredicted poor perfusion by 6.57 cm2 or 72%, and overpredicted necrosis by 13.29 cm2 or 67%, with sensitivity of 90%, specificity 50%, positive predictive value of 56%, and negative predictive value of 88% [17]. This indicates that ICG perhaps is a better adjunct to identify patients who are not at risk for poor perfusion and necrosis, than it is a tool to predict actual flap necrosis.
Our study also demonstrates, that intraoperative ICG does not impact hernia recurrence, with no statistical significance seen in hernia recurrence rates in ICG vs no ICG patients (5.6% vs. 13.7%, p = 0.09), even though the recurrence rate is lower in patients undergoing ICG. In terms of patient characteristics, both cohorts were well matched except in BMI (30.3 vs. 33.2, p = 0.036 in ICG vs. no ICG), and male gender (53% vs. 34%, p = 0.025, respectively). Despite the differences in BMI, we do not note a significant difference in patients with obesity with a BMI ≥ 30, in ICG (56%) compared to no ICG (69%), p = 0.11. It is unclear if this difference potentially contributed to higher incidence of recurrence in the non-ICG cohort.
Additionally, multivariable analysis demonstrated that the two factors having the most impact on incidence of postoperative SSOs were BMI and mesh type. Specifically, every 1 kg/m2 increase in BMI was associated with a 12% increase in surgical site occurrence (aOR: 1.031.121.22, p = 0.01), which is consistent with literature indicating that patients with higher BMIs are at higher risk for wound complications [2, 3]. We also saw that PADM was associated with a 75% reduction in surgical site occurrences (aOR: 0.070.250.94, p = 0.04). These findings corroborate studies showing fewer wound complication rates in those undergoing biologic mesh placement (25–47.7%), especially in potentially contaminated settings with high VHWG class [7, 26,27,28]. Our recurrence rates were not significantly different between PADM (10.7%) and polypropylene mesh (4.7%), p = 0.24. The trend towards a higher incidence of recurrence with PADM could be attributable to increased patient risk factors that necessitated a need for biologic mesh in the first place.
We also found that patients with incisional negative pressure wound therapy (NPWT) applied were at increased risk for developing SSOs, although this was not statistically significant (p = 0.34). This could be due to selection bias as NPWT was applied at the end of the case based on the discretion of the surgeon, which self-selects for those at increased risk for surgical site occurrences.
Although this is one of the largest retrospective studies regarding the usage of ICG in assessment of subcutaneous skin flaps after complex AWR, it is limited due to the retrospective nature of our study by chart review and information gathering based on the electronic medical record. We were also unable to capture recurrences if they occurred outside of our regional, multi-hospital system. Those patients undergoing recurrent hernia operations at the same institution were included in the study. Our patient cohorts were also not perfectly matched in regards to some patient characteristics and comorbidities; even though most of these differences were not significant they could still impact our results. The two study cohorts also differed in the number of patients undergoing panniculectomies, and although in our multivariable analysis this did not affect the primary outcome of surgical site occurrence, it potentially could still have led to differences impacting our results. Ultimately our study was underpowered, and future directions would include a prospective randomized control trial to assess differences between the two techniques.
We have been able to demonstrate that in the subset of patients undergoing perforator-sparing anterior component separation, there is no significant difference in incidence of SSOs and hernia recurrence for those patients undergoing intraoperative ICG assessment and those who are not. Those who had an intraoperative decision change based on ICG did not necessarily have less SSOs than those who did not. ICG angiography is a tool in the surgeon armamentarium for assessing tissue perfusion and possibly preventing wound complications; however, in this particular patient group, it may not be necessary. In our current practice, we have stopped incorporating ICG angiography to assess skin flaps prior to closure in this particular subset of patients. Further study with groups of increased size in a randomized control fashion would provide additional information in regard to overall costs and efficacy.
Intraoperative ICG assessment of subcutaneous skin flaps during perforator-sparing anterior component separation does not significantly decrease surgical site occurrences or hernia recurrence. This highlights the importance of proper surgical technique in abdominal wall reconstruction with preservation of the blood supply to the subcutaneous flaps.
