Abstract
Background
The laparoscopic approach to liver resection has experienced exponential growth in recent years. However, evidence-based guidelines are needed for its safe future progression. The main aim of our study was to perform a systematic review and meta-analysis comparing the short- and long-term outcomes of laparoscopic and open liver resections for colorectal liver metastases (CRLM).
Methods
To identify all the comparative manuscripts between laparoscopic and open liver resections for CRLM, all published English language studies with more than ten cases were screened. In addition to the primary meta-analysis, 3 specific subgroup analyses were performed on patients undergoing minor-only, major-only and synchronous resections. The quality of the studies was assessed using the Scottish Intercollegiate Guidelines Network (SIGN) methodology and Newcastle–Ottawa Score.
Results
From the initial 194 manuscripts identified, 21 were meta-analysed, including results from the first randomized trial comparing open and laparoscopic resections of CRLM. Five of these were specific to patients undergoing a synchronous resection (399 cases), while six focused on minor (3 series including 226 cases) and major (3 series including 135 cases) resections, respectively. Thirteen manuscripts compared 2543 cases but could not be assigned to any of the above sub-analyses, so were analysed independently. The majority of short-term outcomes were favourable to the laparoscopic approach with equivalent rates of negative resection margins. No differences were observed between the approaches in overall or disease-free survival at 1, 3 or 5 years.
Conclusion
Laparoscopic liver resection for CRLM offers improved short-term outcomes with comparable long-term outcomes when compared to open approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is one of the most common tumours worldwide and its incidence is increasing [1]. It is estimated that 30% of patients with CRC will present with liver metastases and a further 20% will develop metastases during their follow-up [2]. Resection of both the liver metastases and the primary tumour has been demonstrated to improve survival [3, 4]; hence, optimization of the management of colorectal liver metastases (CRLM) is essential.
A minimally invasive approach to the resection of CRLM and the primary colonic tumour(s) is now widely practised [5, 6], with recent meta-analyses demonstrating that a laparoscopic approach to liver resections offers improved short-term outcomes with comparable long-term outcomes [7]. However, these studies have all considered differing histopathological lesions as a single homogenous group, which may result in a potential bias and hence inaccuracies in the drawn conclusions.
In the absence of specific evidence-based guidance to the optimal approach for resection of CRLM, an up-to-date meta-analysis incorporating all relevant studies, including data from the first randomized control trial [8] comparing open and laparoscopic liver resections for CRLM, is required. In preparation for The European Guidelines Meeting of Laparoscopic Liver Surgery (EGMLLS), Southampton, 2017 [9], an updated meta-analysis taking into account patterns of resections was prepared to compare open and laparoscopic resections for CRLM.
Patients and methods
-
a.
Aims of the study.
-
i.
Primary aim: to amalgamate, weigh and summarize the current evidence regarding the short- and long-term outcomes of laparoscopic and open liver resections for the management of CRLM by systematic review and meta-analysis.
-
ii.
Secondary aims: to assess the distribution of available studies with regard to resection type and perform secondary subgroup meta-analyses by grouping similar studies in order to increase the level of evidence for specific resection types.
-
i.
-
b.
Search strategy and general considerations: Pubmed, Embase, the Cochrane Library and Web of Science were searched, using the following search strategy ((colorectal[Title] OR colon[Title] OR colonic[Title] OR rectal[Title] OR bowel[Title]) AND (laparoscopic[Title] OR laparoscopy[Title] OR minimally[Title] OR hybrid[Title]) AND (liver[Title] OR hepatic[Title] OR hepatectomy[Title])) and their associated combinations of medical subject heading-MeSH terms. The final search was performed on 20 May 2017. Data from the Oslo laparoscopic versus open liver resection for colorectal metastases (OSLO-COMET) trial were made available by the COMET team (BE and AAF). No IRB approval nor written consent was necessary for this study.
-
c.
