Abstract
Introduction
Laparoscopic pancreatic surgery represents one of the most advanced applications for laparoscopic surgery currently in use. In the past, minimally invasive techniques were only used for diagnostic laparoscopy, staging of pancreatic cancer, and palliative procedures for unresectable pancreatic cancer. With new advances in technology and instrumentation, some sophisticated procedures are currently available, such as the Whipple procedure, one of the most sophisticated applications of minimally invasive surgery.
Materials and methods
A review of the literature shows that 146 laparoscopic Whipple procedures have been published worldwide since 1994. The authors analyzed blood loss, mean operating time, hospital stay, conversion rate, mean age, mortality rate, lymph nodes in the pathologic findings, follow up, and complications.
Results
Mean age was 59.1 years; mean operating time was 439 min. The average blood loss for the reviewed literature was 143 mL; median hospital stay was 18 days; conversion rate was 46%; number of lymph nodes in the pathologic findings was 19; and mortalities related to the procedure was low, 2 patients (1.3%) and the complication rate was 16% (23/46 patients). Complications included 2 hemorrhages, 4 bowel obstructions, 1 stress ulcer, 1 delay of gastric emptying, 4 pneumonias, and 11 leaks.
Conclusion
This review demonstrates that the laparoscopic Whipple procedure is not only feasible but also safe, with low mortality and acceptable rates of complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The laparoscopic approach for tumors located in the head of the pancreas is not universally accepted, because of its technical difficulties and highly trained surgeons required to perform this operation [1].
Laparoscopic pancreatic surgery represents one of the most advanced applications for laparoscopic surgery currently in use. These advances in technology and techniques have widely opened new gates to a wide range of applications in patients with pancreatic disease [1–5].
The first successful pancreatoduodenectomies were performed by Walter Kausch in 1912 and Allan Whipple in 1934. The first laparoscopic pancreatoduodenectomy was described in 1994 [6–10].
Laparoscopic pancreatic surgery uses a relatively new approach. In the past, the minimally invasive techniques were only used for diagnostic laparoscopy to evaluate periampullary malignancies, staging of pancreatic cancer, and palliative procedures for unresectable pancreatic cancer.
With new advances in technology and instrumentation during the past decade, use of laparoscopic procedures for pancreatic disease has expanded to necrosectomy for necrotizing pancreatitis, drainage procedures for pancreatic pseudocysts, and distal resections of pancreatic tumors [11–14]. Currently, it is feasible to perform some sophisticated procedures such as the Whipple procedure, one of the most sophisticated applications of minimally invasive surgery.
This article reviews the literature regarding laparoscopy in the management of benign and malignant pancreatic disease that were treated by a Whipple procedure.
Whipple procedure: surgical technique
The laparoscopic technique for a Whipple procedure is a modification of Longmire and Traverso and pylorus-preserving pancreatoduodenectomy. Under general anesthesia, the patient is placed in a supine position with the legs abducted. Carbon dioxide pneumoperitoneum is established using an open technique through a 10-mm trocar over the umbilicus. A 30° telescope is inserted to examine the peritoneal cavity, liver, stomach, and mesentery vessels.
Then 4 to 6 more trocars are inserted under direct vision in the epigastrium and upper quadrants. The peritoneum covering the common bile duct (CBD) is opened anteriorly and laterally, and is then dissected free posteromedially from the portal vein and the right hepatic artery.
Using a 2-0 nylon suture passed through the abdominal wall in the right subcostal area and passed under the CBD, creating a suspension with minimum retraction. The CBD is then transected at least 2 cm above the pancreatic cystic duct junction using an endoscopic linear stapler. The first portion of the duodenum is then divided 1 cm distal to the pylorus, can be easily identified by looking inferiorly for the veins of Mayo and by palpating a slight induration in the area. After dissection of the gastrocolic ligament is completed, gastroepiploic vessels derived from the gastroduodenal vessels are double clipped with titanium clips.
