Abstract
Background
Higher intra-abdominal pressure may impair cardiopulmonary functions during laparoscopic surgery. While 12–15 mmHg is generally recommended as a standard pressure, the benefits of lower intra-abdominal pressure are unclear. We thus studied whether the low intra-abdominal pressure compared with the standard pressure improves cardiopulmonary dynamics during laparoscopic surgery.
Methods
Patients were randomized according to the intra-abdominal pressure and neuromuscular blocking levels during laparoscopic colorectal surgery: low pressure (8 mmHg) with deep-block (post-tetanic count 1–2), standard pressure (12 mmHg) with deep-block, and standard pressure with moderate-block (train-of-four count 1–2) groups. During the laparoscopic procedure, we recorded cardiopulmonary variables including cardiac index, pulmonary compliance, and surgical conditions. We also assessed postoperative pain intensity and recovery time of bowel movement. The primary outcome was the cardiac index 30 min after onset of laparoscopy.
Results
Patients were included in the low pressure with deep-block (n = 44), standard pressure with deep-block (n = 44), and standard pressure with moderate-block (n = 43) groups. The mean (SD) of cardiac index 30 min after laparoscopy was 2.7 (0.7), 2.7 (0.9), and 2.6 (1.0) L min−1 m−2 in each group (P = 0.715). The pulmonary compliance was higher but the surgical condition was poorer in the low intra-abdominal pressure than the standard pressure (both P < 0.001). Other variables were comparable between groups.
Conclusion
We observed few cardiopulmonary benefits but poor surgical conditions in the low intra-abdominal pressure during laparoscopy. Considering cardiopulmonary dynamics and surgical conditions, the standard intra-abdominal pressure may be preferable to the low pressure for laparoscopic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The laparoscopic surgical space is created by insufflating a gas and increasing the pressure in the abdominal cavity [1, 2]. However, excessively high intra-abdominal pressures may impair cardiovascular or pulmonary functions, decreasing the cardiac output or pulmonary compliance [2,3,4,5,6,7]. Therefore, 12–15 mmHg is generally recommended as a standard intra-abdominal pressure for laparoscopic surgery [1,2,3, 6].
In addition to the intra-abdominal pressure, the neuromuscular block may affect the laparoscopic view [1, 2, 7,8,9,10,11]. Several previous studies suggested that the deep neuromuscular block compared with the moderate block provided acceptable surgical conditions even in the intra-abdominal pressure lower than the standard level [7, 10, 11]. However, cardiopulmonary benefits of this lower intra-abdominal pressure are unclear, although a few animal studies suggested the possibility [12, 13]. We thus studied whether the low intra-abdominal pressure compared with the standard pressure improves cardiopulmonary dynamics during laparoscopic surgery.
Materials and methods
Patients
This prospective, double-blind, single-center, parallel-group, randomized controlled trial was approved by the Institutional Review Board of Seoul National University Hospital (1405-010-576; Seoul, Korea), and registered at ClinicalTrials.gov (NCT02249585). This trial followed the CONSORT guideline (Supplementary Material). After obtaining written informed consents, we enrolled patients aged 20–80 years with American Society of Anesthesiologists physical status I–II, and undergoing elective laparoscopic colorectal surgery. We excluded patients with allergies to anesthetic drugs, neuromuscular dysfunctions, severe cardiovascular or respiratory diseases, irregular cardiac rhythms, or body mass index of > 35 kg m−2. Patients were randomized to one of the three groups depending on the intra-abdominal pressures and neuromuscular blocking levels during laparoscopy: the low pressure with deep-block, standard pressure with deep-block, and standard pressure with moderate-block groups. An assistant not involved in the trial created the randomization in a 1:1:1 ratio and concealed it in sealed opaque envelopes. The group assignment was blinded to patients and investigators.
Anesthesia and surgery
Attending anesthesiologists were instructed about the study protocol but not related to data analyses. Without premedication, patients were monitored with non-invasive blood pressure, pulse oximeter, three-channel electrocardiograph, bispectral index (A-2000 XP; Aspect Medical Systems, Newton, MA, USA), and two-channel cerebral oximeter (Somanetics INVOS oximeter; Covidien, Mansfield, MA, USA). For neuromuscular monitoring, an acceleromyograph (TOF-watch SX; Organon, Dublin, Ireland) was applied to the patient’s left hand to obtain responses of the adductor pollicis muscle. Two stimulating electrodes were attached on the wrist over the ulnar nerve, a temperature sensor on the palm, and an acceleration transducer on the thumb.