Study selection: the inclusion and exclusion criteria are shown in Table 1. Review articles were examined for potential additional references. Duplications were identified by matching author’s names and publication centres. Two reviewers (R.C and I.G-L.) and an independent third one (M.H. or F.C.) in case of match individually assessed each manuscript and rejected those that failed to meet the inclusion criteria.
Table 1 Exclusion and inclusion criteria. TACE: transarterial chemoembolization -
d.
Definitions: considering the aims of our study, the following definitions and patterns were considered:
-
i.
The resection type was based upon the proposal from the Louisville Consensus meeting in 2008 [10] considering minor or major a resection involving ≤ 2 or > 2 Couinaud segments, respectively. Resections including simultaneous colorectal and liver resections were also individually analysed.
-
ii.
Each manuscript was assessed to establish if results reported could be applicable to more than one of the subgroups. If so, the results were separated and individually analysed within their subgroups.
-
iii.
Combined series were defined as those reporting a mixture of minor/major resections that could not be separated and analysed separately and hence could not be included within the above subgroups.
-
i.
-
e.
Variables and endpoints (endpoints in italics).
-
i.
Short-term outcomes (intraoperative parameters): operative time (minutes), operative blood loss (ml) and number of patients requiring blood transfusion (%).
-
ii.
Short-term outcomes (post-operative parameters): total number of early (< 30 days) complications (%), duration of post-operative hospital stay (days), mean resection margin (mm).
-
iii.
Long-term outcomes: 1-, 3- and 5-year overall survival; 1-, 3- and 5-year disease-free recurrence.
-
i.
-
f.
Quality assessment of the studies included in the meta-analysis.
-
i.
First quality assessment: the first quality assessment was performed in accordance with the Scottish Intercollegiate Guidelines Network (SIGN) [11].
-
ii.
Second quality assessment. The second quality assessment was performed in accordance with the Newcastle–Ottawa Quality Assessment Scale (NOS) available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. The criteria for “representativeness of cases” were considered as consecutive or obviously representative series of cases without a potential selection bias. Specifically, no star was given if cases included were not matched by year of inclusion (due to potential selection bias) and/or different surgeons and/or > 10 years of inclusion period (due to potential technical bias). Similarly, equal distribution of type and severity of underlying liver disease was an exclusion criterion to be given a star. For the rating of “Control for important factor”, two stars were given if lap and open cases were matched by age, gender, ASA score, Body mass index, type of resection, number of lesions and size of the lesions. If any of these factors was not specifically mentioned or not correctly matched, only 1 star was given. If 2 or more of these factors were not correctly matched or non-mentioned, no stars were given.
-
i.
-
g.
Statistical analysis: analyses were performed using log odds ratios (OR) with a 95% confidence interval (95% CI) for dichotomous variables and weighted mean differences (WMD) with a 95% CI for continuous variables. For dichotomous variables in which any observed value was 0, OR may be not possible to calculate, and thus, rate differences were used. The standard heterogeneity test, the I-square statistic, was used to assess the consistency of the effect sizes. Based on method reported by DerSimonian and Laird [12], substantial significance was set when p < 0.10 and a random effect model was used [13]. In addition, an I2 value < 25% was defined as low heterogeneity; between 25 and 50% as moderate heterogeneity; and > 50% as high heterogeneity. Publication bias was also assessed visually using a funnel plot. Every calculation for every group had a specific funnel plot. Data that were not significantly heterogeneous (p > 0.1) were calculated using a fixed-effects model by the Mantel–Haenszel method [14]. OpenMEE software based on OpenMetaAnalyst Software was used for statistical analyses [15, 16]. To perform meta-analyses, mean and Standard deviation (SD) were needed and estimations of mean and SD were performed to avoid discarding important studies. According to a recent publication from Wan et al. [17], in the event that a manuscript reported data in different measures than mean and SD, two different scenarios were considered, as reported in our previous meta-analysis [7]. For the meta-analysis, the authors decided to perform calculations only if, at least, 3 series could be identified for each variable, avoiding results obtained derived from analyses of 2 reports.
