Abstract
Background
Patients with lesions in the posterosuperior (PS) segments of the liver have been considered poor candidates for laparoscopic liver resection (LLR). This study aims to compare short-term outcomes of LLR and open liver resections (OLR) in the PS segments.
Methods
This multicenter study consisted of all patients who underwent LLR in the PS segments and all patients who underwent OLR in the PS segments between October 2011 and July 2016. Laparoscopic cases were case-matched with those who had an identical open procedure during the same period based on tumor location (same segment) and the Brisbane classification of the resection. Demographics, comorbid factors, perioperative outcomes, short-term outcomes, necessity of adjuvant chemotherapy, and the interval between surgery and initiation of adjuvant chemotherapy were compared between the two groups. Data were retrieved from a prospectively maintained electronic database.
Results
Both groups were comparable for age, sex, ASA score, maximum tumor diameter, and number of patients with additional liver resections outside the posterior segments. Operative time was similar in both groups (median 140 min; p = 0.92). Blood loss was less in the LLR-group (median: 150 vs. 300 ml in OLR-group). Median hospital stay was 6 days in both groups. There was no significant difference in postoperative complications (OLR-group: 31.4% vs. LLR-group: 25.7%; p = 0.60). There was no significant difference in R0 resections (LLR: 97.2 vs. 100% in OLR; p = 1.00). Tumor-free margins were less in the LLR group (LLR: 5 vs. 9.5 mm in OLR; p = 0.012). Patients undergoing LLR were treated with chemotherapy sooner compared to those undergoing OLR (41 vs. 56 days, p = 0.02).
Conclusion
This study suggests that laparoscopic parenchymal preserving liver resections in the PS segments can be performed with comparable short-term outcomes as similar OLR. The shorter interval to chemotherapy might provide long-term oncologic benefits in patients who underwent LLR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic liver surgery is currently gaining acceptance. Since the first international consensus conference on laparoscopic liver resection (LLR) in Louisville, Kentucky in 2008, LLR has been increasingly employed as a feasible and safe alternative to open liver resection (OLR) [1,2,3,4,5,6,7,8]. During the second international consensus conference held in Morioka, it was stated that minor LLRs had become standard practice. However, laparoscopic major hepatectomies and resections in the posterosuperior (PS) segments were still considered as innovative procedures reserved for experienced surgeons facile with advanced laparoscopic hepatic resections [9]. Patients with lesions in the posterior or superior part of the liver (Segments 7, 8, 4A, and the superior part of segment 6) have been considered poor candidates for LLR due to limited visualization, the risk of major bleeding and difficulty in bleeding control [10, 11]. Therefore, open liver resection remains the gold standard for lesions in these segments in many hepatobiliary units.
To date, few studies have compared open and laparoscopic liver resections in these difficult segments. This case-matched multicenter study aims to compare short-term outcomes of LLR with open liver resections in the PS segments (segment 7, 8, and upper part of segment 6).
Materials and methods
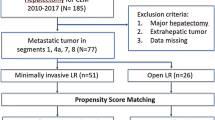
This study consisted of all patients who underwent LLR in the PS segments in a large Belgian supraregional hospital and matched patients who underwent OLR in the PS segments in a large Canadian University hospital between October 2011 and July 2016. The type of resection performed according to the Brisbane classification and the location of the lesion (segment) were used as matching criteria to have comparable groups. A total of 107 patients who underwent a liver resection in the right hemiliver or segment 4A were evaluated. Right hemihepatectomies, trisectionectomies, and resections in segment 5 (non-anatomical resections or segmentectomies) or lower part of segment 6 were excluded. The type of resection performed according to the Brisbane classification and the location of the lesion (segment) were used as matching criteria to have comparable groups.
Demographics, comorbid factors, perioperative outcomes, short-term outcomes, necessity of adjuvant chemotherapy and the interval between surgery and initiation of adjuvant chemotherapy were compared between the two patient groups. The data were retrieved from an institutional review board-approved (CE 16.288 for Canadian Center; AZGS2016073 for Belgian Center), prospectively maintained database in both centers.
Margin status was determined according to the width of the resection margin, defined as the shortest distance from the edge of the tumor to the line of transection. A positive margin was defined as the presence of exposed tumor along the line of transection or the presence of tumor cells at the line of transection detected by histologic examination. If there were multiple lesions, we relied on the smallest margin to define the R status.
