Abstract
Background
Wound complications including infection and necrosis remain common during complex open ventral hernia repair. Advancements or enhancements in imaging technology may abate some of these issues but requires more investigation. Laser-assisted fluorescent imaging with indocyanine green (Spy Elite, LifeCell Corporation, Branchburg, NJ) allows visualization and quantification of perfusion, facilitating management of poorly perfused tissue.
Methods
Ten patients, who underwent large or massive ventral or incisional hernia repair with biologic graft reinforcement and either perforator-sparing components separation or primary open repair, underwent intraoperative laser-assisted fluorescent imaging with indocyanine green from August 2012 to August 2013. The cases were reviewed by an independent data collector with primary outcomes of postoperative skin infection and/or abdominal wall necrosis.
Results
Three (30 %) patients had adequate perfusion, while seven (70 %) patients had inadequate skin perfusion and necessitated excision of additional tissue. Of the patients whose ischemic tissue was removed, four (57 %) patients had an infection and no patients developed necrosis postoperatively. Of the patients who had no removal of additional skin, one (33 %) patient developed an infection and one (33 %) patients developed skin necrosis.
Conclusion
The intraoperative use of laser-assisted fluorescent imaging with indocyanine green may change management of abdominal wall flaps, even in perforator-sparing operations. Our study series is small and cannot suggest statistical significance in the potential benefit of intraoperative imaging, but shows that up to 70 % of patients may require change in management due to poorly perfused tissue flaps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Despite advancement of surgical techniques, complex open ventral or incisional hernia repairs (VIHR) remain a challenge due to frequent wound occurrences. These morbidities include wound infection and skin necrosis. Infection is a common complication that occurs in up to 22 % of ventral hernia repairs [1]. Infection can be associated with up to an 80 % increase in rate of hernia, thus increasing the need for subsequent operation [2]. Patients with comorbidities or a history of contamination are at increased risk for infection, and their hernias are generally repaired with biologic materials [3]. Biologic implants enable revascularization of the patient’s tissue and increase resistance toward infection. With these properties, placement of biologic grafts in VIHR has yielded to fewer infections [4] and has reduced the need for mesh explantation, even in large, contaminated fields [5]. However, the long-term durability of biologic mesh repairs is equivocal, as no difference in recurrence rates has been demonstrated between the use of biologic and synthetic mesh [4].
Complex VIHR are also successfully managed with the component separation technique (CST) [6]. Despite lower recurrences achieved with CST, this technique is associated with a high incidence of skin necrosis and infection [7]. Wound-related complication rates as high as 57 % have been reported with CST [2, 8]. Surgical site occurrences are often attributed to poorly perfused tissue due to disruption of perforator vessels at and around the area of repair during CST [7]. Management of insufficiently perfused skin during complex VIHR is critical to reduce the risk of postoperative wound occurrences. Conventional assessments of tissue perfusion include clinical judgment of tissue color, warmth, capillary refill, and dermal bleeding. Although clinical assessment of tissue vascularization remains a common practice, studies have demonstrated that a subjective approach may inaccurately predict the postoperative outcomes [9, 10].
Intraoperative use of laser-assisted fluorescent imaging (SPY Elite System, LifeCell Corporation, Branchburg, NJ) of tissue perfusion addresses these concerns. This novel technology provides real-time visualization of blood flow and enables surgeons to conduct an objective assessment of perfusion intraoperatively. The imaging system uses intravenously administered indocyanine green (ICG) as a fluorescent agent, which can be quantified by absolute values of fluorescence intensity or by relative luminosity compared to a 100 % luminance area. This quantitative approach facilitates determining tissue viability and management of poorly perfused and potentially necrotic tissue. Intraoperative laser-assisted angiography with ICG has shown clinical application of arterial inflow, venous return, and tissue perfusion in the following procedures: breast reconstruction, free flap analysis, microvascular reconstructive surgery, and coronary artery bypass [9, 11]. This study aims to describe the management of tissues during VIHR assisted and guided by laser-assisted imaging system with ICG, as well as the rate of postoperative wound occurrences.
Materials and methods
Patients
This retrospective case series was approved by the Institutional Review Board. Subjects were selected from a pool of patients who have undergone complex open ventral or incisional hernia repair by a single primary surgeon from August 2012 to August 2013. Eligibility indications include open VIHR with biologic mesh reinforcement, either primary closure or component separation, and intraoperative use of laser-assisted imaging with ICG.
Medical records of subjects were reviewed for demographics and patient characteristics, surgical and perioperative variables, and postoperative progress. Primary outcomes were changes in management as indicated by the use of intraoperative laser-assisted fluorescent imaging and the rate of postoperative wound occurrences. Fischer’s exact test, descriptive and frequency analyses were performed using SAS (Cary, NC).