Results
Eligible studies and final count
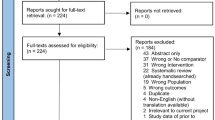
From the initial 194 manuscripts identified in the searches, 36 comparative studies remained after the inclusion and exclusion criteria were applied. Quality assessment was then performed in accordance to SIGN and NOS scales (Table 2). Fifteen manuscripts [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] did not reach a minimum requirement of acceptable quality (by SIGN scoring) or 6 points (by NOS) and were discarded (Fig. 1) resulting in 21 manuscripts to be considered for the systematic review and meta-analysis. Six of these were specific to patients undergoing minor [33,34,35] or major liver resections [33, 36, 37], including 226 and 140 cases, respectively. Five series [38,39,40,41,42] focused on synchronic colorectal and liver resection and included 399 patients. The remaining reports include 13 manuscripts [5, 6, 33, 36, 43,44,45,46,47,48,49,50,51] accounting for 2543 cases that could not be allocated to none of the previous categories and were analysed as “combined” resections. Each of the subgroups underwent separate secondary meta-analyses. All baseline results are depicted in Supplementary Digital Content 1.
Minor-only liver resections
Three manuscripts including 146 laparoscopic and 80 open cases were analysed. Overall complication rate and operative time were similar between both open and laparoscopic groups. Intraoperative blood loss (Het. p value = 0.080; I2 = 60%. SMD = − 0.538 [− 1.003 to − 0.074]; p = 0.023) and hospital stay (Het. p value = 0.728; I2 = 0%. SMD = − 0.363 [− 0.641 to − 0.085]; p = 0.01) were favourable with a laparoscopic approach (Figs. 2 and 3). None of the manuscripts reported data for long-term outcomes (Figs. 4 and 5).
Major-only liver resections
Three manuscripts including 45 laparoscopic and 95 open cases were analysed. With the exception of inpatient stay, which favoured an open approach (Het. p value = 0.150; I2 = 47%. SMD = − 0.545 [0.148–0.943]; p = 0.007), there were no differences in the short-term outcomes between open and laparoscopic approaches (Figs. 2 and 3). Only one manuscript reported results on survival, so no further statistical analyses were performed (Figs. 4 and 5).
Synchronic colorectal and liver resections
Five manuscripts were identified including 212 open versus 187 laparoscopic synchronic colorectal–liver resections. All short-term outcomes were comparable between the groups except for a reduced inpatient stay in the laparoscopic group (Het. p value = 0.184; I2 = 75%. SMD = − 0.709 [− 1.156 to − 0.263]; p = 0.002) (Figs. 2 and 3). Regarding long-term outcomes, only 1-year overall survival had sufficient numbers to allow for meta-analysis, with no significant differences between the approaches (Figs. 4 and 5).
Combined studies
Thirteen manuscripts could not be allocated to any of the three previous subcategories and included 2543 patients (1390 open and 1153 laparoscopic). Short-term outcomes including blood loss, operative time and positive resection margins were not significantly different. However, the rates of complications (Het p = 0.230; I2 = 21.7%. OR = 1.906 [1.504–2.415]; p < 0.001), transfusions (Het p = 0.001; I2 = 0%. OR = 1.653 [1.163–2.349]; p = 0.005) and hospital stay (Het. p = 0.001; I2 = 92.864%. SMD = − 0.3.843 [− 5.533 to − 2.153]; p < 0.001) all favoured a laparoscopic approach (Figs. 2 and 3). Contrary to the previous categories, there were a considerable number of studies reporting long-term outcomes. There were no significant differences observed in the 1-, 3- or 5-year overall and disease-free survival between open and laparoscopic groups (Figs. 4 and 5). In this specific point, we analysed the ratio of minor/major resections in open and laparoscopic approaches. From the 1247 open resections included, 876 were reported as minor, while in the laparoscopic group, 795 out of the 1025 analysed were considered as minor. There was a significant higher rate of minor resections in the laparoscopic group (I2 = 69.22%. OR = 1.804 [1.180–2.760]; p < 0.001) compared to the open groups (Fig. 6).