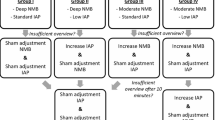
For anesthetic induction, propofol and remifentanil were administered intravenously via effect-site target-controlled infusion (Base Primea; Fresenius Vial, Brezins, France) with Schnider [14] and Minto [15] pharmacokinetic models, respectively. The initial effect-site concentrations were 4 µg mL−1 for propofol and 4 ng mL−1 for remifentanil and lactated Ringer’s solution was infused at 10 mL kg−1 h−1. Before giving rocuronium (Esmeron; MSD, Kenilworth, NJ, USA), the acceleromyograph was calibrated and stabilized: a 50-Hz tetanic stimulation for 5 s followed by serial train-of-four (TOF) measurements within a 5% variation [1, 7, 8, 11, 16]. During TOF stimulation every 10 s, rocuronium 0.8 or 0.4 mg kg−1 was given intravenously to induce deep or moderate neuromuscular blocks, respectively (Fig. 1). At the TOF count of 0 and bispectral index of < 60, the patient’s trachea was intubated with a polyvinylchloride tracheal tube with an inner diameter of 7.0 and 7.5 mm for women and men, respectively. The intra-cuff pressure was adjusted to 20–25 cm H2O (VBM Medizintechnik GmbH, Sulz am Neckar, Germany).
A 20-gauge catheter was inserted into the right radial artery and connected to an arterial waveform analysis system (FloTrac/EV1000, version 4.0; Edwards Life Sciences, Irvine, CA, USA) for hemodynamic monitoring and blood sampling. The FloTrac sensor was placed at the level of the right atrium in the supine position, and adjusted according to the patient’s positional changes. A nasogastric tube and a temperature probe were placed in the stomach and the nasopharynx, respectively.
During anesthetic maintenance, the effect-site concentrations of propofol and remifentanil were titrated within the bispectral index of 40–60, and lactated Ringer’s solution was infused at 5 mL kg−1 h−1. The neuromuscular blocking level was checked every 10 min. Rocuronium 0.3 or 0.15 mg kg−1 was intermittently administered to maintain the deep neuromuscular block with a post-tetanic count 1–2 or the moderate block with a TOF count 1–2, respectively (Fig. 1) [7, 11, 17]. The blocking level was maintained until the end of surgery. During laparoscopic procedures, the patient’s lungs were ventilated (Primus; Dräger, Lübeck, Germany) with a tidal volume of 6–8 mL kg−1 of predicted body weight, PEEP of 5 cm H2O, inspiratory-to-expiratory ratio of 1:2, plateau time of 10%, respiratory rate of 12–16 min−1, and inspired oxygen fraction of 0.5 with a gas flow of 2 L min−1 of oxygen and air. Ephedrine 5 mg, phenylephrine 30 µg, or lactated Ringer’s solution 200 mL were given at a mean blood pressure of < 60 mmHg, urine output of < 0.5 mL kg−1 h−1, or stroke volume variation of > 13%.
Two surgeons (S-YJ and JWP) conducted all laparoscopic procedures under the lithotomy with a 30° head-down position. Carbon dioxide (CO2) was insufflated (Pneumo Sure High Flow Insufflator; Stryker Endoscopy, San Jose, CA) into the abdominal cavity and the intra-abdominal pressure was set at 8 or 12 mmHg for the low or standard pressure levels, respectively (Fig. 1) [2, 6]. If the laparoscopic view was too poor to continue the procedures, the intra-abdominal pressure was increased by 4 mmHg at the surgeon’s request. The intra-abdominal pressure and neuromuscular blocking levels were blinded to the surgeons by concealing the monitor of CO2 insufflator and acceleromyograph.
At the skin closure, an intravenous patient-controlled analgesia (Anaplus; Ewha Biomedics, Goyang-si, Korea) was initiated with a 100-mL mixture of morphine 30–60 mg, fentanyl 1000–1500 µg, and normal saline, and infused at 1 mL h−1, bolus 0.5 mL, and lockout time 15 min until the third postoperative day. Ramosetron 0.3 mg was given to prevent postoperative nausea or vomiting.
