Abstract
Background
A number of technical improvements regarding the pancreatic anastomosis have decreased the morbidity and mortality after pancreaticoduodenectomy. However, postoperative pancreatic fistula (POPF) remains is the most feared complication, and the ideal technique for pancreatic reconstruction is undetermined.
Materials and methods
This study is a retrospective review of a prospectively maintained database. Data were collected from all consecutive robot-assisted pancreaticoduodenectomies (RAPD), performed by a single surgeon, at the University of Illinois Hospital & Health Sciences System, between September 2007 and January 2016.
Results
A total of 28 consecutive patients (16 male and 12 female) who underwent a RAPD were included in this study. Patients had a mean age and mean BMI of 61.5 years (SD = 12.3) and 27 kg/m2 (SD = 4.9), respectively. The mean operative time was 468.2 min (SD = 73.7) and the average estimated blood loss was 216.1 ml (SD = 113.1). The mean length of hospitalization was 13.1 days (SD = 5.4). There was no clinically significant POPF registered.
Conclusion
Trans-gastric pancreaticogastrostomy (TPG) represents a valid and feasible option as a pancreatic digestive reconstruction during RAPD. Initial results showed decreased incidence of POPF with an increased risk of postoperative bleeding. Our experience suggests that TPG might be safer than pancreaticojejunostomy (PJ); further studies are needed in order to confirm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since our group performed the first robotic pancreaticoduodenectomy in 2001 [1], we have witnessed an increase in literature reports on heterogeneous techniques and approaches to this difficult surgery [2, 3]. One of the most important technical aspects of the procedure is the pancreatic anastomosis. The failure of the pancreatic anastomosis is the main cause for postoperative pancreatic fistula (POPF), which is the most feared and dangerous postoperative complication, with high morbidity rates. The management of the pancreatic stump has been extensively investigated [4]. The most commonly used techniques to reestablish pancreatic digestive continuity are pancreaticojejunostomy (PJ) and pancreaticogastrostomy (PG). Lately, several studies have reported that PG could be the safer option [4, 5]. However, authors often use different techniques to create the anastomosis, and the very definition of POPF is not uniform [6]. While the best reconstructive method is still under debate, it is widely accepted that performing a standardized technique on an ongoing basis can reduce the overall incidence of postoperative complications [7].
Our robotic pancreaticoduodenectomy procedure has been refined over the years in order to obtain a fully robotic standardized technique. In our experience, a dunking trans-gastric pancreaticogastrostomy (TPG) would be the safest option. This reconstructive option has been largely described in open surgery, but it is still lacking precise technical statements in robotic surgery.
We hereby describe our techniques and experience.
Materials and methods
This study is a retrospective review of a prospectively maintained database. Data were collected from all consecutive robot-assisted pancreaticoduodenectomies (RAPD), performed by a single surgeon, at the University of Illinois Hospital & Health Sciences System, between September 2007 and January 2016. The study was conducted after Institutional Review Board approval. All patients, 18 years of age or older, who underwent trans-gastric pancreatic reconstruction during a RAPD were included in this study. Patient demographics are summarized in Table 1. Cases of concomitant islet cell transplant, totalization after distal pancreatectomy, and cases converted to open surgery were excluded from the study.
Demographics, American Society of Anesthesiologists (ASA) score, diagnosis, intraoperative, and postoperative data were analyzed. All fistulas were classified according to the International Study Group on Pancreatic Fistula (ISGPF) guidelines [8].
Surgical technique
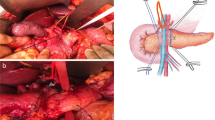
The patient is placed in 20° reverse Trendelenburg position, slightly tilted on the left side, with parted legs to accommodate the assistant surgeon. The 12-mm camera port is positioned in the right pararectal area at the intersection with the transverse umbilical line. A 8-mm trocar for the robotic arm number 1 (R1) is placed in the lower left hypochondrium on the midclavicular line, and another 8-mm trocar for the robotic arm number 2 (R2) is positioned in a specular position on the right side. The 8-mm trocar for the robotic arm number 3 (R3) is placed laterally on the right flank. Additionally, we use two assistant ports, one 12-mm trocar in the periumbilical area and a 5-mm trocar, positioned between the camera port and R2. Once the trocar is placed, the robotic cart is docked reaching from the patient’s head. Trocar placement is shown in Fig. 1.
The steps of the resection portion of the procedure largely resemble those of the open technique originally described by Whipple [9] and also reported for the robotic approach [1].
Before transecting the pancreas, with the harmonic scalpel the robotic port is moved towards the midline using a telescopic technique with the assistant port. This step is crucial when using the harmonic scalpel, a robotic instrument that does not allow for endowrist technology due to the intrinsic characteristics of ultrasounds.
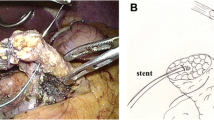
Before dissecting the pancreatic parenchyma, two Prolene® stay sutures are applied at the superior and inferior borders of the pancreas to facilitate retraction during the transection of the pancreatic neck with the harmonic scalpel. Once the dissection is complete, a plastic stent is placed in the main pancreatic duct and secured with a PDS® 5-0 suture. When performing a dunking TPG, the pancreatic stump must be adequately mobilized from the retroperitoneum by at least 5 cm. This step requires accurate hemostasis of the collateral branches of the splenic vessels that can be easily obtained with sutures thanks to the intrinsic advantages of the robotic platform.