Bias analysis
Each sub-analysis was independently assessed for bias in each variable. All the resulting funnel plots are graphically depicted in Supplementary Digital Content 2, 3, 4 and 5.
Discussion
The initial implementation of a laparoscopic approach to liver resections was met with skepticism as to the oncological efficiency of the approach. These concerns have been addressed by small studies [30, 52, 53] but there remains limited high quality evidence provided by randomized controlled trials and meta-analysis to support this. The current study represents the most up-to-date and comprehensive analysis of the differing approaches to liver resection for CRLM and is the first meta-analysis to include data from a randomized controlled trial (OSLO-COMET) [43] specific to this subject.
The results of the current study demonstrate that, in general, the short-term outcomes are in favour of a laparoscopic approach. Regarding long-term outcomes, there are no differences in overall or disease-free survival in any of the series analysed thereby dispelling the concerns of an inferior oncological efficiency of a laparoscopic approach. It is noteworthy that post-operative complications have been suggested to worsen the oncological outcomes of a liver resection and may be even more influential than KRAS status [54]. Hence, a laparoscopic approach may even offer an oncological advantage by reducing post-operative complications. It has also traditionally been argued that laparoscopic liver surgery may not adequately balance the resection margin in CRLM leading to higher rates of R1-positive margins or unnecessary major liver resections. We performed meta-analyses for both resection margin rate and ratio of minor/major resections. Regarding the rate of positive resection margins, there were no differences between laparoscopic and open approaches. Regarding the rate of minor/major resections, there was a higher rate of minor resections in the laparoscopic group. We cannot discriminate from the analysed manuscripts if more complex cases were allocated to open groups, even though the matching was adequate in most of them.
The potential benefits of laparoscopic liver resections have already been demonstrated in smaller studies [5,6,7, 22, 37, 55, 56]. The results of this meta-analysis suggest that for the majority of resection classifications a laparoscopic approach produces better short-term outcomes with equivalent long-term outcomes to an open approach. However, this simplification overlooks important clinical advantages that may be associated with a laparoscopic approach. The reduced complication rate may allow an increased number of patients to have chemotherapy in a timely fashion, while the theoretical reduction in adhesions may allow for an increased number of repeat resections should recurrence occur. Similarly, reduced hospital stay has been found to be lower (not in major liver resections). This finding may also help administration of early chemotherapy with lower probabilities of complications that may delay its use leading to worse oncological prognosis. Finally, technical tools that are exclusive to a laparoscopic approach, such as Indocyanine green (ICG) fluorescence, may help achieve “anatomical parenchymal sparing” resections that may in turn contribute to a reduced ischemic residual that has been reported as a risk factor for recurrence [57].
A meta-analysis of retrospective studies should be approached with caution as several biases must be anticipated and controlled. Several manuscripts with a high quality of evidence could be identified in our search. Actually, excellent propensity score matching analyses [5] and the first RCT [8] could be enrolled in the search and the statistical analysis. This latter manuscript should be the basis of future RCT to be performed in LLR, as the inclusion criteria, statistical analysis and cost-effectiveness results were clearly defined and meticulously analysed. Unfortunately, long-term results were not included among the objectives of the study, which could have made the results of our study stronger. After reviewing the current literature, and despite the fact that several good quality manuscripts were identified, from our point of view it would be desirable that specific comparisons should be reported. We strongly advocate for split results in future publications that may, at least, consider the difficulty of resection.