At the end of surgery, all anesthetic drugs were discontinued, and sugammadex (Bridion; MSD, Kenilworth, NJ, USA) 4 mg kg−1 was administered to reverse the neuromuscular block. When the patient was able to breathe spontaneously and respond to verbal commands at the TOF ratio of > 0.9, the trachea was extubated and the patient was transferred to the postanesthesia care unit. In the postoperative period, fentanyl 50 µg was given as a rescue analgesic at the patient’s request. The patient was discharged from the postanesthesia care unit with a modified Aldrete score of 9–10 [18] and discharged from the hospital when the patient can tolerate food and ambulate safely without severe pain, fever, and complications [19].
Outcomes
We collected baseline data of patients, surgery, and anesthesia. Intraoperative cardiopulmonary variables were recorded at six-time points: 1 min after skin incision and CO2 insufflation in the supine position, and 30, 60, 90, and 120 min after CO2 insufflation in the head-down position. Stroke volume index, cardiac index, mean blood pressure, and heart rate were obtained from the EV1000 platform; plateau and peak inspiratory airway pressures from the Primus anesthesia machine; arterial partial pressures of oxygen and CO2 from the blood gas analyzer (GEM Premier 3000, Model 5700; Instrumentation Laboratory, Lexington, MA, USA); and left or right cerebral oxygen saturations from the INVOS cerebral oximeter. The static and dynamic pulmonary compliances were calculated from the plateau and peak inspiratory pressures, PEEP, and tidal volume [20]. We checked amounts of fluids, transfusion, urine output, estimated blood loss, and inotropic requirements.
After the laparoscopic procedure, the surgeon subjectively assessed the quality of surgical conditions using a five-point scale as follows: optimal, a wide visible laparoscopic working field without any movements or contractions; good, a wide laparoscopic field with sporadic muscle contractions or movements; acceptable, a wide visible laparoscopic field with regular muscle contractions or movements causing some interference with the surgeon’s work; poor, a visible laparoscopic field with continuous muscle contractions or movements causing severe interference with the surgeon’s work; extremely poor, the inability to obtain a visible laparoscopic field because of inadequate muscle relaxation or coughing [8, 16]. We also recorded the insufflated CO2 volume and number of patients receiving elevation of the intra-abdominal pressure during laparoscopy. After surgery, we checked the durations of eye opening or tracheal extubation after the administration of sugammadex, and the length of stay in the postanesthesia care unit or hospital. An investigator (HP) blinded to the group assignment confirmed the TOF ratio of ≥ 1.0 to exclude residual paralysis 30 min after surgery [21]. The investigator also evaluated the pain intensity using an 11-point visual analogue scale (0, no pain; 10, worst pain imaginable), and the sedation level using a seven-point Leiden observer’s assessment of alertness/sedation scale (0, normal alertness; 6, not aroused by a painful stimulus) [16]. We recorded rescue analgesic requirements, recovery time of bowel movement, and any perioperative adverse events.
The primary outcome was the cardiac index 30 min after CO2 insufflation. The secondary outcomes were other cardiopulmonary variables, surgical conditions, and CO2 consumption during laparoscopy; and recovery times and pain intensity after surgery.
Statistical analysis
In our pilot study (n = 10), the mean (SD) of cardiac index was 2.6 (0.6) L min−1 m−2 at 30 min after CO2 insufflation under the intra-abdominal pressure of 12 mmHg and the post-tetanic count of 1–2 during laparoscopic colorectal surgery. To detect a 15% difference in the cardiac index by lowering the pressure to 8 mmHg, 41 patients were needed in each group with a type-I error risk of 0.05 and a power of 0.8 for two-tailed analysis.
Continuous variables were presented as mean (SD) or median (interquartile range) after checking the normality with Shapiro–Wilk test. Repeatedly measured continuous variables were analyzed with linear mixed models, ANOVA or Kruskal–Wallis test, and unpaired t or Mann–Whitney U tests as appropriate. In the mixed model, fixed effects were the group, time, and interaction between group and time, and a random effect was the subject.
Categorical variables were the number of patients (proportion) and were compared with Chi-Squared test or Fisher’s exact test. Effect sizes with 95% CI were calculated. A P-value of < 0.05 was considered significant and adjusted with Bonferroni correction. All analyses were conducted in an intention-to-treat manner. STATA (Special Edition 14.2; Stata Corporation, College Station, TX, USA) was used for sample size calculation, randomization, and statistical analyses.
