Abstract
Introduction
Complex ventral hernia repair (VHR) is associated with a greater than 30 % wound complication rate. Perfusion mapping using indocyanine green fluorescence angiography (ICG-FA) has been demonstrated to predict skin and soft tissue necrosis in many reconstructive procedures; however, it has yet to be evaluated in VHR.
Methods
Patients undergoing complex VHR involving component separation and/or extensive subcutaneous advancement flaps were included in a prospective, blinded study. Patients with active infection were excluded. ICG-FA was performed prior to incision and prior to closure, but the surgeon was not allowed to view it. An additional blinded surgeon documented wound complications and evaluated postoperative photographs. The operative ICG-FA was reviewed blinded, and investigators were then unblinded to determine its ability to predict wound complications.
Results
Fifteen consecutive patients were enrolled with mean age of 56.1 years and average BMI of 34.9, of which 60 % were female. Most (73.3 %) had prior hernia repairs (average of 1.8 prior repairs). Mean defect area was 210.4 cm2, mean OR time was 206 min, 66.6 % of patients underwent concomitant panniculectomy, and 40 % had component separation. Mean follow-up was 7 months. Two patients developed wound breakdown requiring reoperation, while 1 had significant fat necrosis and another a wound infection, requiring operative intervention. ICG-FA was objectively reviewed and predicted all 4 wound complications. Of the 12 patients without complications, 1 had an area of low perfusion on ICG-FA. This study found a sensitivity of 100 % and specificity of 90.9 % for predicting wound complications using ICG-FA.
Conclusion
In complex VHR patients, subcutaneous perfusion mapping with ICG-FA is very sensitive and has the potential to reduce cost and improve patient quality of life by reducing wound complications and reoperation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 350,000 ventral hernia repairs (VHR) are performed in the United States each year with the vast majority arising from prior laparotomies [1, 2]. The increase in obesity and comorbidities in this population has amplified the complexity of VHR and challenges surgeons in their quest for achieving optimal outcomes. The introduction of components separation technique (CST) has made the repair of many complex abdominal wall hernias possible [3]; however, this technique amplifies VHR’s significant morbidity. Open VHR with CST is associated with a high incidence of wound complications with overall rates of up to 57 % in open CST [4]; furthermore, minor complications, including cellulitis, seroma requiring aspiration, and skin breakdown, occur in up to 19 % [5].
The perfusion of the abdominal wall is derived from perforating branches of the superior epigastric and deep inferior epigastric arteries [6]. To prevent postoperative subcutaneous tissue and skin necrosis, surgical identification and preservation of the perforator vessels may be necessary to maintain perfusion of the abdominal wall. As many complex VHR patients have multiple previously failed repairs, periumbilical perforators have frequently been compromised at earlier surgical interventions. In patients with already compromised perfusion, extensive elevation of subcutaneous flaps for wide exposure of anterior abdominal wall, often needed for an open CST, leads to a greater risk of postoperative skin flap complications.
Large and complex abdominal wall defects in obese patients are often best served by a concomitant panniculectomy to provide wide exposure to the abdominal wall for reconstruction, CST, and resection of thin or severely scarred skin and subcutaneous tissue. Panniculectomy is association with increased risk of wound complication [7], with up to 31 % of wounds requiring sharp debridement postoperatively [8], and postoperative wound breakdown requiring treatment in 18 % of patients [9]. Currently, a surgeon’s clinical judgment is considered the gold standard for identification of underperfused skin and soft tissue after panniculectomy or elevation of wide skin flaps for CST; the high frequency of complications indicates that this is not sufficient. To improve outcomes, other means of evaluating flap and soft tissue perfusion have been developed.
Indocyanine green (ICG), initially used as a dye in photography, was first applied clinically in 1957 for liver function tests [10] and gained further popularity in the world of medicine when ophthalmologists began to use it to image choroidal circulation in the early 1970s [11]. Although used in ophthalmology for over 30 years, its role in evaluating circulation gained further attention by plastic and reconstructive surgeons with the innovation of ICG laser-induced fluorescence angiography (ICG-FA) as a new method of evaluating tissue perfusion in animal models [12, 13]. Initial experiences in humans in the late 1990s demonstrated that perfusion mapping with ICG-FA correlated with poor circulation and was sensitive for identifying potential areas of skin breakdown in patients undergoing reconstructive procedures [14–16]. Over the last decade, ICG-FA has been applied to numerous fields of surgery including cardiovascular intraoperative graft evaluation [17, 18], solid organ transplant graft perfusion [19, 20], and ophthalmologic evaluation of chorioretinal disorders [21]. It has been suggested to be beneficial in the intraoperative evaluation of skin and soft tissue reconstruction, particularly in breast reconstruction, with the ability to evaluate tissue perfusion, detect skin perforators, predict skin flap necrosis and loss, and prevent complications of wound healing [22–29].
