Abstract
Introduction
The purpose of this study is to evaluate the utility of using a functional lumen imaging probe (EndoFLIP™) intra-operatively during hiatal hernia repair and fundoplication. Additionally, we hypothesize that these measurements correlate with long-term outcomes.
Methods
A prospectively maintained quality database was queried. Between 2013 and 2018, 175 patients underwent laparoscopic fundoplication, the majority of which also had a hiatal hernia repair. The EndoFLIP™ was used to measure minimum diameter (Dmin), balloon pressure, and distensibility index (DI) at different timepoints throughout the operation. Clinical outcomes were measured up to 2 years after treatment.
Results
Crural closure and fundoplication resulted in a significant increase in balloon pressure and decrease in DI when compared to initial measurements as well as measurements taken after hernia reduction. After 1 year, patients with a final DI < 2.0 mm2/mmHg reported significantly more gas bloat and dysphagia than those with a final DI ≥ 2.0 mm2/mmHg (p = 0.040 and p = 0.025, respectively). This disparity became even more dramatic at 2 years (p = 0.006 and p = 0.004, respectively), with a final DI < 2.0 mm2/mmHg being significantly associated with higher prevalence of daily gas bloat (43.8% vs. 12.0%; p = 0.03). Additionally, patients with a final DI between 2.0 and 3.5 mm2/mmHg reported significantly lower Reflux Symptom Index scores at one year compared to those with a final DI < 2.0 or > 3.5 mm2/mmHg (p = 0.042).
Conclusion
EndoFLIP™ measurements correlate well with patient outcomes, with a final DI between 2 and 3.5 mm2/mmHg potentially being ideal. The EndoFLIP™ can be a useful adjunct in the operating room by providing objective measurements of esophageal distensibility after crural closure and fundoplication.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gastroesophageal reflux disease (GERD) affects up to 30% of the adult Western population [1]. Disruption of the anti-reflux barrier allows gastric contents to enter the esophagus which can result in life-altering symptoms and increased risk of malignancy [1, 2]. For patients with medically refractory GERD, surgery is a safe and effective option, and laparoscopic fundoplication is the gold standard anti-reflux operation [3, 4]. By returning the gastroesophageal junction (GEJ) to its normal intra-abdominal location, re-approximating the crura, and creating a new flap valve with fundoplication, surgeons are able to recreate the complex anti-reflux barrier.
Despite its effectiveness, many patients are still reluctant to undergo surgery due to potential adverse outcomes such as dysphagia and gas bloat [5]. Although there are several techniques to minimize these risks (e.g., use of a bougie, “floppy” Nissen), rates of dysphagia and gas bloat are still reported to be as high as 20% [6, 7]. Although the exact mechanism is not known, it has been suggested that an overly tight crural closure or fundoplication can increase the risk of gas bloat and dysphagia by preventing the surgically altered GEJ to relax sufficiently [8, 9]. Until now, there has not been an objective way to evaluate the tightness of crural closure or fundoplication in real-time during the operation.
The endoluminal functional lumen imaging probe (EndoFLIP™) (Medtronic; Dublin, Ireland) is a balloon-based catheter that uses impedance planimetry technology to evaluate the diameter, cross-sectional area, and distensibility of any sphincter in response to volume-controlled distention. Its use in the ambulatory setting for evaluating GEJ competency has been well described [10]; however, its use in the operating room to evaluate GEJ distensibility after crural closure and fundoplication has not been fully explored. Additionally, correlation between EndoFLIP™ measurements and long-term patient outcomes after fundoplication has never been described.
The aim of our study was to evaluate the utility of using the EndoFLIP™ intra-operatively to assess esophageal distensibility during laparoscopic fundoplication. We hypothesize that the EndoFLIP™ catheter can detect geometric changes in the GEJ after fundoplication and that final EndoFLIP™ measurements will correlate with patient outcomes.