Results
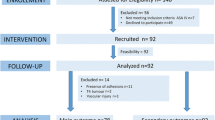
After screening 150 patients, 131 patients were included in the low pressure with deep-block (n = 44), standard pressure with deep-block (n = 44), and standard pressure with moderate-block (n = 43) groups between December 2014 and October 2016 (Fig. 1). However, at 90 and 120 min after CO2 insufflation, we obtained cardiopulmonary data only from 103 and 70 patients because of the shorter laparoscopic procedure time (Fig. 1). The characteristics of patients, surgery, and anesthesia were comparable between groups except for the dose of rocuronium (Table 1). No patients received conversion to open laparotomy or transfusion.
The mean (SD) of the cardiac index at 30 min after CO2 insufflation was 2.7 (0.7), 2.7 (0.9), and 2.6 (1.0) L min−1 m−2 in the low pressure with deep-block, standard pressure with deep-block, and standard pressure with moderate-block groups (P = 0.715, ANOVA) and similar throughout the entire laparoscopic procedure (Fig. 2B, P = 0.192, linear mixed model). The stroke volume index (P = 0.213), mean blood pressure (P = 0.814), and heart rate (P = 0.543) were also comparable between groups (Fig. 2). The static and dynamic pulmonary compliances were significantly higher in the low pressure group than in the standard pressure groups at 1, 30, and 60 min after CO2 insufflation (Fig. 3A, B, P < 0.001, ANOVA). The arterial partial pressures of oxygen (Fig. 3C, P = 0.651) and CO2 (Fig. 3D, P = 0.338), or the left (P = 0.745) and right (P = 0.712) cerebral oxygen saturations were similar between groups.
Mean (circle) and SD (bar) of pulmonary variables before and 1, 30, 60, 90, and 120 min after onset of laparoscopy. *Mean difference (95% CI) 5.8 (1.9–9.7) mL cm−1 H2O, P = 0.004 and 5.6 (1.5–9.7) mL cm−1 H2O, P = 0.007 compared with the standard pressure with deep- and moderate-block groups, respectively, by unpaired t test. †4.1 (1.7–6.5) mL cm−1 H2O, P = 0.001 and 4.6 (2.2–7.1) mL cm−1 H2O, P < 0.001. ‡4.2 (1.2–7.2) mL cm−1 H2O, P = 0.006 and 4.0 (1.0–7.1) mL cm−1 H2O, P = 0.009. §5.1 (1.6–8.6) mL cm−1 H2O, P = 0.005 and 5.8 (1.8–9.7) mL cm−1 H2O, P = 0.004. ¶4.9 (2.3–7.4) mL cm−1 H2O, P < 0.001 and 5.9 (3.4–8.4) mL cm−1 H2O, P < 0.001. ║3.6 (1.2–6.0) mL cm−1 H2O, P = 0.003 and 4.0 (1.3–6.6) mL cm−1 H2O, P = 0.004
The laparoscopic surgical condition was better in the deep neuromuscular block than in the moderate block under the standard intra-abdominal pressure, but worse in the low pressure than the standard pressure under the deep-block (Fig. 4). Moreover, in 12/44 (27%) patients in the low pressure with deep-block group, the surgeon asked to increase the intra-abdominal pressure because of the unacceptable laparoscopic view (Table 2). The insufflated CO2 volume was comparable between groups (Table 2).
After surgery, recovery times and pain intensity were similar between groups (Table 2). No patients showed residual paralysis or severe complications.
Discussion
In this randomized controlled trial, the low intra-abdominal pressure (8 mmHg) compared with the standard pressure (12 mmHg) showed similar hemodynamic responses and higher pulmonary compliances, but poor surgical conditions during laparoscopic colorectal surgery.
The intra-abdominal pressures higher than 15 mmHg may compress the inferior vena cava or mediastinum and decrease the cardiac preload, and thus the stroke volume and cardiac output [2, 3]. These effects can be aggravated in cardiovascular-compromised patients [2, 3, 22, 23]. However, because our participants were relatively healthy without severe cardiovascular diseases, they might be hemodynamically tolerable even in the increased intra-abdominal pressures. Furthermore, the leg-up (i.e., lithotomy) and head-down position may facilitate venous return to the heart [3, 6, 24], compensating for negative inotropic effects of the elevated intra-abdominal pressure. These could explain our findings of similar hemodynamic status between the low and standard intra-abdominal pressures during laparoscopic colorectal surgery.