To date, the evaluation of ICG-FA in complex OVHR is limited to a single-patient case study and thus requires further investigation for validation [30]. The aim of the present study is to perform the first evaluation of ICG-FA during complex OVHR, which the authors hypothesize will provide aid as a sensitive and specific modality to identify and predict postoperative skin and soft tissue complications such as wound dehiscence, skin breakdown, and fat necrosis. The secondary objectives of this study are to define an optimal objective threshold, in absolute perfusion units, to predict wound complications and to serve as a guideline for the use of ICG-FA in complex VHR. The financial impact of these wound-related complications will also be examined.
Methods
Indocyanine green
ICG is a water-soluble tricarbocyanine dye that is not metabolized and is completely eliminated through the liver after intravenous injection and excreted unchanged in the bile [15, 31]. While in the plasma, it binds strongly to plasma proteins to remain in the intravascular space. In the absence of capillary protein leakage, it is exclusively distributed within the intravascular space [32], making it an optimal marker for monitoring vascular perfusion in healthy patients. ICG has a short half-life of 3–5 minutes in humans [33] and has a safe pharmacological profile with a low incidence of side affects. The most severe of these adverse effects is very rare anaphylaxis, and a slightly higher incidence of adverse reactions in patients with end-stage renal disease (ESRD) undergoing hemodialysis [31, 34], despite hepatic clearance. In a large ophthalmologic study of 1226 consecutive patients exposed to ICG, there were very low adverse reaction rates: 0.15 % mild reactions, 0.2 % moderate reactions, 0.05 % severe reactions, and no deaths [35]. ICGs short half-life and safe clinical profile allow for repeat evaluations during the same surgical procedure, unlike other dyes such as fluorescein, which remain in the tissue for over 12 h and thus can only be utilized once [22].
ICG-FA
ICG absorbs light in the near infrared range, with a maximum at 805 nm, and fluoresces with a maximum at 835 nm [15]. Because of the near infrared wavelength, ICG-FA does not require protective eyewear or other safety equipment [22]. The use of an excitation light induces fluorescence from the ICG containing blood vessels within the deep dermal plexus and subcutaneous fat; this differs from fluorescein, which is only found in the superficial dermis [15]. The combination of these properties with the relative transparent nature of the skin to the ICG fluorescence wavelength allows recording by a suitable camera [15]. The authors utilized the SPY Elite® intraoperative perfusion assessment system (Lifecell Corp., Branchburg NJ, USA) for ICG-FA. SPY Elite® is currently used for capturing and viewing fluorescence images for the visual assessment of blood flow as an adjunctive method for the evaluation of tissue perfusion in plastic, reconstructive, cardiovascular, and gastrointestinal surgical procedures [33]. The application in complex VHR falls within the indication for evaluation of soft tissue perfusion.
The SPY Elite® system utilizes a laser diode array to illuminate a maximum field of 18.5 × 13.5 cm2 and a charge coupled device camera that can capture images at 3.75–30 frames per second with a recording duration of 30 s to four and a half minutes [22, 33]. Additionally, the system includes computer software for capturing, enhancing visualization, and archiving created images for review and reporting [36]. The SPY Elite® system utilizes a 255 unit grayscale for measuring fluorescence emitted from ICG and allows for set levels of contour to separate areas based on their absolute or relative fluorescent intensity. The measurement of fluorescent intensity can be reported as a percentage relative to other tissue within the field (relative perfusion unit) or as an absolute value based on the 255 point grayscale (absolute perfusion unit).
The present study utilized absolute perfusion units over relative perfusion units to achieve objective measures of perfusion. To define the optimal perfusion threshold for the standardized dose of 5 mg of ICG and identify the threshold that maximized sensitivity and specificity for predicting wound complications after VHR, multiple levels were tested. Identifying the optimal threshold is essential for further utilization of ICG-FA in complex VHR.