Using a right-angled dissector under the pylorus, a window of 1 cm2 is created that allows passage of an endoscopic linear 12-mm stapler. Similarly, the duodenojejunal junction is transected as close to the proximal jejunum as possible, with the sample stapler to the right of the mesenteric vessels. The proximal jejunum retracts in the retroperitoneum and is freed at the ligament of Treitz. After transection, the gastroduodenal artery is exposed as the antrum of the stomach is pushed toward the left upper quadrant. The artery is dissected from the pancreatic neck and more so superiorly near its origin. From the hepatic artery, it is double-clipped with titanium clips and divided. The pancreas above the mesenteric vein and the portal vein is then transected using scissors. This sections the inferior and superior pancreatic vessel arcades controlled with a combination of metallic clips and cautery. A 10-mm, ultrasonic dissector can be used. The pancreatic duct is easily seen because of the magnification. It is left open and can be cannulated with a 5-Fr pediatric feeding tube.
The uncinate process is resected from the mesenteric vessels by using an endoscopic linear stapler with two cartridges 60 mm long or using the ultrasonic dissector. The resected specimen is then inserted into a bag and left in the lower quadrant of the abdomen for later extraction.
At this time, 3 anastomoses need to be created, and a good 2-handed technique with fast intracorporeal knot tying is necessary. The proximal jejunal loop is prepared for this task by further mobilizing the Treitz ligament, and several vessels are taken with the hook cautery and metallic clips. The loop is passed and advanced behind the mesenteric vessels through the ligament of Treitz.
The pancreas to jejunum anastomosis is created first because of its need for precision and delicate sutures, and it is easier to perform with the free jejunal loop. The anastomosis is created in 2 layers: an outer layer with interrupted 3-0 silk and an inner layer with 4-0 absorbable sutures placed with a semi-curved needle, taking the duct to the antimesenteric side of the jejunum through the whole wall. Then 4 to 6 interrupted sutures are positioned beginning posteriorly.
The hepaticojejunostomy is created in a similar fashion with intracorporeal sutures starting posteriorly, using 6 to 10 sutures. The distance between each anastomosis is 10 cm. The pylorojejunostomy is created using a 3-0 absorbable monofilament suture. The gallbladder can be removed after the sutures are placed. The specimen is extracted through the 18-mm umbilical trocar, the largest.
Drains are positioned below and above the anastomosis and passed through the trocar sites in the right subcostal and right paramedian areas. A feeding jejunostomy is needed and a nasogastric tube is placed and its position verified. The fascial wounds are closed with 2-0 absorbable sutures [9].
Methods
The following variables of each article: blood loss, mean operating time, hospital stay, conversion rate, mean age, mortality, lymph nodes in the pathology, follow up, and complications. Complications were determined to be bleeding, bowel obstruction, stress ulcer, delay gastric emptying, leak, and pneumonia.
Results
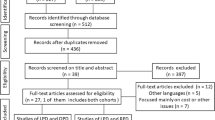
A review of the literature shows that 146 laparoscopic Whipple procedures have been published since 1994. The 14 publications with 146 Whipple procedures are listed in Table 1.
-
Mean age was 59 (43–71) years.
-
Mean operating time was 439.28 min. Range was 284 min [1] to 660 min [19] (Table 2).
Table 2 Blood loss, operation time and hospital stay -
Blood loss average for the reviewed literature was 142.8 mL, with a range of 50 mL [16] to 770 ml [15] (see Table 2).
-
The average number of hospital-stay days was 18, 7 days [18] to 39 [15] (see Table 2).
-
Conversion rates were 46% (12 to 100%).
-
Mean number of lymph nodes in the pathology was 19 (13–26).
-
Mortality rates related with the procedure were low, only 2 (1.3%) patients died during the peri operative period.
-
Follow up was an average of 11 months (2–32).