The high pressure in the abdomen can be transmitted to the thorax by pushing up the diaphragm, thereby reducing the efficiency of mechanical ventilation [2, 4,5,6, 25, 26]. We observed that the lower intra-abdominal pressure provided higher pulmonary compliances but not better oxygenation nor CO2 elimination [2, 11, 27]. Thus, the lower intra-abdominal pressure seems to have only limited benefits for pulmonary functions.
Deeper neuromuscular blocks can reduce abdominal muscle tones, thereby expanding the laparoscopic working spaces under the same intra-abdominal pressure [1, 2, 8, 9]. However, it remains controversial whether the deep-block enables laparoscopic procedures to be performed even in the low intra-abdominal pressure [10, 28, 29]. In our study, the deeper block provided better surgical conditions in the standard intra-abdominal pressure, but unacceptable conditions in the low pressure. Moreover, in 12 patients with a pressure of 8 mmHg, the surgeon requested higher pressure because of unacceptable laparoscopic views. Therefore, the intra-abdominal pressure is more likely to affect the laparoscopic conditions compared with the neuromuscular blocking level, especially under lower pressures.
Although CO2 is commonly used for laparoscopic surgery, its systemic absorption may cause adverse effects such as embolism, acidosis, or hemodynamic instability [2, 6, 11, 26, 30]. Theoretically, lower intra-abdominal pressures require a lower volume of CO2 [26, 31], but we found no difference between groups. This is probably because the intra-abdominal pressure was increased from 8 to 12 mmHg in the 12 patients, and their data were included for the intention-to-treat analysis.
Intraoperative deep neuromuscular blocks may lead to postoperative residual paralysis and delayed recovery [32,33,34]. However, because sugammadex is known to completely antagonize any level of rocuronium-induced neuromuscular block within 3 min [32, 34, 35], we observed similar durations to tracheal extubation or discharge from the postanesthesia care unit without recurarization in all groups. Higher intra-abdominal pressures may compress the nerves or vessels in the abdominal cavity, thus can intensify postoperative pain or prolong the recovery of bowel movement, but previous findings were inconsistent [2, 3, 6, 10, 17, 36]. We also observed no differences in the postoperative outcomes although the analyses were underpowered.
Our study has limitations. The pulmonary artery catheter is the gold standard for cardiac output monitoring, but we did not use it because of its invasiveness [37, 38]. The arterial waveform analysis is known to have low accuracy and precision to predict the absolute value of cardiac output and can be biased by hemodynamic instability [39]. However, it can estimate the cardiac output reliably in a stable hemodynamic status with a regular cardiac rhythm as in our study [37, 40, 41]. In addition, although cardiopulmonary responses can be affected by the patient’s condition, position, or anesthetic type during laparoscopy [3, 6, 25, 26]. we only investigated relatively healthy patients in the lithotomy and head-down positions under the intravenous anesthesia. Our findings thus may not be extrapolated to other clinical situations.
Nevertheless, we found few advantages in cardiopulmonary dynamics, but poor surgical conditions in the low intra-abdominal pressure compared with the standard pressure during laparoscopic colorectal surgery even under the deep neuromuscular block. Therefore, when considering cardiopulmonary effects and surgical conditions, the standard intra-abdominal pressure may be preferable to the low pressure for successful laparoscopic colorectal surgery and patient safety.