In order to increase the quality of evidence reported by this meta-analysis, several steps were taken to improve the analyses. The use of resection categories permitted sub-analyses that allow for more homogeneous groups for comparison, while the division of outcomes into short- and long-term enabled the specific examination of the intra- and post-operative period and the oncological efficiency of the approaches. Finally, the current meta-analysis included several steps that tried to minimize biases. As per the EGMLLS methodology, we initially performed an extensive literature review with strong quality discrimination. For this purpose, we used two well-validated quality assessment tools to obtain the best quality of evidence: the SIGN methodology and the NOS. Manuscripts that were rated as low quality in the SIGN method and/or received less than 6 stars in the NOS were discarded. Finally, all meta-analyses performed to date use the methodology of Hozo et al. [58]; however, we have chosen to use the methodology of Wan et al. [17], which has recently been demonstrated to achieve more precise calculations of mean and standard deviation that in turn allows for more accurate conclusions to be drawn.
The results of this meta-analysis support the use of a minimally invasive approach for the resection of CRLM. A laparoscopic approach has no detrimental impact on long-term outcomes and provides improved short-term outcomes for the majority of resection classifications.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66:683–691. https://doi.org/10.1136/gutjnl-2015-310912
Jones RP, Jackson R, Dunne DFJ, Malik HZ, Fenwick SW, Poston GJ, Ghaneh P (2012) Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg 99:477–486. https://doi.org/10.1002/bjs.8667
‘t Lam-Boer J, Ali Al C, Verhoeven RHA, Roumen RMH, Lemmens VEPP, Rijken AM, De Wilt JHW (2015) Large variation in the utilization of liver resections in stage IV colorectal cancer patients with metastases confined to the liver. Eur J Surg Oncol 41:1217–1225. https://doi.org/10.1016/j.ejso.2015.05.014
Ahmad A, Reha J, Somasundar P, Espat NJ, Katz SC (2016) Predictors of surgical non-referral for colorectal liver metastases. J Surg Res 205:198–203. https://doi.org/10.1016/j.jss.2016.06.037
Martínez-Cecilia D, Cipriani F, Vishal S, Ratti F, Tranchart H, Barkhatov L, Tomassini F, Montalti R, Halls M, Troisi RI, Dagher I, Aldrighetti L, Edwin B, Abu Hilal M (2017) Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg 265:1192–1200. https://doi.org/10.1097/SLA.0000000000002147
Cipriani F, Rawashdeh M, Stanton L, Armstrong T, Takhar A, Pearce NW, Primrose J, Abu Hilal M (2016) Propensity score-based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg 103:1504–1512. https://doi.org/10.1002/bjs.10211
Ciria R, Cherqui D, Geller DA, Briceño J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777. https://doi.org/10.1097/SLA.0000000000001413
Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET Randomized Controlled Trial. Ann Surg 267:199–207. https://doi.org/10.1097/SLA.0000000000002353
Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceño J, Gayet B, D’Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien P-A, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D (2017) The Southampton Consensus Guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 268:1–18. https://doi.org/10.1097/SLA.0000000000002524
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han H-S, Wakabayashi G, Belli G, Kaneko H, Ker C-G, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey J-N, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS, World Consensus Conference on Laparoscopic Surgery (2009) The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 250(5):825–830