Materials and methods
Data collection
After institutional review board approval, a prospectively maintained quality gastroesophageal (GE) database was queried for all patients undergoing laparoscopic fundoplication and EndoFLIP™ evaluation between 2013 and 2018. The GE database is maintained by research associates who prospectively collect clinical information on patients who present to our clinic with any gastroesophageal chief complaint. Data from pre-operative (e.g., usage of PPIs, symptomatology), intra-operative (e.g., operative length, blood loss), and post-operative time periods are prospectively collected through the electronic medical record. Additionally, online surveys are sent to all patients prior to surgery, as well as at various time points after their operation.
Operative protocol
All patients underwent a comprehensive esophageal work-up including upper endoscopy, manometry, and radiographic imaging (either barium swallow or CT scan) prior to surgery. pH testing was performed unless the indication for surgery was a symptomatic paraesophageal hernia.
All operations were performed laparoscopically by a single surgeon in a standardized manner. Once pneumoperitoneum (15 mmHg) was established, the crus was dissected, the hiatal hernia (if present) was reduced, the esophagus was mobilized to ensure at least 3 cm of intra-abdominal length, and the crura were re-approximated with permanent, posterior sutures. For patients with paraesophageal hernias or very large crural defects, mesh was used to buttress the repair. All fundoplications were performed over a bougie, the sizes of which ranged from 50 to 60 French, and was selected based on esophageal size. Patients with abnormal esophageal motility underwent Toupet instead of Nissen fundoplication.
Intra-operative EndoFLIP™ protocol
An EndoFLIP™ 1.0 unit and an 8 cm catheter (EF-325) were used for this protocol. Prior to usage, the catheter pre-check process was completed and the pressure transducer was referenced to atmospheric pressure. During the operation, the EndoFLIP™ catheter was placed transorally into the stomach and inflated to 20 ml. The catheter was pulled back until an hourglass shape was seen on the monitor, indicating the balloon was straddling the lower esophageal sphincter. The balloon was then inflated to 30 ml of volume and given 30 s to stabilize. All measurements were recorded with the patient in reverse Trendelenburg. Minimum diameter (Dmin), cross-sectional area (CSA), intra-balloon pressure, and distensibility index (DI) were recorded at the following timepoints:
-
1.
After intubation—“initial”
-
2.
After hiatal mobilization and/or hernia reduction
-
3.
After crural closure
-
4.
After fundoplication and bougie removal—“final”
Unfortunately, during this study period, there were variations in our EndoFLIP™ protocol. At times measurements were taken prior to establishing pneumoperitoneum whereas other times they were taken after insufflation. Additionally, some patients were evaluated with a 30 ml volume fill, whereas others were evaluated with a 40 ml volume. The impact of pneumoperitoneum and varying fill volumes on EndoFLIP™ catheter measurements is well known [11, 12], so we have taken extensive precautions to be consistent in our comparisons of the data. There were no complications related to EndoFLIP™ catheter usage.
Clinical follow-up
All patients are seen in clinic 3 weeks after surgery for their post-operative check. Additionally, Reflux Symptom Index (RSI), GERD-health related quality of life (GERD-HRQL), and Dysphagia Score surveys are emailed to patients at 3 weeks, 6 months, 1 year, 2 years, 5 years, 7 years, and 10 years after surgery. Gas bloat was evaluated based on the answer to question #9 on the GERD-HRQL (i.e., Do you have bloating or gassy feelings), and dysphagia frequency was evaluated based on question #7 (i.e., Do you have difficulty swallowing)? Responses in the GERD-HRQL range from a score of 0 to 5: 0—no symptoms, 1—symptoms noticeable but not bothersome, 2—symptoms noticeable and bothersome but not daily, 3—symptoms bothersome every day, 4—symptoms affect daily activity, 5—symptoms are incapacitating. The Dysphagia Score is used to evaluate the severity of dysphagia on a 5-point scale: 1—I am able to eat a normal diet/no dysphagia, 2—I am able to swallow some solid foods, 3—I am able to swallow only semi-solid foods, 4—I am able to swallow liquids only, 5—I am unable to swallow anything/total dysphagia. The RSI survey evaluates “atypical” symptoms of reflux, and a score > 13 is suggestive of severe reflux [13]. The GERD-HRQL is intended to quantify “typical” reflux symptoms [14]. Survey responses are recorded in the GE database.