Inclusion and exclusion criteria
To be eligible for the clinical study, patients had to be at least 18 years old and have a complex ventral hernia requiring tissue advancement flaps at the time of open ventral hernia repair. Patients were ineligible if they had an iodine allergy, ESRD, active wound or mesh infection, or American Society of Anesthesiologists’ physical status classification of IV or V [37]. Pregnant patients were also excluded.
All patients provided written informed consent to participate in a study approved by the Carolinas Medical Center’s Institutional Review Board. All patient, operative, and follow-up data were prospectively collected and stored in a REDCap database [38].
Study design
The purpose of the study was to assess the ability of ICG-FA to predict wound complications and establish a standardized approach or guideline for its use in complex VHR. To achieve this aim without introducing bias, complex VHR was performed in the authors’ standard fashion with the surgical team blinded to the ICG-FA results. ICG-FA was performed prior to incision and prior to closure and not viewed by the operative team. The ICG-FA data were stored for later review on a secure server. Pre-incision ICG-FA was performed with the hypothesis that patients with altered subcutaneous perfusion from prior surgical procedures and the current abdominal wall reconstruction may benefit from operative planning to avoid creation of subcutaneous flaps that are poorly perfused. Pre-closure ICG-FA was hypothesized to predict subsequent necrosis of the skin or subcutaneous tissue.
Digital photographs of the operative field were included in the design to facilitate in documentation of the subcutaneous dissection for later analysis; pictures were taken and stored prior to incision, immediately after incision, prior to closure, and upon completion of VHR. Patients’ abdomens were photographed daily as inpatients and at each follow-up visit. All photographs were also stored on a secure server.
Patients were followed closely postoperatively for complications, and two blinded physicians reviewed all postoperative information and photographs to detect wound complications, including wound breakdown, cellulitis, necrosis, and dehiscence. Blinded physicians then reviewed all ICG-FA imaging, using objective perfusion measures to identify poorly perfused tissue in the patients. Finally, the investigators were unblinded so that the ability of ICG-FA to predict wound complications could be evaluated. In addition, hospital and follow-up charges were queried from the institutional billing database.
To establish a guideline for the use of ICG-FA in complex VHR, several thresholds were evaluated to identify the optimal value. Using each absolute perfusion unit threshold, the patients with ICG-FA imaging suggestive of poor perfusion were then compared to the patients with wound complications. Sensitivity and specificity were determined from the true positives (imaging accurately predicted wound complications), false positives (imaging predicted wound complication that did not occur), false negatives (imaging did not predict complications that occurred), and true negatives (imaging did not predict complication and none occurred). The threshold with the maximum specificity and sensitivity was determined as the optimal threshold.
Open ventral hernia repair
As the present study was designed to blindly assess subcutaneous perfusion, all VHR were performed in the standard fashion employed by the authors prior to the study design. All patients received prophylactic antibiotics according to surgical care improvement project (SCIP) guidelines [39], as well as pre-operative injection of 5000 units of subcutaneous heparin injection as a prophylactic measure to prevent deep venous thrombosis (DVT). All patients were placed in the supine position and received sequential compression devices prior to induction of anesthesia. Foley bladder catheters were universally placed, and the patients were prepped and draped widely. The patient’s abdomen was photographed with a digital camera. After a “time-out” to identify patient and procedure and prior to incision, 5 mg of ICG (the recommended dosage for SPY Elite® ICG-FA in plastic and reconstructive procedures [33]) was intravenously injected by peripheral or central venous access. ICG-FA was then performed; none of the surgical team was allowed to view the real-time imaging of the entire abdomen. Midline or transverse incisions were performed at the discretion of the surgical team, based on prior scars or need for concomitant panniculectomy. When applicable and appropriate, previous scar excision was performed. A second digital photograph was taken after incision. Electrosurgery was utilized to dissect the subcutaneous tissues to the hernia defect. After completed dissection, the hernia sac was entered, and adhesiolysis was performed circumferentially. The defect area was determined after previous mesh excision if needed.