Anesthesia protocol
In both centers, a similar protocol for anesthesia was used. Before patient induction, an epidural was inserted into the 10–11 thoracic epidural space. Both centers routinely restricted fluids, reduced tidal volume, and administered a sufficient amount of muscle relaxant to mildly reduce central venous pressure (CVP) during parenchymal transection. Hypovolemic phlebotomy was performed in patients undergoing open or laparoscopic liver resection. A volume of whole blood corresponding to approximately 0.7% of the patient’s weight was withdrawn before the start of hepatic parenchymal division and stored in a standard blood collection bag in order to lower CVP and prevent back bleeding. The collected blood was reinfused into the patient after parenchymal transection.
Liver resection—technical aspects
Open liver resections
All OLRs were performed by one of seven HPB surgeons. A right-sided subcostal or median incision was used. The liver was inspected, palpated, and evaluated segment-by-segment with intraoperative ultrasound (IOUS) to exclude contraindications to hepatic resection and to delineate the target lesion. The number, size, and relationship to segmental portal and hepatic veins of all lesions were evaluated. A cholecystectomy was performed as needed. Pringle maneuvre was only performed selectively. The liver was mobilized as needed, to adequately expose the liver. For anatomical segment 7 and 8 resections and for anatomical posterior sectionectomies, a full mobilization of the right hemiliver was performed. Margins of resection were marked on the liver surface under guidance of IOUS. The choice of parenchymal transection technique was based on the individual surgeon’s preference. Integrated bipolar and ultrasonic 5 mm scissors [THUNDERBEAT® (Olympus Medical Systems Corp., Tokyo, Japan)] or a 5-mm bipolar sealing device [Ligasure Dolphin tip (Covidien, Mansfield, MA, USA)] or the bipolar ENSEAL® Tissue Sealer [Ethicon Endo-Surgery Inc., Cincinnati, OH, USA)] were used associated with Kelly clamp crushing technique. Arterial and portal venous branches or large hepatic veins were clipped or tied. When portal or hepatic branches were too large to apply clips, they were stapled using a 45 mm linear vascular stapler (TA stapler, Covidien, Mansfield, MA, USA) and divided. After checking for hemostasis and biliary leak, a silicone drain was placed in most patients.
Laparoscopic liver resections
All LLRs were performed by the same laparoscopic liver surgeon. Operative details of laparoscopic liver resections have been described previously [12]. All patients were placed in semiprone position. The patient was placed on a vacuum mattress in a left lateral position. Once the patient was draped, the table was turned by 30°–45° toward the semiprone position. A 5 (or 6) trocar approach was used and trocars were always placed in two rows. A laparoscopic IOUS was performed (Flex 700, BK-Medical, Flex Focus 700, bk Ultrasound, Peabody, MA, USA.). A cholecystectomy was only performed when cholelithiasis was present or in patients who underwent a segment 6 resection or posterior sectionectomy. As in OLRs the right hemiliver was mobilized to adequately expose the liver. For anatomical segment 7 and 8 resections, anatomical posterior sectionectomies, non-anatomical resections in segment 7 or high up in segment 8 a full mobilization of the right hemiliver was performed. When an anatomical posterior sectionectomy had to be performed, the Glissonean approach was used. The fissure between segments 5 and 6 (Rouviere's sulcus) was opened and the pedicle to the posterior sector was isolated and transected using a linear vascular stapler (ECHELON FLEX powered vascular stapler (PVS), Ethicon, Cincinnati, OH, USA) (Fig. 1). In the first five patients, parenchymal transection was performed with the 5 mm bipolar sealing device: Ligasure Dolphin tip (Covidien, Mansfield, MA, USA). In all other cases, parenchymal transection was performed using the ENSEAL G2 articulating curved tissue sealer, a new generation of articulating curved bipolar device (ENSEAL® G2 Tissue Sealers by Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). For deep seated lesions, the sealing device was used in combination with the cavitron ultrasonic surgical aspirator (CUSA, Plainsboro, NJ, USA). Arterial and portal venous branches or large hepatic veins were clipped using 5 mm Hemolock clips (TFX Medical, Durham, NC, USA) and divided using the bipolar tissue sealer. When portal or hepatic branches were too large to apply clips, they were divided using a 35 or 45 mm linear vascular stapler. Once the surgical specimen was fully detached, it was placed in a retrieval bag (Memo Bag, Teleflex Medical, Park, Athlone, Ireland) and extracted through a 5 cm Pfannenstiel incision in most cases or through a previous existing midline incision. After checking for hemostasis and biliary leak, a fibrinogen and thrombin-coated collagen patch (TachoSil, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland) was applied to the cut surface of the liver. A silicone drain was placed in most patients.