Statistical analysis
The Wilcoxon signed-rank test was used to compare EndoFLIP™ measurements over the course of the operation and survey responses over time. The Wilcoxon rank-sum, Kruskal–Wallis, and Fisher’s exact test were used to compare survey responses between groups of EndoFLIP™ measurements. Multiple comparisons for the Kruskal–Wallis test were computed using the Dwass, Steel, Critchlow–Fligner method. All statistical analysis was performed using two-tailed tests with SAS 9.3 (SAS Institute, Cary, NC). A p value of < 0.05 was considered statistically significant.
Results
Patient demographics
Between 2013 and 2018, 175 consecutive patients underwent laparoscopic fundoplication ± hiatal hernia repair with EndoFLIP™ evaluation. Demographics of the cohort along with intra-operative details are shown in Table 1. Indications for surgery included GERD (48.6%) or symptomatic paraesophageal hernia (51.4%). GERD was characterized objectively by either positive pH testing (i.e., DeMeester score > 14.72), LA grade C or D esophagitis, histologically proven Barrett’s esophagus or histologic evidence of esophagitis.
EndoFLIP™ catheter detected changes in esophageal distensibility during surgery
When comparing all measurements taken with pneumoperitoneum and 30 ml volume fill, initial measurements of intra-balloon pressure and DI changed significantly after fundoplication (Fig. 1). Final Dmin was 8.7 ± 1.9 mm compared to 8.6 ± 2.5 mm initially, final intra-balloon pressure was 36.3 ± 11.2 mmHg compared to 28.6 ± 10.9 mmHg initially, and final DI was 1.9 ± 0.9 mm2/mmHg compared to 2.6 ± 2.3 mm2/mmHg initially.
Patient outcomes were affected by final distensibility
Outcomes of 71 patients were examined for correlation with pre-operative or post-operative Dmin, CSA or DI. All included patients had a hiatal hernia repair, and final EndoFLIP™ measurements recorded with a 30 ml volume fill and without pneumoperitoneum. On average, the final Dmin was 8.6 ± 1.8 mm, intra-balloon pressure was 28.8 ± 9.4 mmHg, and DI was 2.3 ± 1.5 mm2/mmHg.
When evaluating gas bloat, patients with a final DI < 2 mm2/mmHg reported higher gas bloat scores at 1 year compared to those with a DI ≥ 2 mm2/mmHg (1.9 ± 1.2 vs. 1.2 ± 0.3, p = 0.040) (Fig. 2). At 2 years, this disparity became even more pronounced (2.3 ± 0.3 vs. 1.2 ± 0.2, p = 0.006), with 43.8% of patients with a DI < 2 mm2/mmHg reporting daily gas bloat versus 12.0% of patients with a DI > 2 mm2/mmHg (p = 0.03).
Patients with a final DI < 2.0 mm2/mmHg reported significantly less gas bloat at 1 and 2 years after surgery. Final measurements based on a 30 ml volume fill and without pneumoperitoneum. Scale: 0—no symptoms; 1—symptoms noticeable but not bothersome; 2—symptoms noticeable and bothersome but not daily; 3—symptoms bothersome every day; 4—symptoms affect daily activity; 5—symptoms are incapacitating. p Values represent comparisons of responses between patients based on their final DI at 1 and 2 years after surgery. WPO weeks post-operative, MPO months post-operative, YPO years post-operative
Similarly, patients with a final DI < 2 mm2/mmHg reported significantly more frequent dysphagia at 1 year compared to those with a final DI ≥ 2.0 mm2/mmHg (0.7 ± 0.2 vs. 0.2 ± 0.1, p = 0.024), and this difference became even larger at 2 years (1.7 ± 0.3 vs. 0.2 ± 0.1, p = 0.004) (Fig. 3). Although this significant difference is not seen when evaluating Dysphagia Score, there continues to be a trend toward slightly worse dysphagia at 2 years for patients with a final DI < 2.0 mm2/mmHg (1.2 ± 0.1 vs. 1.0 ± 0, p = 0.065) (Fig. 4).