-
Complication rates were 16% of patients (23/146).
-
Complications were 2 hemorrhages, 4 bowel obstructions, 1 stress ulcer, 1 delay of gastric emptying, 4 pneumonias, and 11 leaks (Fig. 1) [16–20].
-
Histopathological findings were:
-
Ampulloma (n = 62)
-
Pancreatic adenocarcinoma (n = 45)
-
Chronic pancreatitis (n = 13)
-
Neuroendocrine tumors (n = 5)
-
Cystadenocarcinoma (n = 5)
-
Metastasis (n = 4)
-
Low bile duct carcinoma (n = 3)
-
Pancreatic mixed carcinoma (n = 1)
-
Cholangiocarcinoma (n = 3)
-
Mesenchymal tumor (n = 1)
-
Duodenal adenocarcinoma (n = 2)
-
Serous cystadenoma (n = 1) (Fig. 2)
-
Discussion
Some articles in the literature report that performing a Whipple procedure laparoscopically is feasible, but differences with the open technique have not been documented [4, 21, 22]. These authors say that the laparoscopic approach for distal pancreatectomy is easier than the Whipple procedure and is more accepted [4].
The total number of laparoscopic pancreatic resections remain small and many reports are often based on limited experience. In a recent multi-institutional study, 127 pancreatic resections in 27 European centers were described [23]. Most were distal resections. Another study from France, in a single institution, has 48 cases of laparoscopic pancreatic surgery, and only one was a Whipple procedure and one total pancreatectomy, the rest were distal resections or enucleations [24]. As stated previously, laparoscopic pancreatectomy series remain small, particularly those on Whipple procedures.
A retrospective review from Italy, a study of 19 patients with cephalic pancreatic cancer, was published in 2008, and 13 cases could be finished laparoscopically with good results [10].
In the review of the literature, we found good results, but the laparoscopic approach is not universally accepted as the best one because of long operating time, technically difficult procedure, and a lack of reduction in length of hospital stay.
When the results from this review were compared with those of a meta-analysis with regard to comparison between two open techniques, published by Diener and colleagues [21], the operating time was determined to be similar (mean of 493.28 laparoscopic vs. 401 open); blood loss was higher in the open technique (819.6 open vs. 142.8 laparoscopic); hospital stay was similar in comparing both techniques (20.9 days for the open group vs. 18.7 for the laparoscopic group); and mortality rate was also similar (1.54 open vs. 1.3 laparoscopic). The complications were also similar. The difference was that we noted more wound infections in the open group (16%). Also, the pancreatic fistula, which was 18% for the laparoscopic procedure, compared with the literature of open techniques increased from 2% to 30% [10].
Therefore, when techniques were compared for all the items reviewed, no large differences were noted between them. The principle disadvantage of the laparoscopic approach is the difficult and lengthy learning curve required. The principle advantage of the laparoscopic Whipple procedure is that it is a minimally invasive with all the advantages that that offers. However, although no benefit seemed to be derived from use of a laparoscopic approach, randomized controlled trials must be performed to achieve strong conclusions.
One major concern always raised in cases of malignant disease treated by laparoscopy is whether this surgery is an adequate cancer operation, allowing tumor-free margins and sufficiently extensive lymph node dissection while being minimally invasive [16]. In review of the literature, the authors observed that extended lymphadenectomy is not necessary, because it does not prolong patient survival. Laparoscopic surgery causes less immunodeficiency with the open approach, which seems to be an important advantage for cancer patients.
Conclusion
This review demonstrates that the laparoscopic Whipple procedure is not only feasible but safe, with low mortality and acceptable complication rates. However, the laparoscopic Whipple procedure should be performed in selected cases by a highly skilled laparoscopic surgeon to be feasible, safe, and achieve good results. In case of doubt or difficulties in the dissection, an open resection should be performed.