Laparoscopic anatomical posterior sectionectomy—Glissonean approach. A and B mobilizing right hemiliver (B = anatomical landmarks). C Intraoperative ultrasound. D Hepatotomy above and below sulcus of Rouviere. E Clamping of posterior sectoral pedicle with vascular clamp—ischemic demarcation between anterior sector and posterior sector. F Stapling of right posterior sectoral pedical using 35 mm ECHELON FLEX powered vascular stapler before parenchymal transection
Postoperative care
Postoperative management in both centers included avoidance of using nasogastric tubes, early dietary advancement, and early ambulation. Drains were removed on day 2 postoperatively except when there was evidence of bile leak. For postoperative pain management, all patients received an epidural patient-controlled analgesia device. Regular laboratory tests were performed on postoperative days 1, 3, and 5.
Statistical analysis
Data are described using mean and standard deviation (SD) in case of normally distributed data, and with median and range when data are not normally distributed. Comparison of continuous variables between groups is performed with Unpaired Student’s T-test when data are normally distributed and Mann-Whitney U test when data are not normally distributed. Association between categorical variables is evaluated with the (exact) chi-square test. All analyses are performed with Statistica 64, StatSoft Inc., Tulsa, OK, USA and/or SAS 9.3, SAS Institute Inc., Cary, NC, USA.
Results
Demographic data and indications for liver resection
Out of 107 patients, 35 LLRs could be matched with 35 OLRs based on the type of resection performed according to the Brisbane classification and the location of the lesion (segment). Indication of liver resection in the open group was colorectal liver metastases in all cases. In the laparoscopic group 21 patients underwent a LLR for colorectal liver metastases, 8 patients for hepatocellular carcinoma, 5 for other malignant tumors (in two cases for neuroendocrine liver metastases, in two cases for metachronous solitary liver metastasis of a gastric adenocarcinoma and one intrahepatic cholangiocarcinoma) and 1 patient for an adenoma.
Types of resection according to Brisbane classification, surgical outcomes, and intervals between surgery and chemotherapy are shown in Table 1.
Besides matching criteria, the LLR and OLR groups were comparable for age, sex, ASA score, maximum tumor diameter, and number of patients with additional liver resections outside the posterior segments. There was significantly less blood loss in the laparoscopic group (p = 0.028), although 2 patients in the laparoscopic group required blood transfusion of 1 unit of packed cells versus none in the open group. One laparoscopic procedure was converted to open surgery due to uncertainty of the tumor-free margin.
There were no significant differences in postoperative complications between the two groups (Table 2). There were two cases with a postoperative bile leak in both groups. In 3 patients the bile leak was adequately drained by the drain that was placed during the operation. In one patient in the LLR group, a transpapillary stent was placed due to the high output of the bile leak. In this patient, the drain and transpapillary stent were removed after 42 days. There were two other Clavien-Dindo grade III complications: one patient in the OLR group had a burst abdomen requiring reintervention and in the LLR group one patient who underwent an anatomical segment VIII resection developed a subphrenic abscess requiring CT-guided percutaneous drainage.
There was no significant difference in R0 resections, although one patient had an R1 resection in the LLR group and tumor-free margins were less in the LLR group. There was no difference in hospital stay.
In total 39 patients required adjuvant chemotherapy: 18 patients in the LLR group and 21 patients in the OLR group. Patients undergoing LLR were treated with chemotherapy sooner compared to those undergoing OLR (p = 0.02).
Discussion
Historically, LLR has remained limited for resecting lesions in the PS segments because of concerns regarding limited visualization, the risks of bleeding, and difficulty in bleeding control [10, 11]. LLRs in the PS segments were identified as an independent risk factor for conversion in a large series of laparoscopic liver resections [13].