A gastrotomy in the posterior gastric wall, usually in the lower body of the stomach or the antrum, is made using a combination of the monopolar hook and the harmonic scalpel. The position of the gastric opening should be carefully evaluated when the stomach is lying naturally, while also considering the length of the pancreatic stump (Fig. 2).
The incision should be at least 1/3 smaller than the diameter of the pancreatic stump. This improves continence and hemostasis of the anastomosis. A purse string of prolene 2-0 can be used and gently tied to the gastric wall around the stump. Afterwards, a longitudinal anterior gastrotomy is performed as shown in Fig. 3.
The pancreatic stump is gently brought inside the gastric cavity by pulling the two previously placed sutures on the inferior and superior borders of the gland. The pancreatic body should protrude inside the stomach by at least 3–4 cm. During this step, the scope is moved in the left assistant port to have a more favorable angle for viewing (Figs. 4, 5).
The anterior gastrotomy allows for favorable access and facilitates the fixation of the pancreatic capsule to the stomach inside the lumen. This is the most important step in the procedure. Multiple short running sutures of PDS 4-0 are used to secure the pancreas to the gastric mucosa. More interrupted stitches are added to perfect the hemostasis. If bleeding occurs, that stitch should be reinforced with another stitch on top. Special attention should be paid to this anastomosis that requires meticulous hemostasis, since the pancreatic juice could loosen the stitches, increasing the chances of postoperative bleeding (Fig. 6).
After achieving accurate control of the hemostasis, the anterior gastrotomy is closed with a single running layer suture of PDS 3-0 (Figs. 7 and 8).
We routinely place two drains, one near the PG, and the other near the hepaticojejuno anastomosis (Fig. 9).
Postoperative management
Patients are usually extubated within the first 24 h. Ocreotide is initiated at the time of the pancreatic transection to decrease the pressure of the secretions. The stomach should be decompressed for a few days, leaving a nasogastric tube in place for four to five postoperative days. The Amylase level of the drainage is measured on postoperative days two and five. A Gastrografin series study is performed on postoperative day five. If POPF is ruled out and normal gastric emptying is confirmed, the NG tube is removed and the Ocreotide is discontinued. Patients start oral feeding and are discharged with good oral tolerance and pain control.
Results
A total of 28 patients (16 male and 12 female) were included in this study, with a mean age and BMI of 61.5 years (SD = 12.3) and 27 kg/m2 (SD = 4.9), respectively. One patient had ASA 1, 18 patients had ASA 2, and nine patients had ASA 3. Data are summarized in Table 1.
The mean operative time was 468.2 min (SD = 73.7) and the average estimated blood loss was 216.1 ml (SD = 113.1). The mean length of hospitalization was 13.1 days (SD = 5.4). There were no clinically significant postoperative pancreatic fistulas (POPF) registered. Only a POPF Grade A was listed and the patient was discharged home without drains on postoperative day 11.
Two patients had delayed gastric emptying (DGE) Grade C with the NG tube removed on postoperative days 21 and 23, respectively. Three patients had bleeding. One patient required emergent reoperation within the first 24 h, and the other two patients were managed conservatively with blood transfusions, and a coiled embolization of the pancreatic magna artery by an endovascular approach, respectively.
Discussion
There has been a constant improvement in postoperative outcomes of Pancreaticoduodenectomies (PD), which can be related to better perioperative management and operative techniques [10,11,12,13].
Still, the pancreatico digestive reconstruction is a weak point in the operation, in both the open and laparoscopic fashion. The incidence of postoperative pancreatic fistulas (POPF) in open PD varies from 8 to 26% [14,15,16] and the specific mortality is up to 8% [17].
Many different kinds of digestive reconstructions have been described in an attempt to improve the POPF rate, however, the ideal technique is still under debate [8, 18].
The quality of the pancreatic stump and the diameter of the pancreatic duct have been identified as risk factors for developing POPF [4, 19,20,21,22].
Pancreaticogastrostomy has been originally described by Waugh and Clagett [23]. Since that first description, other authors began reporting their experience [7, 18, 24].
The technique seems safe as far as the risk of POPF, but a higher incidence of postoperative bleeding has been reported [18, 24].
The laparoscopic PD, originally described by Gagner [25], failed in gaining wider acceptance because of the technical challenges connected with the limitations of laparoscopy. Only a few centers worldwide were able to develop a routine practice. After the introduction of Robotic-Assisted Surgery in the early 2000s and the first robotic Whipple performed by Giulianotti in 2001 [26], an increase of experience grew around the world [27].
The majority of reconstructions in robotics are PJ, and the incidence of POPF is reported in the range of 18% [28].
Pancreaticogastrostomy was initially utilized during the robotic Whipple with a tradition posterior approach but, in our hands, failed to improve the incidence of related complications (27.3%).