The majority of patients underwent preperitoneal, open ventral hernia repair with prosthetic mesh [40]. One patient had an onlay-type repair. For preperitoneal repairs, the peritoneum was dissected from the anterior abdominal wall to the paracolic gutters, pubis, and xiphoid. The peritoneum was closed with a running absorbable suture. Prosthetic mesh was placed in the preperitoneal space and secured using interrupted permanent transfascial sutures. Lightweight or midweight polypropylene mesh was used in most cases, but there were no restrictions on prosthetic choice in the present study.
For patients in whom the fascia could not be primarily reapproximated without undue tension, a component separation was performed as described previously by Ramirez et al. [3], consisting of wide subcutaneous dissection to facilitate release of the external oblique aponeurosis lateral to the rectus sheath and/or posterior rectus sheath release 2 cm lateral to the linea alba. At times, posterior rectus sheath release was performed by incising the sheath from the xiphoid to the arcuate line, alone or in combination with the external oblique release. Preperitoneal mesh was inserted as described above and secured lateral to the cut edge of the external oblique in effort to prevent herniation at the site of component separation.
Drains were placed on top of the mesh, and the fascia was closed over the mesh with a running suture. The subcutaneous tissues were thoroughly irrigated. Two subcutaneous drains were placed over the fascia. Prior to subcutaneous tissue closure, ICG-FA was performed again using 5 mg of ICG. The surgical team was blinded to the imaging. The surgical team was able to modify the flaps prior to imaging if clinical judgment suggested ischemia, but this did not occur in the present study. A third digital photograph was taken at the time of ICG-FA. The deep dermal tissues were then closed with an interrupted absorbable suture and the skin was closed using an absorbable subcuticular stitch or staples. A final digital photograph was taken after closure.
Results
Fifteen patients were included in the study, with a mean follow-up of 7 months. Patient-specific data are presented in Table 1. The mean age was 56.1 years, and 60 % of patients were female. The mean BMI was 34.9, with 73.3 % having BMI >30. The vast majority of patients had a prior hernia repair (73.3 %), with a mean of 1.8 prior repairs. Diabetes was present in 26.7 % of patients, and 20 % are currently using tobacco. Prior wound infection had occurred in 13.3 %, but none had a prior mesh infection.
Operative data are also presented in Table 1. The mean operative time was 206 min, and average defect size was 210 cm2. Most patients had polypropylene mesh implanted; two had biologic graft. The mean area of prosthetic mesh implanted was 826 cm2. Concomitant panniculectomy was performed in 66.6 % of cases, and component separation was necessary in 40 %.
Average length of stay was 5.7 days. Postoperative complications are presented in Table 2. Seroma was the most common complication seen postoperatively (N = 4, 26.7 %). Two of these patients required drain placement under ultrasound guidance. Two patients were found to have clinically significant fat necrosis, and they required a return to the operating room during their outpatient follow-up for debridement. Wound infection occurred in the two patients with fat necrosis, as well as one of the patients with a seroma drained by interventional radiology. Only one other patient developed a wound infection, which did not respond to antibiotics and required operative intervention. One additional patient developed cellulitis that responded to antibiotic therapy. There was no hernia recurrence, mesh infection, or death in the study.
ICG-FA
Upon extensive review of the ICG-FA imaging data, an absolute perfusion unit threshold of ten was found to be most appropriate given the previously stated criteria concerning true and false positives, and this number was used with contour lines to identify areas of underperfusion (falling below ten) within the study sample. Again, this threshold was determined to be the most appropriate for the dose of ICG in this study, as it maximized the sensitivity and specificity of ICG-FA. This yielded four true positives for the present study, one false positive, ten true negatives, and no false negatives. ICG-FA with a 5 mg ICG dose and an absolute perfusion unit threshold of ten yielded a sensitivity of 100 % and a specificity of 90.9 % for predicting postoperative wound complications.
True positives
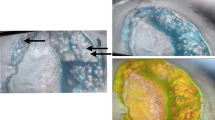
Four patients had wound complications with ICG-FA imaging suggestive of poor perfusion. Patient #14 had a low transverse incision with excision of chronic, non-infected granulation tissue above the umbilicus (Fig. 1). The pre-closure ICG-FA is shown in Fig. 2. There is an area of poor perfusion on the right side of the superior flap. The patient had wound breakdown and fat necrosis, who required operative debridement on postoperative day 21 (Fig. 3). The area of wound breakdown and fat necrosis corresponded exactly to the pre-closure ICG-FA at the time of initial hernia repair.