Currently, there is a shift towards parenchymal-sparing surgery with a decrease in major hepatectomy and an increasing use of multiple simultaneous hepatic resections [14, 15]. The use of a parenchymal-sparing approach has been associated with decreased mortality, and parenchymal preserving resections of colorectal liver metastases offer a prolonged survival compared to major hepatectomies [14, 15]. The possibility of subsequent resections during long-term follow-up of cancer patients is maintained without compromising the oncological outcome [16, 17]. Repeat parenchymal preserving liver resections are increasingly being offered to these patients as it can even positively impact survival [18, 19]. Technical aspects of the laparoscopic parenchymal-sparing approach in all liver segments have been described by Gayet et al. [20]. However, in the recommendations for laparoscopic liver resections published in 2015, there was a concern among the jury during the Morioka consensus meeting that larger procedures resecting more liver parenchyma are sometimes favored if the procedure is done laparoscopically because a smaller parenchyma-sparing operation may be more complex laparoscopically [9]. In both centers involved in this study, non-anatomical resections, segmentectomies, sectionectomies, and multiple resections are often used in order to spare uninvolved surrounding parenchyma and to avoid trisegmentectomies or hemihepatectomies.
The feasibility and safety of LLRs for lesions located in the PS segments are still under debate and laparoscopic parenchymal preserving liver resections in these segments are still considered as innovative procedures reserved for experienced surgeons facile with advanced laparoscopic hepatic resections [9, 21]. However, it may be appropriate to create two subcategories of laparoscopic major hepatectomies to reflect differences in intraoperative and postoperative outcomes between traditional major hepatectomies (trisegmentectomy and hemihepatectomy) and complex LLR in PS segments [22]. A prospective randomized trial comparing open and laparoscopic parenchymal preserving resections in these segments is difficult to initiate due to the inherent technical difficulty of randomizing these patients.
All patients in the laparoscopic group were positioned in the semiprone position. The advantages of positioning the patient in semiprone instead of supine position have been well described by several groups including our own experience [12, 23,24,25]. By placing the patient in the semiprone position, a maximum amount of space is created between the right subphrenic region, creating extra working space.
To the best of our knowledge, the current case-matched study comparing open and laparoscopic liver resections in the PS segments is one of the largest evaluating short-term outcomes. When comparing LLR and OLR in the PS segments, the level of operative difficulty in the two groups should be taken into account. As shown by the validated difficulty scoring system for laparoscopic liver resections, the level of difficulty of a LLR is defined by several factors: tumor size, the extent of the liver resection, tumor location, proximity of the lesion to major vessels and liver function [26]. Tumor location and extent of resection are seen as major factors related to the difficulty of LLR, since laparoscopic resections in these PS segments require a longer operative time than resections in the peripheral segments [1, 21, 27]. Therefore, the type of resection according to the Brisbane terminology and location of the lesion were both used as matching criteria to have comparable groups. Furthermore, there was no significant difference in ASA classification, tumor size, or in number of patients with cirrhosis in the matched groups.
The use of Pringle maneuver or pedicle clamping is sometimes used to evaluate the learning curve for laparoscopic liver resections [28,29,30]. In the laparoscopic group of the current series, the Pringle maneuver was not used; however, in 8 patients of the open group, a Pringle maneuver was used.
The finding of decreased blood loss with the laparoscopic approach has been reported in previous studies comparing laparoscopic and open resections in the peripheral segments but not in the PS segments [31]. As both centers use the technique of hypovolemic phlebotomy, other factors might be responsible for the difference in intraoperative blood loss. In LLRs, the laparoscope might offer a better exposure with a magnified view, while the pneumoperitoneum pressure reduces hepatic vein bleeding from the cut surface [32, 33]. Furthermore, the caudal-cranial transection of the hepatic parenchyma with magnification in LLR might results in better identification of intraparenchymal vascular structures compared to the open anterior approach. Reverse Trendelenburg position in LLRs further decreases the venous pressure and improves exposure by shifting the stomach, transverse colon, and omentum away from the liver hilum. By placing the patient in a semiprone position, the right hepatic vein is also in a higher position than the inferior vena cava. This also reduces the risk of bleeding from the right hepatic vein in LLR in the posterior segments [32].
In the present study, no significant difference in hospital stay and postoperative morbidity was noted which is in line with other case-match studies evaluating OLR and LLR [34,35,36,37,38].
In the current series, there was no significant difference in R0 resections but 1 patient in the LLR group had a positive tumor margin (p = 1.00). This patient underwent a laparoscopic liver resection for a colorectal liver metastasis for a well-defined lesion located in proximity of the vena cava. The specimen showed a well-defined tumor but with submillimetric margin. In fact, a tumor-free margin of less than 8 mm towards the vena cava was noted on preoperative imaging. Due to proximity to the vena cava, the CUSA device was used for resection. It is known that the CUSA device aspirates cells and promotes necrosis (of about 7 mm) at the cutting edge due to its thermal energy. An experimental model demonstrated that the CUSA device is the one providing the deeper parenchymal ablation [39]. In this patient, no additional resection was performed. Follow-up was the treatment of choice. Research shows that R1 resections due to vessel detachment of colorectal liver metastases achieve equivalent outcomes compared to R0 resections [40]. At 24 months post-surgery, there is no recurrence of disease.