References
Madsen MV, Gatke MR, Springborg HH, Rosenberg J, Lund J, Istre O (2015) Optimising abdominal space with deep neuromuscular blockade in gynaecologic laparoscopy-a randomised, blinded crossover study. Acta Anaesthesiol Scand 59:441–447
Ozdemir-van Brunschot DM, van Laarhoven KC, Scheffer GJ, Pouwels S, Wever KE, Warle MC (2016) What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg Endosc 30:2049–2065
Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, Fuchs KH, Jacobi Ch, Jansen FW, Koivusalo AM, Lacy A, McMahon MJ, Millat B, Schwenk W (2002) The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc 16:1121–1143
Suh MK, Seong KW, Jung SH, Kim SS (2010) The effect of pneumoperitoneum and Trendelenburg position on respiratory mechanics during pelviscopic surgery. Korean J Anesthesiol 59:329–334
Park JS, Ahn EJ, Ko DD, Kang H, Shin HY, Baek CH, Jung YH, Woo YC, Kim JY, Koo GH (2012) Effects of pneumoperitoneal pressure and position changes on respiratory mechanics during laparoscopic colectomy. Korean J Anesthesiol 63:419–424
Gurusamy KS, Vaughan J, Davidson BR (2014) Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006930.pub3
Koo BW, Oh AY, Seo KS, Han JW, Han HS, Yoon YS (2016) Randomized clinical trial of moderate versus deep neuromuscular block for low-pressure pneumoperitoneum during laparoscopic cholecystectomy. World J Surg 40:2898–2903
Martini CH, Boon M, Bevers RF, Aarts LP, Dahan A (2014) Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br J Anaesth 112:498–505
Dubois PE, Putz L, Jamart J, Marotta ML, Gourdin M, Donnez O (2014) Deep neuromuscular block improves surgical conditions during laparoscopic hysterectomy: a randomised controlled trial. Eur J Anaesthesiol 31:430–446
Staehr-Rye AK, Rasmussen LS, Rosenberg J, Juul P, Lindekaer AL, Riber C, Gätke MR (2014) Surgical space conditions during low-pressure laparoscopic cholecystectomy with deep versus moderate neuromuscular blockade: a randomized clinical study. Anesth Analg 119:1084–1092
Kim MH, Lee KY, Lee KY, Min BS, Yoo YC (2016) Maintaining optimal surgical conditions with low insufflation pressures is possible with deep neuromuscular blockade during laparoscopic colorectal surgery: a prospective, randomized, double-blind, parallel-group clinical trial. Medicine 95:e2920
Shuto K, Kitano S, Yoshida T, Bandoh T, Mitarai Y, Kobayashi M (1995) Hemodynamic and arterial blood gas changes during carbon dioxide and helium pneumoperitoneum in pigs. Surg Endosc 9:1173–1178
Perry Y, Reissman P, Blumental M, Lyass S, Pizov R (2003) Pressure-related hemodynamic effects of CO2 pneumoperitoneum in a model of acute cardiac failure. J Laparoendosc Adv Surg Tech A 13:341–347
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, Youngs EJ (1998) The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 88:1170–1182
Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, Billard V, Hoke JF, Moore KH, Hermann DJ, Muir KT, Mandema JW, Shafer SL (1997) Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 86:10–23
Boon M, Martini CH, Aarts LP, Bevers RF, Dahan A (2013) Effect of variations in depth of neuromuscular blockade on rating of surgical conditions by surgeon and anesthesiologist in patients undergoing laparoscopic renal or prostatic surgery (BLISS trial): study protocol for a randomized controlled trial. Trials 14:63
Kopman AF, Naguib M (2015) Laparoscopic surgery and muscle relaxants: is deep block helpful? Anesth Analg 120:51–58
Aldrete JA (1995) The post-anesthesia recovery score revisited. J Clin Anesth 7:89–91
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
Gommers D, Vilstrup C, Bos JA, Larsson A, Werner O, Hannappel E, Lachmann B (1993) Exogenous surfactant therapy increases static lung compliance, and cannot be assessed by measurements of dynamic compliance alone. Crit Care Med 21:567–574
Capron F, Alla F, Hottier C, Meistelman C, Fuchs-Buder T (2004) Can acceleromyography detect low levels of residual paralysis? A probability approach to detect a mechanomyographic train-of-four ratio of 0.9. Anesthesiology 100:1119–1124
Stuttmann R, Vogt C, Eypasch E, Doehn M (1995) Haemodynamic changes during laparoscopic cholecystectomy in the high-risk patient. Endosc Surg Allied Technol 3:174–179
Hein HA, Joshi GP, Ramsay MA, Fox LG, Gawey BJ, Hellman CL, Arnold JC (1997) Hemodynamic changes during laparoscopic cholecystectomy in patients with severe cardiac disease. J Clin Anesth 9:261–265