ICG-FA imaging of patient 14. Left looking from above at the inferior flap, with the cranial portion of the abdomen at the bottom of the image, there are no areas with absolute perfusion units <10. Right view of the superior flap, with the cranial portion of the abdomen at the bottom of the image. There is an area of poor perfusion on the patients’ right side. The area contained within the green contour has absolute perfusion units <10. Incidentally noted is an area of poor perfusion in the midline fascial closure, which is routinely noted and does not appear to have clinical correlation (color figure online)
Fat necrosis in patient 14 fat necrosis on the right side of the superior flap corresponding to poor perfusion noted in this area (Fig. 2)
Patient #8 had extensive subcutaneous dissection (Fig. 4). The pre-closure ICG-FA is shown in Fig. 5. There is an area of poor perfusion in the subcutaneous tissue. This patient presented on postoperative day 31 with continued subcutaneous drain output, a significant subcutaneous mass effect, and small areas of wound breakdown. He was taken to the operating room and found to have massive fat necrosis underneath the superior flap, which required one additional debridement in the operating room and skin grafting in his third postoperative return to the OR.
Pre-closure ICG-FA for patient 8. Left looking from above at the inferior flap, there are no areas with absolute perfusion units < 10. Right an upward view of the superior flap with a large area of poor perfusion in the subcutaneous tissue and abdominal wall. The area contained within the green contour has absolute perfusion units < 10 (color figure online)
Patient #2 had small areas of poor perfusion on the left side of the superior flap (Fig. 6) and the midline of the superior flap. These corresponded to areas of wound breakdown noted on follow-up visit (Fig. 6).
Patient #13 had an area of poor perfusion on the right side of his subcutaneous tissue after a vertical midline incision. This patient developed an infection of the abdominal wall who required readmission and operative debridement of his abscess cavity.
False positive
One patient had an area of poor perfusion noted on ICG-FA that did not correspond to clinically detectable wound breakdown or necrosis. This patient had an area of poor perfusion exclusively in the subcutaneous tissue and recovered well with no evident complications, including no seroma detected and no interventions required.
Financial impact
The mean hospital charges were similar for the four true positive patients and the ten true negative patients: $65,677 versus $57,000 (p = 0.480). Follow-up charges were also examined. Patient #14 had $20,800 in follow-up charges ($74,100 total charges, including initial hospitalization) due to the return to the operating room for debridement. Patient #8 had $93,900 in follow-up charges ($135,800 total charges) due to three trips to the operating room for debridement and eventual skin grafting. Patient #2 had only $100 in follow-up charges ($63,300 total charges), as the very small areas of wound breakdown were managed in the outpatient clinic. Patient #13 had follow-up charges of $62,505 ($166,813 total charges) for multiple clinic visits and readmission for debridement of his abdominal wall abscess cavity. The follow-up charges for the ten true negative patients were lower than the charges for the four true positives: $8800 versus $44,326 (p = 0.077), which led to lower total charges ($65,700 vs $110,004, p = 0.090). The differences in the follow-up charges between the true positives and true negatives were not statistically significant due to low sample size. The possibility of preventing these complications, however, certainly yields an opportunity for significant healthcare cost savings.
Discussion
This study is the first to evaluate the ability of intraoperative ICG-FA to predict postoperative wound complications in patients undergoing complex VHR. Patel et al. [41] demonstrated reduced wound complications with ICG-FA in 5 patients. The authors have also provided the first guideline for the use of ICG-FA in this field: an absolute perfusion unit threshold of ten with a 5 mg dose of ICG with the SPY Elite® system. This threshold identified all patients in the sample who developed a wound complication, corresponding to a sensitivity of 100 %. Additionally, only one patient in the study was identified as having an area of poor perfusion with ICG-FA that did not develop a clinically detectable wound breakdown or necrosis, providing an overall specificity of 90.9 %. Certainly, ICG-FA has the potential to augment surgeons’ judgment and help identify areas of poor perfusion which otherwise may have been unidentified, as they were in the present study.