SPY elite system
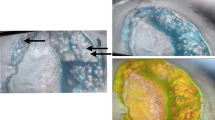
The SPY Elite System consists of a mobile cart, articulating arm, and an imaging head, which contains a laser light source, video camera, still image camera for color photos, and controls for various imaging functions. The articulating arm allows positioning of the camera over the area of interest without contamination of the sterile field. The mobile cart has two display monitors, one for the surgeon and the other for the system operator to utilize. The surgeon has the option on the imaging head to start and stop capture, zoom in and zoom out, and to take color snapshots. The SPY system utilizes a fluorescent agent (ICG or fluorescein) that is administered by peripheral or central venous access, and which adheres to plasma protein. A laser diode array is emitted by the system to view the fluorescence agent in a maximum field of 18.5 × 13.5 cm2. A charge-coupled device camera can be configured to capture images at 3.75–30 frames per second, which are then viewed on a high definition monitor in real-time for evaluation in the OR. The software allows basic quantitative analyses that enable it to annotate, color, and contour image sequences. The toolkit is available for analysis and algorithms for measurement of fluorescence intensity if the system software is insufficient. The fluorescence luminosity can be quantified by absolute values generated by the SPY system or by relative values comparing to a 100 % luminance area selected by the user. A relative luminosity percentage of <30 % is indicative of poor perfusion to the examined area. Image sequences are archived automatically as reports to a separate hard drive for review are needed. The ultimate evaluation and responsibility of diagnosis, however, rests with the clinician utilizing the imaging system.
Results
Ten subjects (6 male and 4 female) underwent complex open VIHR with intraoperative use of fluorescent angiography. The mean age of the subjects was 48 years. Twenty percent of the patients were obese, 20 % were smokers, and 30 % had diabetes. Additionally, 70 % had past history of gastrointestinal violation, 40 % had a history of wound infection, and 20 % had an active infection at the time of operation (Table 1).
All repairs were performed with reinforcement with biologic dermal matrix (Strattice, LifeCell Corporation, Branchburg, NJ). In one patient, the defect was closed primarily. In nine patients CST was performed to achieve abdominal closure. The mean hernia size was 393 cm2. The average size of the underlay mesh was 664 cm2 and overlay mesh was 388 cm2.
With assistance from fluorescent angiography seven (70 %) patients had a change in management of surgery. In these patients, the imaging system indicated perfusion less than 25–30 % along the edges of skin flaps or area of repair. The areas identified with poor perfusion were excised from the tissue flaps. The remaining three (30 %) patients had greater than 40 % perfusion and did not necessitate further debridement (Table 2). No adverse events were associated with administration of ICG and use of intraoperative angiography. One patient experienced respiratory distress and emergent intubation post-operatively. The average length of stay at the hospital was 5 days.
Patients were followed for a mean length of 3.16 months. Of the patients who had tissue debrided during surgery, none developed necrosis. Four (57 %) patients experienced postoperative infection. Of these patients, two (50 %) had an active infection at the time of surgery and one (25 %) had a prior history of infection. Comorbidities of these patients include obesity (25 %), diabetes (50 %), and smoking (50 %). Conversely, of patients who did not have a change in management of surgery, one developed a surgical site infection (33 %), and skin necrosis (33 %).
Discussion
Wound complications are a driving force for the evolution of surgical techniques. Even with utilization of biological materials and modifications to components separation, wound occurrences remain frequent after complex open VIHR. However, new technology with laser-assisted fluorescent angiography may lessen these issues. Laser-assisted fluorescent angiography provides a quantitative analysis of blood flow and an objective evaluation of tissue perfusion. This technology may assist in differentiating between potentially ischemic and viable tissue, an important factor in wound occurrences. Although management of insufficiently perfused tissue is ultimately the surgeon’s responsibility, this adjunctive method to assess tissue perfusion aids in the surgeon’s decision-making for maneuvering tissue reconstructions [9]. The use of intraoperative laser-assisted fluorescent angiography has been suggested to be superior to using clinical judgment alone for determining the extent of perfusion. Indeed, several studies have reported improved clinical outcomes when surgery was guided by the laser imaging with ICG [9, 10, 12].
The use of laser imaging with ICG has been used in various clinical settings. The technology has been increasingly popular in reconstructive surgeries that affect tissue vasculature, including free flap, pedicle flap, and implant surgeries. However, the utility of this system in complex VIHR has yet to be elucidated. At present, only one case report of one patient has demonstrated the use of laser-assisted intraoperative imaging in open ventral hernia repair with components separation. The surgery was uncomplicated and no recurrence, infection, or necrosis occurred postoperatively [7].
Our study presents a series of patients with complex hernias who underwent open VIHR with biologic mesh reinforcement, with either primary closure of the defect or component separation. According to our study, up to 70 % of patients undergoing complex open VIHR required a change of management of surgery. A higher rate of infection was observed in patients who had additional skin resection (57 vs. 33 %). However, assessment of the infection group as a whole demonstrated that the change in management of surgery did not affect this outcome (p = 1.00). Prior infection (p = 0.17), active infection (p = 0.44), and comorbidities that may be associated with poor perfusion (p = 0.53) were more common but were not significant. In addition, none of the patients with additional tissue excision developed necrosis. This finding supports other studies where necrosis was minimized when tissue reconstruction was guided by intraoperative fluorescent angiography.