Another interesting finding in the current study is the shorter interval between liver resection and chemotherapy in the LLR group. A similar finding was reported in a recent case-matched study of patients who underwent open or minimal invasive liver resection for colorectal liver metastases [41]. In univariable analysis, surgical approach, postoperative complications, blood loss, length of stay, and number of lesions were associated with timing to chemotherapy, but in multivariable analysis, the surgical approach was still associated with timing to chemotherapy and postoperative complications resulted in a delay of chemotherapy. This might provide long-term oncologic benefits in patients who underwent LLR. A recent large meta-analysis of large series of patient who underwent laparoscopic or open resection of their colorectal cancer clearly demonstrated that the laparoscopic approach was associated with earlier initiation of adjuvant chemotherapy [42]. In the current study, there were no differences in postoperative complications. A longer waiting interval between liver resection and adjuvant chemotherapy might be explained by a faster functional recovery after LLR.
Limitations of the current study include the retrospective nature and the fact that LLRs and OLRs were performed at different centers. Furthermore, all OLRs were performed by seven different, high-volume hepatobiliary surgeons while all LLRs were performed by a single surgeon creating significant heterogeneity between techniques for parenchymal transection. Moreover, differences between Canadian and Belgian health care systems could have an impact on interval between surgery and chemotherapy.
In conclusion, this study suggests that laparoscopic parenchymal preserving liver resections in the PS segments can be performed with comparable short-term outcomes as similar OLR. Furthermore, less blood loss and a shorter interval to adjuvant chemotherapy were noted in the laparoscopic group.
References
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker C-G, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey J-N, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, Mcmasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the Louisville statement. Ann Surg 250:825–830
Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, Rotman N, Fagniez P-L (2000) Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 232:753–762
Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, Tranchart H, Franco D, Cherqui D (2010) Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 211:16–23
Vigano L, Tayar C, Laurent A, Cherqui D (2009) Laparoscopic liver resection: a systematic review. J Hepato-Biliary-Pancreat Surg 16:410–421
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2,804 patients. Ann Surg 250:831–841
Koffron AJ, Auffenberg G, Kung R, Abecassis M (2007) Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 246:385–392
Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM (2008) Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 248:475–486
Chen HY, Juan CC, Ker CG (2008) Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol 15:800–806
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Dulucq JL, Wintringer P, Sabilini C, Berticelli J, Mahajna A (2005) Laparoscopic liver resections: a single center experience. Surg Endosc 19:886–891
Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL (2003) Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 138:763–769
D’Hondt M, Yoshihara E, Vansteenkiste F, Steelant PJ, Van Ooteghem B, Pottel H, Devriendt D, Van Rooy F (2016) Laparoscopic parenchymal preserving hepatic resections in semiprone position for tumors located in the posterosuperior segments. Langenbecks Arch Surg 401:255–262
Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B (2014) Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB 16:75–82
von Heesen M, Schuld J, Sperling J, Grünhage F, Lammert F, Richter S, Schilling M, Kollmar O (2012) Parenchyma-preserving hepatic resection for colorectal liver metastases. Langenbecks Arch Surg 397:383–395
Gold JS, Are C, Kornprat P, Jarnagin WR, Gönen M, Fong Y, Dematteo RP, Blumgart LH, D’Angelica M (2008) Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 247:109–117
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C (2016) Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg 263:146–152
Montalti R, Tomassini F, Laurent S, Smeets P, Man M, Geboes K, Libbrecht L, Troisi R (2015) Impact of surgical margins on overall and recurrence-free survival in parenchymal-sparing laparoscopic liver resections of colorectal metastases. Surg Endosc 29(9):2736–2747
Abu Hilal M, Lodge P (2008) Pushing back the frontiers of resectability in liver cancer surgery. Eur J Surg Oncol 34:272–280