Petrie JC, Grimshaw JM, Bryson A (1995) The Scottish Intercollegiate Guidelines Network Initiative: getting validated guidelines into local practice. Health Bull (Edinb) 53:345–348
Simonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clinical Trials 7:177–188
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH (2012) Closing the gap between methodologists and end-users: r as a computational back-end. J Stat Softw 49:1–15
Wan X, Wang W, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. arXiv
Mala T, Edwin B, Gladhaug I, Fosse E, Søreide O, Bergan A, Mathisen Ø (2002) A comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases. Surg Endosc 16:1059–1063. https://doi.org/10.1007/s00464-001-9176-5
Huh JW, Koh YS, Kim HR, Cho CK, Kim YJ (2011) Comparison of laparoscopic and open colorectal resections for patients undergoing simultaneous R0 resection for liver metastases. Surg Endosc 25:193–198. https://doi.org/10.1007/s00464-010-1158-z
Chen K-Y, Xiang G-A, Wang H-N, Xiao F-L (2011) Simultaneous laparoscopic excision for rectal carcinoma and synchronous hepatic metastasis. Chin Med J 124:2990–2992. https://doi.org/10.3760/cma.j.issn.0366-6999.2011.19.006
Karagkounis G, Akyuz M, Guerron AD, Yazici P, Aucejo FN, Quintini C, Miller CM, Vogt DP, Fung JJ, Berber E (2016) Perioperative and oncologic outcomes of minimally invasive liver resection for colorectal metastases: a case-control study of 130 patients. Surgery 160:1097–1103. https://doi.org/10.1016/j.surg.2016.04.043
Allard M-A, Cunha AS, Gayet B, Adam R, Goere D, Bachellier P, Azoulay D, Ayav A, Navarro F, Pessaux P, Colorectal Liver Metastases-French Study Group (2015) Early and long-term oncological outcomes after laparoscopic resection for colorectal liver metastases: a propensity score-based analysis. Ann Surg 262:794–802. https://doi.org/10.1097/sla.0000000000001475
Tohme S, Goswami J, Han K, Chidi AP, Geller DA, Reddy S, Gleisner A, Tsung A (2015) Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg 19:2199–2206. https://doi.org/10.1007/s11605-015-2962-5
Langella S, Russolillo N, D’Eletto M, Forchino F, Tesoriere Lo R, Ferrero A (2015) Oncological safety of ultrasound-guided laparoscopic liver resection for colorectal metastases: a case-control study. Updates Surg 67:147–155. https://doi.org/10.1007/s13304-015-0325-0
Kubota Y, Otsuka Y, Tsuchiya M, Katagiri T, Ishii J, Maeda T, Tamura A, Kaneko H (2014) Efficacy of laparoscopic liver resection in colorectal liver metastases and the influence of preoperative chemotherapy. World J Surg Oncol 12:351. https://doi.org/10.1186/1477-7819-12-351
Guerron AD, Aliyev S, Agcaoglu O, Aksoy E, Taskin HE, Aucejo F, Miller C, Fung J, Berber E (2013) Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc 27:1138–1143. https://doi.org/10.1007/s00464-012-2563-2
Doughtie CA, Egger ME, Cannon RM, Martin RCG, McMasters KM, Scoggins CR (2013) Laparoscopic hepatectomy is a safe and effective approach for resecting large colorectal liver metastases. Am Surg 79:566–571
Cannon RM, Scoggins CR, Callender GG, McMasters KM, Martin RCG (2012) Laparoscopic versus open resection of hepatic colorectal metastases. Surgery 152:567–573. https://doi.org/10.1016/j.surg.2012.07.013discussion 573–4
Topal B, Tiek J, Fieuws S, Aerts R, Cutsem E, Roskams T, Prenen H (2012) Minimally invasive liver surgery for metastases from colorectal cancer: oncologic outcome and prognostic factors. Surg Endosc 26:2288–2298. https://doi.org/10.1007/s00464-012-2176-9
Abu Hilal M, Underwood T, Zuccaro M, Primrose J, Pearce N (2010) Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg 97:927–933. https://doi.org/10.1002/bjs.7034
Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B (2009) Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg 250:849–855. https://doi.org/10.1097/SLA.0b013e3181bcaf63