References
Dulucq JL, Wintringer P, Stabilini C, Feryn T, Perissat J, Mahajna A. Are major laparoscopic pancreatic resections worthwhile? A prospective study of 32 patients in a single institution. Surg Endosc. 2005;19(8):1028–34.
Olivié D, Lepanto L, Billiard JS, Audet P, Lavallée JM. Predicting resectability of pancreatic head cáncer with multi-detector CT. Surgical and pathologic correlation. JOP. 2007;8(6):753–8.
Mori T, Abe N, Sugiyama M, Atomi Y. Laparoscopic pancreatic surgery. J Hepatobiliary Pancreat Surg. 2005;12(6):451–5.
Masson B, Sa-Cunha A, Laurent C, Rault A, Collet D. Laparoscopic pancreatectomy: report of 22 cases. Ann Chir. 2003;128(7):452–6.
Spanknebel K, Conlon KC. Advances in the surgical management of pancreatic cancer. Cancer J. 2001;7(4):312–23.
Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology. 1997;113(3):983–94.
Schäfer M, Müllhaupt B, Clavien PA. Evidence based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236(2):137–48.
Gagner M, Pomp A. Laparoscopic pancreatic resection: is it a worthwhile? J Gastrointest Surg. 1997;1(1):20–5. (discussion 25-6).
Gagner M, Pomp A. Laparoscopic pylorus preserving pancreatoduodenectomy. Surg Endosc. 1994;8(5):408–10.
Pugliese R, Scandroglio I, Sansonna F, Maggioni D, Costanzi A, Citterio D, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech. 2008;18(1):13–8.
Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20(7):1045–50.
Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, Madhankumar MV. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205(2):222–30.
Milone L, Turner P, Gagner M. Laparoscopic surgery for pancreatic tumors, an uptake. Minerva Chir. 2004;59(2):165–73.
Staudacher C, Orsenigo E, Baccari P, Di Palo S, Crippa S. Laparoscopic assisted duodenopancreatectomy. Surg Endosc. 2005;19(3):352–6.
Lu B, Cai X, Lu W, Huang Y, Jin X. Laparoscopic pancreaticoduodenectomy to treat cancer of the ampulla the Vater. JSLS. 2006;10(1):97–100.
Zheng MH, Feng B, Lu AG, Li JW, Hu WG, Wang ML, et al. Laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma of common bile duct: a case report and literature review. Med Sci Monit. 2006;12(6):CS57–60.
Sa Cunha A, Rault A, Beau C, Laurent C, Collet D, Masson B. A single institution prospective study of laparoscopic pancreatic resection. Arch Surg. 2008;143(3):289–95. (discussion 295).
Gentileschi P, Gagner M. Laparoscopic pancreatic resection. Chir Ital. 2001;53(3):279–89.
Ammori BJ. Laparoscopic hand assisted pancreaticoduodenectomy: initial UK experience. Surg Endosc. 2004;18(4):717–8.
Huang J, Yeo C, Sohn T, Lillemoe KD, Sauter PK, Coleman J, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231(6):890–8.
Diener M, Knaebel P, Heukaufer C, Antes G, Buchler M, Seiler C. A systematic review and meta-analysis of pylorus preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245(2):187–200.
Raut C, Tseng J, Sun C, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60.
Eubanks S, Swanstron L, Soper N. Mastery of endoscopic and laparoscopic surgery. Lippincott Williams & Wilkins 2000, Chapter 32. Laparoscopic Pancreatic Surgery. Michel Gagner 291–305.
Pappas T, Pryor A, Harnisch M. Atlas of laparoscopic surgery. 3rd ed. New York: Springer; 2008.
Assalia A, Gagner M. Laparoscopic pancreatic surgery for islet cell tumors of the pancreas. World J Surg. 2004; 28(12): 1239–47.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gagner, M., Palermo, M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg 16, 726–730 (2009). https://doi.org/10.1007/s00534-009-0142-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0142-2