Tuttle TM, Curley SA, Roh MS (1997) Repeat hepatic resection as effective treatment for recurrent colorectal liver metastases. Ann Surg Oncol 4:125–130
Ishizawa T, Gumbs AA, Kokudo N, Gayet B (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256(6):959–964
Cho JY, Han H-S, Yoon Y-S, Shin S-H (2008) Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 144:32–38
Di Fabio F, Samim M, Di Gioia P, Godeseth R, Pearce NW, Abu Hilal M (2014) Laparoscopic major hepatectomies: clinical outcomes and classification. World J Surg 38:3169–3174
Gumbs AA, Gayet B (2008) Video: the lateral laparoscopic approach to lesions in the posterior segments. J Gastrointest Surg 12(7):1154
Ikeda T, Yonemura Y, Ueda N, Kabashima A, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, Kakeji Y, Morita M, Tsujitani S, Maehara Y (2011) Pure laparoscopic right hepatectomy in the semiprone position using the intrahepatic Glissonian approach and a modified hanging maneuver to minimize intraoperative bleeding. Surg Today 41:1592–1598
Ikeda T, Mano Y, Morita K, Hashimoto N, Kayashima H, Masuda A, Ikegami T, Yoshizumi T, Shirabe K, Maehara Y (2013) Pure laparoscopic hepatectomy in semiprone position for right hepatic major resection. Hepato-Biliary-Pancreat Sci 20:145–150
Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G (2014) A novel difficulty scoring system for laparoscopic liver resection. J Hepato-biliary-pancreat Sci 21:745–753
Xiang L, Xiao L, Li J, Chen J, Fan Y, Zheng S (2015) Safety and feasibility of laparoscopic hepatectomy for hepatocellular carcinoma in the posterosuperior liver segments. World J Surg 39:1202–1209
Chan FK, Cheng KC, Yeung YP (2014) Laparoscopic liver resection: lessons learnt after 100 cases. Hong Kong Med J 20:386–392
Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D (2009) The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 250:772–782
Chang S, Laurent A, Tayar C, Karoui M, Cherqui D (2007) Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 94:58–63
Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL (2003) Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg 196:236–242
Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF (2014) Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepato-biliary-pancreat Sci 21:723–731
Makabe K, Nitta H, Takahara T, Hasegawa Y, Kanno S, Nishizuka S, Sasaki A, Wakabayashi G (2014) Efficacy of occlusion of hepatic artery and risk of carbon dioxide gas embolism during laparoscopic hepatectomy in a pig model. J Hepato-biliary-pancreat Sci 21:592–598
Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B (2009) Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg 250:849–855
Iwahashi S, Shimada M, Utsunomiya T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Mori H, Kanamoto M, Yamada S (2014) Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc 28:80–84
Cheung TT, Poon RT, Yuen WK, Chok KS, Tsang SH, Yau T, Chan SC, Lo CM (2013) Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ J Surg 83:847–852
Guerron AD, Aliyev S, Agcaoglu O, Aksoy E, Taskin HE, Aucejo F, Miller C, Fung J, Berber E (2013) Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc 27:1138–1143
Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, de Hemptinne B, Geboes K, Troisi RI (2014) Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol 40:536–544
Hammond JS, Muirhead W, Zaitoun AM, Cameron IC, Lobo DN (2012) Comparison of liver parenchymal ablation and tissue necrosis in a cadaveric bovine model using the Harmonic Scalpel, the LigaSure, the Cavitron Ultrasonic Surgical Aspirator and the Aquamantys devices. HPB 14:828–832
Viganò L, Procopio F, Cimino MM, Donadon M, Gatti A, Costa G, Del Fabbro D, Torzilli G (2016) Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? Ann Surg Oncol 23(4):1352–1360
Tohme S, Goswami J, Han K, Chidi AP, Geller DA, Reddy S, Gleisner A, Tsung A (2015) Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg 19:2199–2206
Malietzis G, Mughal A, Currie AC, Anyamene N, Kennedy RH, Athanasiou T, Jenkins J (2015) Factors implicated for delay of adjuvant chemotherapy in colorectal cancer: a meta-analysis of observational studies. Ann Surg Oncol 22:3793–3802
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Mathieu D’Hondt, Esther Tamby, Isabelle Boscart, Simon Turcotte, Isabelle Parmentier, Hans Pottel, Réal Lapointe, Sander Ovaere, Franky Vansteenkiste, and Franck Vandenbroucke-Menu have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
D’Hondt, M., Tamby, E., Boscart, I. et al. Laparoscopic versus open parenchymal preserving liver resections in the posterosuperior segments: a case-matched study. Surg Endosc 32, 1478–1485 (2018). https://doi.org/10.1007/s00464-017-5835-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5835-z