Kim JT, Kim HS, Lim YJ, Bahk JH, Lee KH, Kim CS, Kim SD, Jeon Y (2008) The influence of passive leg elevation on the cross-sectional area of the internal jugular vein and the subclavian vein in awake adults. Anaesth Intensive Care 36:65–68
Hofer CK, Zalunardo MP, Klaghofer R, Spahr T, Pasch T, Zollinger A (2002) Changes in intrathoracic blood volume associated with pneumoperitoneum and positioning. Acta Anaesthesiol Scand 46:303–308
Henny CP, Hofland J (2005) Laparoscopic surgery: pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surg Endosc 19:1163–1171
Sefr R, Puszkailer K, Jagos F (2003) Randomized trial of different intraabdominal pressures and acid-base balance alterations during laparoscopic cholecystectomy. Surg Endosc 17:947–950
Kopman AF, Naguib M (2016) Is deep neuromuscular block beneficial in laparoscopic surgery? No, probably not. Acta Anaesthesiol Scand 60:717–722
Madsen MV, Staehr-Rye AK, Claudius C, Gatke MR (2016) Is deep neuromuscular blockade beneficial in laparoscopic surgery? Yes, probably. Acta Anaesthesiol Scand 60:710–716
Egawa H, Morita M, Yamaguchi S, Nagao M, Iwasaki T, Hamaguchi S, Kitajima T, Minami J (2006) Comparison between intraperitoneal CO2 insufflation and abdominal wall lift on QT dispersion and rate-corrected QT dispersion during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 16:78–81
Caesar Y, Sidlovskaja I, Lindqvist A, Gislason H, Hedenbro JL (2016) Intraabdominal pressure and postoperative discomfort in laparoscopic Roux-en-Y gastric bypass (RYGB) surgery: a randomized study. Obes Surg 26:2168–2172
Jones RK, Caldwell JE, Brull SJ, Soto RG (2008) Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology 109:816–824
Murphy GS (2006) Residual neuromuscular blockade: incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol 72:97–109
Geldner G, Niskanen M, Laurila P, Mizikov V, Hübler M, Beck G, Rietbergen H, Nicolayenko E (2012) A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia 67:991–998
Pühringer FK, Rex C, Sielenkämper AW, Claudius C, Larsen PB, Prins ME, Eikermann M, Khuenl-Brady KS (2008) Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: an international, multicenter, randomized, dose-finding, safety assessor-blinded, phase II trial. Anesthesiology 109:188–197
Schwarte LA, Scheeren TW, Lorenz C, De Bruyne F, Fournell A (2004) Moderate increase in intraabdominal pressure attenuates gastric mucosal oxygen saturation in patients undergoing laparoscopy. Anesthesiology 100:1081–1087
Button D, Weibel L, Reuthebuch O, Genoni M, Zollinger A, Hofer CK (2007) Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth 99:329–336
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K (2013) Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003408.pub3
Geisen M, Ganter MT, Hartnack S, Dzemali O, Hofer CK, Zollinger A (2017) Accuracy, precision, and trending of 4 pulse wave analysis techniques in the postoperative period. J Cardiothorac Vasc Anesth. https://doi.org/10.1053/j.jvca.2017.09.006
Cannesson M, Attof Y, Rosamel P, Joseph P, Bastien O, Lehot JJ (2007) Comparison of FloTrac cardiac output monitoring system in patients undergoing coronary artery bypass grafting with pulmonary artery cardiac output measurements. Eur J Anaesthesiol 24:832–839
Lorsomradee S, Lorsomradee S, Cromheecke S, De Hert SG (2007) Uncalibrated arterial pulse contour analysis versus continuous thermodilution technique: effects of alterations in arterial waveform. J Cardiothorac Vasc Anesth 21:636–643
Acknowledgements
We thank the Medical Research Collaborating Centre for their advices about the statistical analyses.
Funding
This study was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corporation. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Kook Hyun Lee and Jeong-Hwa Seo received a research grant from Merck Sharp & Dohme Corporation (Grant No. 51350). Youn Joung Cho, Hyesun Paik, Seung-Yong Jeong, Ji Won Park, Woo Young Jo, and Yunseok Jeon have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cho, Y.J., Paik, H., Jeong, SY. et al. Lower intra-abdominal pressure has no cardiopulmonary benefits during laparoscopic colorectal surgery: a double-blind, randomized controlled trial. Surg Endosc 32, 4533–4542 (2018). https://doi.org/10.1007/s00464-018-6204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6204-2