The trans-gastric pancreatogastrostomy (TPG) anastomosis was introduced later in an attempt to decrease the incidence of POPF, and our results did not show any clinically significant POPFs, but did demonstrate a higher risk for bleeding.
There are few technical tips that have been learned and seem relevant:
-
The preparation of the pancreatic stump
-
The posterior opening of the stomach
-
The way the pancreatic stump is anchored to the stomach
-
The stomach decompressions
-
The utilization of peripancreatic drainages
The peripancreatic drainages are controversial because minor pancreatic leaks are not activated like in PJ, but for pancreaticogastrostomy, minor leaks could be managed conservatively, avoiding reoperations [29].
In conclusion, TPG represents a valid and feasible option as a pancreatic digestive reconstruction during robotic-assisted PD. Initial results showed decreased incidence of POPF with an increased risk of postoperative bleeding. Our experience suggests that TPG might be safer than PJ; however, further studies are needed in order to confirm.
References
Fernandes E, Giulianotti PC (2013) Robotic-assisted pancreatic surgery. J Hepatobiliary Pancreat Sci 20(6):583–589
Cirocchi R et al (2013) A systematic review on robotic pancreaticoduodenectomy. Surg Oncol 22(4):238–246
Baker EH et al (2015) Robotic pancreaticoduodenectomy for pancreatic adenocarcinoma: role in 2014 and beyond. J Gastrointest Oncol 6(4):396–405
Guerrini GP et al (2015) Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis. J Invest Surg 29:175–184
Chen Z et al (2014) Pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis of randomized control trials. Eur J Surg Oncol 40(10):1177–1185
Guerrini GP, Fusai G (2016) Should we consider pancreaticogastrostomy the best method of reconstruction after pancreaticoduodenectomy? Eur J Surg Oncol 42(2):315–316
Shrikhande SV et al (2016) Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 161:1221–1234
Bassi C et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13
Whipple AO, Parsons WB, Mullins CR (1935) Treatment of carcinoma of the ampulla of vater. Ann Surg 102(4):763–779
Coppola A, Stauffer JA, Asbun HJ (2016) Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg 68(3):217–224
Adams DB (2009) The pancreatic anastomosis: the danger of a leak, which anastomotic technique is better? J Gastrointest Surg 13(7):1182–1183
Cheng Y et al (2016) Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database Syst Rev 2:CD009621
Gagner M, Palermo M (2009) Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg 16(6): 726–730
Denbo JW et al (2012) Toward defining grade C pancreatic fistula following pancreaticoduodenectomy: incidence, risk factors, management and outcome. HPB (Oxford) 14(9):589–593
Addeo P et al (2014) Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 16(1):46–55
Grobmyer SR et al (2010) Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 210(1):54–59
Liao CH et al (2016) Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J Surg 40:1218–1225
Keck T et al (2016) Pancreatogastrostomy versus pancreatojejunostomy for reconstruction after pancreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg 263(3):440–449
Ansorge C et al (2012) Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. Br J Surg 99(8):1076–1082
Belyaev O et al (2011) Histomorphological features of the pancreatic remnant as independent risk factors for postoperative pancreatic fistula: a matched-pairs analysis. Pancreatology 11(5):516–524
Belyaev O et al (2010) Assessment of pancreatic hardness-surgeon versus durometer. J Surg Res 158(1):53–60
Liu QY et al (2014) Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol 20(46):17491–17497
Waugh JM, Clagett OT (1946) Resection of the duodenum and head of the pancreas for carcinoma; an analysis of thirty cases. Surgery 20:224–232
Qin H et al (2016) Pancreaticogastrostomy has advantages over pancreaticojejunostomy on pancreatic fistula after pancreaticoduodenectomy. A meta-analysis of randomized controlled trials. Int J Surg 36(Pt A):18–24
Gagner M, Pomp A (1994) Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 8(5):408–410
Giulianotti PC et al (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138(7):777–784
Boone BA et al (2015) Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 150(5):416–422
Hogg ME et al (2016) Grading of surgeon technical performance predicts postoperative pancreatic fistula for pancreaticoduodenectomy independent of patient-related variables. Ann Surg 264(3):482–491
Kunstman JW et al (2017) Pancreaticoduodenectomy can be performed safely with rare employment of surgical drains. Am Surg 83(3):265–273
Acknowledgements
We would like to thank and acknowledge Zaid Zayyad for his contribution with designing the drawings in the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Raquel Gonzalez-Heredia, Sofia Esposito, Mario Masrur, Antonio Gangemi, and Francesco M. Bianco have no conflicts of interest or financial ties to disclose. Dr. Giulianotti is a consultant for Covidien LP. and Ethicon, Inc.; he has a proctoring agreement and Grant support as Chief of the Division.
Rights and permissions
About this article
Cite this article
Giulianotti, P.C., Gonzalez-Heredia, R., Esposito, S. et al. Trans-gastric pancreaticogastrostomy reconstruction after pylorus-preserving robotic Whipple: a proposal for a standardized technique. Surg Endosc 32, 2169–2174 (2018). https://doi.org/10.1007/s00464-017-5916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5916-z