The four patients whose wound complications were predicted consumed an average of nearly $45,000 each in follow-up costs. Recent data presented to the American College of Surgeons [42] support the high cost of wound complications found in the present study. The study of 500 consecutive VHR demonstrated increased follow-up charges, up to $62,000 more than patients with no complications, for patients with wound complications, wound infections, and mesh infections through 1 year postoperatively. The high cost of these complications is notable, as these are potential savings in healthcare expenditures if wound complications can be prevented.
The results of this study, utilizing ICG-FA to identify areas of underperfusion in complex VHR, follow prior demonstration of its efficacy in skin and soft tissue reconstructive procedures. The majority of prior ICG-FA studies have occurred over the last decade. One of the initial series from 2004 evaluated the use of ICG-FA in patients undergoing extremity reconstruction and free flap transfers and found it to be superior in prognostic predictability compared to traditional clinical parameters of perfusion (skin turgor, tissue color, capillary refill, tissue temperature, and bleeding after puncture) [43]. Comparisons of ICG-FA to other measures of tissue perfusion and viability, such as fluorescein dye, have also been performed. A series of 51 tissue expander implant breast reconstructions where ICG-FA was compared to fluorescein dye angiography demonstrated that ICG-FA was superior at predicting tissue necrosis with a higher specificity, as well as improved negative and positive predictive value [23]. The present study found that ICG-FA was associated with a high sensitivity (100 %) and specificity (90.9 %) for predicting postoperative complications in complex VHR. The results are similar to the high sensitivity (90 %) and specificity (100 %) reported in a recent series by Phillips et al. [23], which evaluated intraoperative ICG-FA for prediction of mastectomy skin flap necrosis in breast reconstruction.
ICG-FA has also been demonstrated to be beneficial in identifying perforators in patients undergoing perforator-based island flap surgery for reconstruction of skin defects. A series of 14 patients by Azuma et al. [28] demonstrated that ICG-FA precisely identified the perforators in each patient and also provided information on the direction of blood flow. The blood supply to the abdominal wall is derived from perforators of the superior epigastric and deep inferior epigastric arteries [6], which may be of importance to identify with ICG-FA intraoperatively as it has been proposed to be possibly disrupted during the extensive undermining and elevation of skin flaps during components separation technique in complex VHR [30], although this remains to be substantiated.
Not only can ICG-FA serve in predicting underperfusion, studies have shown that it can also lead to beneficial intraoperative changes in management to prevent postoperative skin necrosis and soft tissue breakdown. In a series by Mothes et al. [43], the planned postoperative management was altered in 47.2 % of cases based on discrepancies between ICG-FA and clinical findings. Other studies in breast and soft tissue reconstruction have also reported the benefit of ICG-FA for modifying operative technique [26, 36] to prevent complications. The present study did not involve changes in intraoperative management based on ICG-FA findings, as the focus was on studying its prognostic ability and identifying an absolute threshold predictive of wound complications in complex VHR; however, a follow-up randomized controlled trial is underway.
Using a standardized dose of 5 mg of ICG, an absolute perfusion unit threshold of ten was found to be the most advantageous. This threshold differs from a prior study examining absolute perfusion units in breast reconstruction [23]. The difference between the two studies is likely due to different ICG doses (5 vs 17.5 mg) and tissues evaluated; skin and subcutaneous tissue of the abdominal wall has not been evaluated in this context until the present study.
An important feature of implementing any predictive tool is minimizing the false negative rate. Using a threshold of ten absolute perfusion units, there were no false negatives in the present study. Lowering the threshold would reduce the already low false positive rate, but this is less important for the application of ICG-FA in complex VHR. Utilizing ICG-FA in an unblinded fashion, a surgeon would debride any areas of poor perfusion, including any that would never lead to a wound complication (false positives). This debridement is certainly preferable to a wound complication that would be missed by utilizing a lower threshold. In contrast, the false positive rate increases with higher thresholds, such as 20, which could lead to excessive and unnecessary debridement with a higher rate of false positives and lower specificity. Additionally, absolute perfusion units were chosen over relative perfusion units to utilize objective measures of perfusion, which is consistent with a recent consensus paper on the review of SPY ICG-FA that recommended absolute perfusion units as they may provide a greater utility for the quantification of perfusion over relative values [22]. There has been prior demonstration of increased variability with the use of relative perfusion units [23]. The present study is the first to evaluate the perfusion unit threshold in complex VHR, and the authors have demonstrated that an absolute perfusion unit threshold of 10 for a 5 mg ICG dose is optimal for predicting wound complications.