Iwahashi S, Shimada M, Utsunomiya T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Mori H, Kanamoto M, Yamada S (2014) Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc 28:80–84. https://doi.org/10.1007/s00464-013-3165-3
Nachmany I, Pencovich N, Zohar N, Ben-Yehuda A, Binyamin C, Goykhman Y, Lubezky N, Nakache R, Klausner JM (2015) Laparoscopic versus open liver resection for metastatic colorectal cancer. Eur J Surg Oncol 41:1615–1620. https://doi.org/10.1016/j.ejso.2015.09.014
Cheung TT, Poon RTP, Yuen WK, Chok KSH, Tsang SHY, Yau T, Chan SC, Lo CM (2013) Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ J Surg 83:847–852. https://doi.org/10.1111/j.1445-2197.2012.06270.x
Inoue Y, Hayashi M, Tanaka R, Komeda K, Hirokawa F, Uchiyama K (2013) Short-term results of laparoscopic versus open liver resection for liver metastasis from colorectal cancer: a comparative study. Am Surg 79:495–501
Hasegawa Y, Nitta H, Sasaki A, Takahara T, Itabashi H, Katagiri H, Otsuka K, Nishizuka S, Wakabayashi G (2015) Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: a comparative analysis of 168 consecutive cases at a single center. Surgery 157:1065–1072. https://doi.org/10.1016/j.surg.2015.01.017
Topal H, Tiek J, Aerts R, Topal B (2012) Outcome of laparoscopic major liver resection for colorectal metastases. Surg Endosc 26:2451–2455. https://doi.org/10.1007/s00464-012-2209-4
Tranchart H, Fuks D, Viganò L, Ferretti S, Paye F, Wakabayashi G, Ferrero A, Gayet B, Dagher I (2016) Laparoscopic simultaneous resection of colorectal primary tumor and liver metastases: a propensity score matching analysis. Surg Endosc 30:1853–1862. https://doi.org/10.1007/s00464-015-4467-4
Ratti F, Catena M, Di Palo S, Staudacher C, Aldrighetti L (2016) Impact of totally laparoscopic combined management of colorectal cancer with synchronous hepatic metastases on severity of complications: a propensity-score-based analysis. Surg Endosc 30(11):4934–4945. https://doi.org/10.1007/s00464-016-4835-8
Lin Q, Ye Q, Zhu D, Wei Y, Ren L, Zheng P, Xu P, Ye L, Lv M, Fan J, Xu J (2015) Comparison of minimally invasive and open colorectal resections for patients undergoing simultaneous R0 resection for liver metastases: a propensity score analysis. Int J Colorectal Dis 30:385–395. https://doi.org/10.1007/s00384-014-2089-2
Jung KU, Kim HC, Cho YB, Kwon CHD, Yun SH, Heo JS, Lee WY, Chun H-K (2014) Outcomes of simultaneous laparoscopic colorectal and hepatic resection for patients with colorectal cancers: a comparative study. J Laparoendosc Adv Surg Tech 24:229–235. https://doi.org/10.1089/lap.2013.0475
Hu M-G, Ou-yang C-G, Zhao G-D, Xu D-B, Liu R (2012) Outcomes of open versus laparoscopic procedure for synchronous radical resection of liver metastatic colorectal cancer: a comparative study. Surg Laparosc Endosc Percutan Tech 22:364–369. https://doi.org/10.1097/SLE.0b013e31825af6b2
Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B, Oslo-CoMet Study Group (2017) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET Randomized Controlled Trial. Ann Surg 267(2):199–207. https://doi.org/10.1097/sla.0000000000002353
Untereiner X, Cagniet A, Memeo R, Tzedakis S, Piardi T, Severac F, Mutter D, Kianmanesh R, Marescaux J, Sommacale D, Pessaux P (2016) Laparoscopic hepatectomy versus open hepatectomy for colorectal cancer liver metastases: comparative study with propensity score matching. Hepatobiliary Surg Nutr 5:290–299. https://doi.org/10.21037/hbsn.2015.12.06
Lewin JW, O’Rourke NA, Chiow AKH, Bryant R, Martin I, Nathanson LK, Cavallucci DJ (2016) Long-term survival in laparoscopic vs open resection for colorectal liver metastases: inverse probability of treatment weighting using propensity scores. HPB (Oxford) 18:183–191. https://doi.org/10.1016/j.hpb.2015.08.001
Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, Hirokawa F, Taniai N, Watanabe M, Katou M, Nagano H, Honda G, Baba H, Kokudo N, Konishi M, Hirata K, Yamamoto M, Uchiyama K, Uchida E, Kusachi S, Kubota K, Mori M, Takahashi K, Kikuchi K, Miyata H, Takahara T, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepato-Biliary Pancreat Sci 22:711–720. https://doi.org/10.1002/jhbp.261
de’Angelis N, Eshkenazy R, Brunetti F, Valente R, Costa M, Disabato M, Salloum C, Compagnon P, Laurent A, Azoulay D (2015) Laparoscopic versus open resection for colorectal liver metastases: a single-center study with propensity score analysis. J Laparoendosc Adv Surg Tech 25:12–20. https://doi.org/10.1089/lap.2014.0477
Qiu J, Chen S, Pankaj P, Wu H (2014) Laparoscopic hepatectomy is associated with considerably less morbidity and a long-term survival similar to that of the open procedure in patients with hepatic colorectal metastases. Surg Laparosc Endosc Percutan Tech 24:517–522. https://doi.org/10.1097/SLE.0b013e31829cec2b
Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, De Hemptinne B, Geboes K, Troisi RI (2014) Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol 40:536–544. https://doi.org/10.1016/j.ejso.2014.01.005
Qiu J, Chen S, Pankaj P, Wu H (2013) Laparoscopic hepatectomy for hepatic colorectal metastases: a retrospective comparative cohort analysis and literature review. PLoS ONE 8:e60153. https://doi.org/10.1371/journal.pone.0060153
Nguyen KT, Marsh JW, Tsung A, Steel JJL, Gamblin TC, Geller DA (2011) Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 146:348–356. https://doi.org/10.1001/archsurg.2010.248
Cipriani F, Shelat VG, Rawashdeh M, Francone E, Aldrighetti L, Takhar A, Armstrong T, Pearce NW, Abu Hilal M (2015) Laparoscopic parenchymal-sparing resections for nonperipheral liver lesions, the diamond technique: technical aspects, clinical outcomes, and oncologic efficiency. J Am Coll Surg 221:265–272. https://doi.org/10.1016/j.jamcollsurg.2015.03.029
Abu Hilal M, Di Fabio F, Abu Salameh M, Pearce NW (2012) Oncological efficiency analysis of laparoscopic liver resection for primary and metastatic cancer: a single-center UK experience. Arch Surg 147:42–48. https://doi.org/10.1001/archsurg.2011.856
Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, Vauthey J-N, Conrad C (2016) Comprehensive complication index predicts cancer-specific survival after resection of colorectal metastases independent of RAS mutational status. Ann Surg. https://doi.org/10.1097/sla.0000000000002018
McPhail MJW, Scibelli T, Abdelaziz M, Titi A, Pearce NW, Abu Hilal M (2009) Laparoscopic versus open left lateral hepatectomy. Expert Rev Gastroenterol Hepatol 3:345–351. https://doi.org/10.1586/egh.09.36
Cipriani F, Rawashdeh M, Ahmed M, Armstrong T, Pearce NW, Abu Hilal M (2015) Oncological outcomes of laparoscopic surgery of liver metastases: a single-centre experience. Updates Surg 67:185–191. https://doi.org/10.1007/s13304-015-0308-1
Yamashita S, Venkatesan AM, Mizuno T, Aloia TA, Chun YS, Lee JE, Vauthey J-N, Conrad C (2017) Remnant liver ischemia as a prognostic factor for cancer-specific survival after resection of colorectal liver metastases. JAMA Surg 152:e172986. https://doi.org/10.1001/jamasurg.2017.2986
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Drs. Ciria, Ocaña, Gómez-Luque, Cipriani, Halls, Fretland, Okuda, Aroori, Briceño, Aldrighetti, Edwin and Abu Hilal have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript and research performed in the context of the European Guidelines Meeting of Laparoscopic Liver Surgery held in Southampton-United Kingdom from the 9th to 11th of February 2017.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ciria, R., Ocaña, S., Gomez-Luque, I. et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 34, 349–360 (2020). https://doi.org/10.1007/s00464-019-06774-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06774-2