Patients with a final DI < 2.0 mm2/mmHg reported significantly more frequent dysphagia at 1 and 2 years after surgery. Final measurements based on a 30 ml fill volume and without pneumoperitoneum. Scale: 0—no symptoms; 1—symptoms noticeable but not bothersome; 2—symptoms noticeable and bothersome but not daily; 3—symptoms bothersome every day; 4—symptoms affect daily activity; 5—symptoms are incapacitating. p Values represent comparisons of responses between patients based on their final DI at 1 and 2 years after surgery. WPO weeks post-operative, MPO months post-operative, YPO years post-operative
Regardless of final distensibility (DI), all patients had some initial dysphagia, but symptoms improved over the course of the first year. However, dysphagia for patients with a final DI < 2.0 mm2/mmHg seemed to worsen slightly over the course of the second year. Final measurements based on a 30 ml fill volume and without pneumoperitoneum. Scale: 1—no dysphagia; 2—able to swallow some solid foods; 3—able to swallow only semi-solid foods; 4—able to swallow liquids only; 5—unable to swallow anything. p Values represent comparisons of responses between patients based on their final DI at 2 years after surgery. WPO weeks post-operative, MPO months post-operative, YPO years post-operative
When evaluating RSI scores, patients with a final DI between 2 to 3.5 mm2/mmHg reported significantly lower scores at 1 year compared to those with a DI < 2 or > 3.5 mm2/mmHg (Fig. 5). While not statistically significant, the trend persists at 2 years, with a final DI between 2 and 3.5 mm2/mmHg being associated with lower RSI scores. There was no association between final DI and total GERD-HRQL scores.
Patients with a final DI between 2 and 3.5 mm2/mmHg report lowest RSI scores at 1 and 2 years. Final measurements based on a 30 ml volume fill and without pneumoperitoneum. Min score: 0; max score: 45. RSI > 13 suggestive of severe reflux. p Values represent comparisons of responses between patients based on their final DI at 1 and 2 years after surgery. WPO weeks post-operative, MPO months post-operative, YPO years post-operative
Our cohort includes nine patients who were undergoing re-do surgery, and we acknowledge that this is a very different patient population. With this in mind, repeat analysis removing these nine patients did not alter our results, so the decision was made to include them in the study.
Additional analysis between EndoFLIP™ measurements and surgical characteristics
Additional comparison between final EndoFLIP™ measurements and surgical factors were performed, using only final measurements that were completed with a 30 ml volume fill and without insufflation (Table 2). Final EndoFLIP™ measurements did not differ whether a hiatal hernia repair was performed or not. Additionally, there were no difference in outcomes between the two groups, likely due to the small number of patients who did not have a hiatal hernia repair. Similarly, when comparing patients who ended up recurring versus those who did not, there was also no significant difference between final EndoFLIP™ measurements. Overall, there were 13 (7.4%) patients who recurred, and recurrence rates were similar between biosynthetic and porcine small intestine submucosa mesh (7.1 vs. 9.1%, p = 0.966). Lastly, aside from intra-balloon pressure, there was no significant difference in final measurements between patients undergoing a partial (Toupet) fundoplication compared to those undergoing a complete (Nissen) fundoplication.
Discussion
The goal of anti-reflux surgery is to recreate the complex anatomic zone of the GEJ. Particularly in patients with hiatal hernia, GEJ distensibility is increased which means GEJ opening occurs under significantly lower distention pressure [15]. By reducing the distensibility of the GEJ, surgery is able to recreate the anti-reflux barrier, but calibration of distensibility has not been possible until now. This study demonstrates that the EndoFLIP™ can detect changes in esophageal distensibility during laparoscopic fundoplication and that final measurements are associated with patient outcomes. These findings suggest that there may be an “ideal” distensibility range for which surgeons can target in order to maximize patient outcomes.