Interestingly, one (10 %) patient without a change in surgical management developed necrosis postoperatively. In our patients, tissue with less than 30 % perfusion was suspected as poorly perfused and was excised. This threshold is commonly applied in clinical practice and was determined from previous studies investigating laser imaging-assisted breast reconstructions. In a series of mastectomy flap surgeries by Newman et al., the threshold between viable tissue and possible necrosis was approximately 25 % relative perfusion [13]. The study also reported that absolute values of fluorescent intensity did not consistently differentiate between patients who developed necrosis and those who did not. Although the SPY is capable of providing two different outputs, the absolute value may be less reliable than the relative value of tissue perfusion. In another study by Holm et al., delayed wound healing was demonstrated with less than 60 % relative perfusion [5]. Thus, it is possible that the threshold may differ for abdominal flap reconstructions as the distribution of perforator vessels vary throughout the body. Further investigation is necessary to ascertain the optimal value for adequate perfusion to minimize necrosis, without removing excessive amount of skin to permit abdominal wall closure.
Our case series is limited in sample size and by its retrospective nature. Our study lacks the statistical power to suggest reduced wound complications with the use of intraoperative fluorescent imaging. Although we cannot demonstrate statistical significance in the potential benefit of intraoperative imaging, our findings suggest that up to 70 % of patients undergoing complex open VIHR may require a change in management due to poorly perfused tissue flaps. Future studies would warrant a larger subject pool for statistical analysis, as well as a prospective long-term study for variable control, decrease of selection bias, and evaluation of long-term results and benefits of the imaging system.
The use of real-time laser-assisted fluorescent imaging system with ICG is growing rapidly in surgery, with widespread applicability to different departments such as cardiac surgery, neurosurgery, and reconstructive surgery [7]. The SPY system requires little learning curve and is straightforward to use. From our experience, the time of use from injection of ICG to capture and review the fluorescent images was less than 5 min. We were able to operate the system with only a single in-service staff and surgeons. However, the financial aspects may be a barrier to use. The retail value of the SPY system is approximately $300,000; the disposable cost ranges from $500 to $1500, depending on the institution contractual agreement. Although intraoperative laser-assisted fluorescent angiography may be a promising modality to manage tissue with low perfusion intraoperatively, the financial impact of avoiding complications due to use of the system requires further investigation.
References
Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK (2013) Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg 217:974–982. doi:10.1016/j.jamcollsurg.2013.08.003
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398. doi:10.1056/NEJM200008103430603
Ventral Hernia Working Group, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558. doi:10.1016/j.surg.2010.01.008
Darehzereshki A, Goldfarb M, Zehetner J, Moazzez A, Lipham JC, Mason RJ, Katkhouda N (2014) Biologic versus nonbiologic mesh in ventral hernia repair: a systematic review and meta-analysis. World J Surg 38:40–50. doi:10.1007/s00268-013-2232-1
Sbitany H, Kwon E, Chern H, Finlayson E, Varma MG, Hansen SL (2013) Outcomes Analysis of Biologic Mesh Use for Abdominal Wall Reconstruction in Clean-Contaminated and Contaminated Ventral Hernia Repair. Ann Plast Surg. doi:10.1097/SAP.0000000000000030
Ramirez OM, Ruas E, Dellon AL (1990) “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86:519–526
Wang HD, Singh DP (2013) The use of indocyanine green angiography to prevent wound complications in ventral hernia repair with open components separation technique. Hernia J Hernias Abdom Wall Surg 17:397–402. doi:10.1007/s10029-012-0935-0
Morris LM, LeBlanc KA (2013) Components separation technique utilizing an intraperitoneal biologic and an onlay lightweight polypropylene mesh: “a sandwich technique”. Hernia 17:45–51. doi:10.1007/s10029-012-0949-7
Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, Sacks JM, Zenn MR (2013) Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 7:1. doi:10.1186/1750-1164-7-1
Phillips BT, Lanier ST, Conkling N, Wang ED, Dagum AB, Ganz JC, Khan SU, Bui DT (2012) Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 129:778e–788e. doi:10.1097/PRS.0b013e31824a2ae8
Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY (2013) Use of intraoperative indocyanin-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg 47:476–480. doi:10.3109/2000656X.2013.787085
Komorowska-Timek E, Gurtner GC (2010) Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 125:1065–1073. doi:10.1097/PRS.0b013e3181d17f80
Newman MI, Jack MC, Samson MC (2013) SPY-Q analysis toolkit values potentially predict mastectomy flap necrosis. Ann Plast Surg 70:595–598. doi:10.1097/SAP.0b013e3182650b4e
Disclosures
Shawn Tsuda is a consultant for LifeCell Corporation. Jonathan Cho, Audriene May, and Heidi Ryan have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cho, J., May, A., Ryan, H. et al. Intraoperative use of fluorescent imaging with indocyanine green changes management of abdominal wall flaps during open ventral hernia repair. Surg Endosc 29, 1709–1713 (2015). https://doi.org/10.1007/s00464-014-3868-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3868-0