References
van ‘t Riet M, Steyerberg EW, Nellensteyn J et al (2002) Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg 89:1350–1356
Bosanquet D, Ansell J et al (2015) Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14618 patients. PLoS ONE 10(9):e0138745
Isatsu K, Yokoyama Y et al (2014) Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg 101:1439–1447
Ramirez OM, Ruas E, Dellon AL (1990) ‘‘Components separation’’ method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86:519–526
Ko JH, Wang EC, Salvay DM, Paul BC, Dumanian GA (2009) Abdominal wall reconstruction: lessons learned from 200 ‘‘components separation’’ procedures. Arch Surg 144(11):1047–1055
Clarke JM (2010) Incisional hernia repair by fascial component separation: results in 128 cases and evolution of technique. Am J Surg 200:2–8
Ventral Hernia Working Group, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558
Albright E (2011) Component separation technique for hernia repair: a comparison of open and endoscopic techniques. Am Surg 77(7):839–843
Saulis AS, Dumanian G (2002) Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg 109:2275–2280
Holm C, Mayer E, Höfter E, Becker U, Pfeiffer J, Mühlbauer W (2002) Intraoperative evaluation of skin-flap via- bility using laser-induced fluorescence of indocyanine green. Br J Plast Surg 55(8):635–644
Azuma R, Morimoto Y, Masumoto K, Nambu M, Takikawa M, Yanagibayashi S, Yamamoto N, Kikuchi M, Kiyosawa T (2008) Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg 122:1062–1067
Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY (2013) Use of intraoperative indocyanine-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg 47:476–480
Cho J, May A, Ryan H, Tsuda S (2014) Intraoperative use of fluorescence imaging with indocyanine green changes management of abdominal wall flaps during open ventral hernia repair. Surg Endosc 29:1709–1713
Wang HD, Singh DP (2013) The use of indocyanine green angiography to prevent wound complications in ventral hernia repair with open components separation technique. Hernia J Hernias Abdom Wall Surg 17:397–402
Wormer BA, Huntington CR, Ross SW (2015) A prospective randomized double-blinded controlled trial evaluating indocyanine green fluorescence angiography on reducing wound complications in complex abdominal wall reconstruction. J Surg Res 202:461–472
Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, Sacks JM, Zenn MR (2013) Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 7:1
Phillips BT, Lanier ST, Conkling N, Wang ED, Dagum AB, Ganz JC, Khan SU, Bui DT (2012) Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 129:778e–788e
Mothes H, Dönicke T, Friedel R, Simon M, Markgraf E, Bach O (2004) Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma 57(5):1018–1024
Rübben A, Eren S, Krein R, Younossi H, Böhler U, Wienert V (1994) Infrared videoangiofluorography of the skin with indocyanine green–rat random cutaneous flap model and results in man. Microvasc Res 47(2):240–251
Desai ND, Miwa S, Kodama D, Koyama T, Cohen G, Pelletier MP, Cohen EA, Christakis GT, Goldman BS, Fremes SE (2006) A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg 132(3):585–594
Sanchez EQ, Chinnakotla S, Jhan T, Nikirin D, Vasani S, Randall HB, McKenna GJ, Ruiz R, Onaca N, Levy MF, Goldstein RM, Docherty JC, Hurd DK, Klintmalm GB (2008) Intraoperative imaging of pancreas transplant allografts using indocyanine green with laser fluorescence. Proc (Bayl Univ Med Cent) 21(3):258–260
Lifecell corp (2012) SPY Elite intraoperative perfusion assessment system: SPY Elite Pack and SPY Elite Kit Instructions for Use. Branchburg, Lifecell corp
Colavita PD, Wormer BA, Belyansky I, Lincort A, Getz SB, Heniford BT, Augenstein VA (2015) Intraoperative indocyanine green fluorescence angiography to predict wound complications in complex ventral hernia repair. Hernia 20:139–149
Chatterjee A, Krishnan NM, Van Vliet MM, Powell SG, Rosen JM, Ridgway EB (2013) A comparison of free autologous breast reconstruction with and without the use of laser-assisted indocyanine green angiography: a cost-effectiveness analysis. PRS 131:693e–701e
Jenson KK, Henriksen NA, Jorgensen LN (2014) Endoscopic component separation for ventral hernia causes fewer wound complications compared to open components separation: a systematic review and meta-analysis. Surg Endosc 28:3046–3052
Garvey PT, Giordano SA, Baumann DP, Liu J, Butler CE (2017) Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg 224(3):341–350
Butler CE, Campbell KT (2011) Minimally invasive component separation with inlay bioprosthetic mesh for complex abdominal wall reconstruction. Plast Reconstr Surg 128(3):698–709
Patel KM, Bhanot P (2012) Complications of acellular dermal matrices in abdominal wall reconstruction. J Plast Surg Hand Surg 130:216S–224S
Acknowledgements
Filiz Greenberg, BS, CPC, CPPM.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Parag Bhanot is a consultant to Allergan. Drs. Jenny Shao, Yewande Alimi, and Dylan Conroy have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, J.M., Alimi, Y., Conroy, D. et al. Outcomes using indocyanine green angiography with perforator-sparing component separation technique for abdominal wall reconstruction. Surg Endosc 34, 2227–2236 (2020). https://doi.org/10.1007/s00464-019-07012-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07012-5