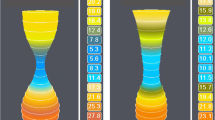
When comparing initial EndoFLIP™ measurements to final measurements, we saw that minimum diameter did not change significantly. While this seems counter-intuitive, it is consistent with findings by Kwiatek et al. who demonstrated that fundoplication actually creates a longer zone of constriction, resulting in increased intra-balloon pressure [11]. Since distensibility is calculated by dividing CSA by intra-balloon pressure, increasing pressure subsequently decreases distensibility. Using the EndoFLIP™ intra-operatively provides a visual representation of this change (Fig. 6). After hernia reduction, the GEJ appears wider and more patulous. After crural repair, Dmin decreases and intra-balloon pressure increases. After fundoplication, although the minimum diameter does not decrease, the length of the narrowing increases which results in an overall increase in intra-balloon pressure and further decrease of distensibility.
Visual representation of the changes in gastroesophageal junction shape at different timepoints of the operation. Although the minimum diameter does not change after fundoplication, the length of the narrowing (i.e., lengthening of the valve) increases which increases intra-balloon pressure and subsequently decreases DI
To our knowledge, Kim et al. is the only other group that has reported patient outcomes based on final EndoFLIP™ measurements after crural repair and fundoplication [16]. They reported a final Dmin of 5.97 mm, CSA of 28.28 mm2, and DI of 1.26 mm2/mmHg, using a 30 ml volume fill and insufflation. The presence of pneumoperitoneum likely accounts for the difference between their average final measurements compared to ours. At 1 month follow-up, they reported that all patients had resolution of reflux symptoms without any significant dysphagia. Ilczyszyn and Botha also recorded EndoFLIP™ measurements after fundoplication and reported an average final DI of 0.972 mm2/mmHg based on a 30 ml volume fill and no pneumoperitoneum [12]. DeHaan et al. reported a final DI of 1.6 mm2/mmHg after fundoplication; however, these measurements were based on a 30 ml volume fill with pneumoperitoneum [17] and neither of these studies included patient outcomes.
Additionally, the variability in protocols for recording EndoFLIP™ measurements amongst studies highlights the need for additional standardization of EndoFLIP™ usage in the operating room. While we used final measurements obtained with a 30 ml volume fill and without pneumoperitoneum, it remains unclear whether these measurement settings correlate best with outcomes. There are no studies that have obtained measurements with various settings (e.g., 30 ml ± pneumoperitoneum, 40 ml ± pneumoperitoneum) and reported which is best at predicting outcomes. While the gastrointestinal literature suggests that a 40 ml volume fill correlates best with symptoms in patients with achalasia [18], this is an area requiring further research, particularly in the surgical realm.
Ours is the first study to compare EndoFLIP™ measurements with long-term patient outcomes. We demonstrated that final distensibility is in fact associated with outcomes, and that a final DI between 2 and 3.5 mm2/mmHg (based on a 30 ml volume fill without pneumoperitoneum) may produce the best result by minimizing gas bloat, reflux, and dysphagia. For both frequency and severity of dysphagia, it appears that patients with a final DI < 2.0 mm2/mmHg improve over the course of the first year after surgery; however, their dysphagia worsens over the course of the second year. This may be due to an achalasia-like pathophysiology, in which the low distensibility acts as an esophageal outflow obstruction, gradually causing impaired motility and worse dysphagia. Unfortunately, we do not have follow-up manometry studies in these patients to confirm this theory; however, this progression highlights the need for additional studies reporting long-term outcomes.
Although final EndoFLIP™ measurements seemed to be associated with RSI scores, they were not associated with GERD-HRQL scores. This may be due to the different focus of the two surveys. The RSI focuses mainly on “atypical” GERD symptoms such as coughing, hoarseness and throat clearing, while the GERD-HRQL aims to quantify “typical” GERD symptoms such as heartburn and regurgitation. It is possible that recreating the anti-reflux barrier alone, by returning the GEJ to its normal intra-abdominal position, re-approximating the crura, and creating a new flap valve, sufficiently reduces typical GERD symptoms, regardless of distensibility. However, when it comes to atypical symptoms, targeting a DI between 2 and 3.5 mm2/mmHg allows for preserved esophageal clearance and reduction of atypical symptoms. This mechanism likely accounts for why RSI scores in patients with a final DI < 2.0 mm2/mmHg also fail to improve over time.