When determining suitable criteria for our study design, patients with ESRD or iodine allergy were excluded from our study. Patients with iodine allergy may have allergic reactions to ICG, as it contains 5.0–9.5 % sodium iodide as a contaminant [44], although studies report it is of little significance [31]. Patients with ESRD appear to be at the greatest risk of developing an adverse reaction to ICG as demonstrated in a study that found a 9.3 % incidence of adverse reactions to ICG in dialysis patients [34]. Additionally, a review of adverse reactions of patients receiving ICG found that 41.2 % of all reported reactions occurred in patients undergoing hemodialysis [31]. There were no adverse reactions to ICG in the 15 patients in the present study. These results add to the well-demonstrated safe clinical profile of ICG with an overall rate of one adverse reaction to every 42,000 doses [31].
There are limitations which must be addressed to substantiate any conclusions from this preliminary study of ICG-FA in complex VHR. This is a small series and lacks intraoperative intervention analysis on the treatment outcomes of ICG-FA, as it was designed to examine wound complication prediction and identification of a threshold for defining adequate perfusion; prevention of complications is beyond the scope of the present design. The false positive, as well as the true negatives may have had complications such as fat necrosis that were not detected on physical examination. Additionally, the mean follow-up was limited to 7 months. This is not adequate to detect all complications after VHR, such as recurrence. The authors do believe, however, that it is an adequate time-frame to detect wound complications. The authors also obtained pre-incision ICG-FA, which was not analyzed in the presented data. There were no areas of ischemia identified in the pre-incision imaging, but the imaging was stored and analyzed to plan for a randomized, controlled trial to assess the ability to prevent wound complications that is currently underway.
In the first series to evaluate the predictive use of ICG-FA intraoperatively during complex VHR, the authors have found that ICG-FA can successfully predict areas of skin and soft tissue breakdown and necrosis with a sensitivity of 100 % and a specificity of 90.9 % using an absolute perfusion unit threshold of ten. Although this is a small, preliminary series of patients, the results are promising. Future investigations on the role of ICG-FA in complex VHR are necessary, including operative intervention based on ICG-FA findings. The potential to identify and intervene to prevent wound complications in this patient population will provide substantial clinical impacts for patient quality of life and reduce the financial impact of poor outcomes. Preventing only four complications in the true positive group may have saved an average of nearly $45,000 in follow-up cost for each patient in the present study. With the high rates of postoperative wound complications in patients undergoing complex VHR, any promising opportunity to reduce the incidence of complications should be examined.
References
Poulose BK et al (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
Flum DR, Horvath K, Koepsell T (2003) Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg 237(1):129–135
Ramirez OM, Ruas E, Dellon AL (1990) “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86(3):519–526
Albright E et al (2011) The component separation technique for hernia repair: a comparison of open and endoscopic techniques. Am Surg 77(7):839–843
Ko JH et al (2009) Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg 144(11):1047–1055
Huger WE Jr (1979) The anatomic rationale for abdominal lipectomy. Am Surg 45(9):612–617
Harth KC, Blatnik JA, Rosen MJ (2011) Optimum repair for massive ventral hernias in the morbidly obese patient–is panniculectomy helpful? Am J Surg 201(3):396–400 discussion 400
Saxe A et al (2008) Simultaneous panniculectomy and ventral hernia repair following weight reduction after gastric bypass surgery: is it safe? Obes Surg 18(2):192–195 discussion 196
Downey SE et al (2005) Review of technique for combined closed incisional hernia repair and panniculectomy status post-open bariatric surgery. Surg Obes Relat Dis 1(5):458–461
Leevy CM, Stein SW, Cherrick GR, Davidson CS (1959) Indocyanine green clearence: a test of liver excretory function. Clin Res 7:290–294
Flower RW, Hochheimer BF (1972) Clinical infrared absorption angiography of the choroid. Am J Ophthalmol 73(3):458–459
Eren S et al (1995) Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg 96(7):1636–1649
Rubben A et al (1994) Infrared videoangiofluorography of the skin with indocyanine green–rat random cutaneous flap model and results in man. Microvasc Res 47(2):240–251
Holm C et al (2002) Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery 22(7):278–287
Holm C et al (2002) Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 55(8):635–644
Still J et al (1999) Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg 42(3):266–274
Desai ND et al (2006) A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg 132(3):585–594
Takahashi M et al (2004) SPY: an innovative intra-operative imaging system to evaluate graft patency during off-pump coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 3(3):479–483
Sanchez EQ et al (2008) Intraoperative imaging of pancreas transplant allografts using indocyanine green with laser fluorescence. Proc (Bayl Univ Med Cent) 21(3):258–260
Sekijima M et al (2004) An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc 36(7):2188–2190
Stanga PE, Lim JI, Hamilton P (2003) Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology 110(1):15–21 quiz 22–3
Gurtner GC et al (2013) Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 7(1):1
Phillips BT et al (2012) Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 129(5):778e–788e
Moyer HR, Losken A (2012) Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 129(5):1043–1048
Newman MI et al (2011) An investigation of the application of laser-assisted indocyanine green fluorescent dye angiography in pedicle transverse rectus abdominus myocutaneous breast reconstruction. Can J Plast Surg 19(1):e1–e5
Komorowska-Timek E, Gurtner GC (2010) Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 125(4):1065–1073
Pestana IA et al (2009) Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg 123(4):1239–1244
Azuma R et al (2008) Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg 122(4):1062–1067
Giunta RE et al (2005) Prediction of flap necrosis with laser induced indocyanine green fluorescence in a rat model. Br J Plast Surg 58(5):695–701
Wang HD, Singh DP (2012) The use of indocyanine green angiography to prevent wound complications in ventral hernia repair with open components separation technique. Hernia 17(3):397–402
Benya R, Quintana J, Brundage B (1989) Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn 17(4):231–233
Ishihara H et al (1998) Detection of capillary protein leakage by glucose and indocyanine green dilutions during the early post-burn period. Burns 24(6):525–531
Lifecell corp (2012) SPY Elite intraoperative perfusion assessment system: SPY Elite Pack and SPY Elite Kit Instructions for Use. Branchburg, Lifecell corp
Iseki K et al (1980) Shock caused by indocyanine green dye in chronic hemodialysis patients. Clin Nephrol 14(4):210
Hope-Ross M et al (1994) Adverse reactions due to indocyanine green. Ophthalmology 101(3):529–533
Newman MI, Samson MC (2009) The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg 25(1):21–26
Keats AS (1978) The ASA classification of physical status–a recapitulation. Anesthesiology 49(4):233–236
Harris PA et al (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Jones RS, Brown C, Opelka F (2005) Surgeon compensation: “Pay for performance,” the American College of Surgeons National Surgical Quality Improvement Program, the Surgical Care Improvement Program, and other considerations. Surgery 138(5):829–836
Novitsky YW et al (2006) Open preperitoneal retrofascial mesh repair for multiply recurrent ventral incisional hernias. J Am Coll Surg 203(3):283–289
Patel KM et al (2013) Use of intraoperative indocyanin-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg 47(6):476–480
American Burn Association (1990) Hospital and prehospital resources for optimal care of patients with burn injury: guidelines for development and operation of burn centers. J Burn Care Rehabil 11(2):98–104
Mothes H et al (2004) Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma 57(5):1018–1024
McEvoy GK (ed) (1988) Cardiac function. Indocyanine green. Drug info 88: American Hospital Formulary Service. American Society of Hospital Pharmacists, Bethesda, pp 1190–1191
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The study was conducted with a grant from LifeCell for materials only. PC declares no other conflicts of interest. BW declares no other conflicts of interest. IB declares no pertinent conflicts of interest, but is a speaker for W. L. Gore and Lifecell. AL declares no other conflicts of interest. SG declares no other conflicts of interest. BH has received grants from LifeCell and W. L. Gore. BH has received personal fees from W. L. Gore, LifeCell, Ethicon, and Davol. VA is a consultant for LifeCell.
Rights and permissions
About this article
Cite this article
Colavita, P.D., Wormer, B.A., Belyansky, I. et al. Intraoperative indocyanine green fluorescence angiography to predict wound complications in complex ventral hernia repair. Hernia 20, 139–149 (2016). https://doi.org/10.1007/s10029-015-1411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-015-1411-4