There are several limitations to our study. As previously stated, our EndoFLIP™ usage protocol during this study period was not consistent; therefore, measurements were not always recorded the same way for all patients. Despite this, we have made a conscious effort to compare measurements in a consistent manner, although this may have decreased our sample sizes in some instances. Additionally, we do not have objective data such as pH studies to correlate with EndoFLIP™ measurements—this is an area for future research. Lastly, lack of standardization between institutions regarding EndoFLIP™ protocol in the operating room makes it difficult to compare results between studies and limits generalizability.
In conclusion, usage of the EndoFLIP™ in the operating room is safe and feasible. Our study is the first to demonstrate that final distensibility after laparoscopic fundoplication is associated with long-term patient outcomes. These findings suggest that the EndoFLIP™ has potential to assist surgeons in calibrating crural and wrap tightness to minimize adverse effects of anti-reflux surgery. Additional research to confirm our ideal range of distensibility as well as the correlation between objective studies and final EndoFLIP™ measurements is warranted.
References
Richter JE, Rubenstein JH (2018) Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology 154:267–276
Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS (1982) Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 307:1547–1552
Mehta S, Bennett J, Mahon D, Rhodes M (2006) Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: seven-year follow-up. J Gastrointest Surg 10:1312–1316 (discussion 1316)
Dallemagne B, Weerts J, Markiewicz S, Dewandre JM, Wahlen C, Monami B, Jehaes C (2006) Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 20:159–165
Humphries LA, Hernandez JM, Clark W, Luberice K, Ross SB, Rosemurgy AS (2013) Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc 27:1537–1545
Patterson EJ, Herron DM, Hansen PD, Ramzi N, Standage BA, Swanström LL (2000) Effect of an esophageal bougie on the incidence of dysphagia following nissen fundoplication: a prospective, blinded, randomized clinical trial. Arch Surg 135:1055–1061 (discussion 1061)
Catarci M, Gentileschi P, Papi C, Carrara A, Marrese R, Gaspari AL, Grassi GB (2004) Evidence-based appraisal of antireflux fundoplication. Ann Surg 239:325–337
Yadlapati R, Hungness ES, Pandolfino JE (2018) Complications of antireflux surgery. Am J Gastroenterol 113:1137–1147
Richter JE (2013) Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 11:465–471 (quiz e39)
Kwiatek MA, Pandolfino JE, Hirano I, Kahrilas PJ (2010) Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP). Gastrointest Endosc 72:272–278
Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, Pandolfino JE (2010) Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg 14:268–276
Ilczyszyn A, Botha AJ (2014) Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus 27:637–644
Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the reflux symptom index (RSI). J Voice 16:274–277
Velanovich V (2007) The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 20:130–134
Pandolfino JE, Shi G, Curry J, Joehl RJ, Brasseur JG, Kahrilas PJ (2002) Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol 282:G1052–G1058
Kim MP, Meisenbach LM, Chan EY (2018) Tailored fundoplication with endoluminal functional lumen imaging probe allows for successful minimally invasive hiatal hernia repair. Surg Laparosc Endosc Percutan Tech 28:178–182
DeHaan RK, Davila D, Frelich MJ, Gould JC (2017) Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg Endosc 31:193–198
Pandolfino JE, de Ruigh A, Nicodème F, Xiao Y, Boris L, Kahrilas PJ (2013) Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil 25:496–501
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Michael B. Ujiki is a speaker for Medtronic, consultant for Olympus, on the Boston Scientific advisory board, a consultant and speaker for Apollo Medical Devices and a speaker for Gore Medical. Dr. Bailey Su, Ms. Stephanie Novak, Dr. Zachary M. Callahan, Ms. Kristine Kuchta and Ms. JoAnn Carbray have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, B., Novak, S., Callahan, Z.M. et al. Using impedance planimetry (EndoFLIP™) in the operating room to assess gastroesophageal junction distensibility and predict patient outcomes following fundoplication. Surg Endosc 34, 1761–1768 (2020). https://doi.org/10.1007/s00464-019-06925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06925-5