Abstract
Background
Since the first report of laparoscopic liver resection, by Gagner et al. 1992, an increasing number of small prospective studies have been published. They have shown encouraging results for the feasibility and safety of the procedure. This paper prospectively evaluated the results of a single center’s experience with elective liver resections.
Methods
From January 1995 to January 2004 a prospective study of laparoscopic liver resections was undertaken in 31 patients with preoperative diagnosis of benign lesions (13 cases, 42.4%), hepatocellular carcinoma in absence of complicated cirrhosis (three cases, 9.1%), and liver metastases (15 cases, 45.5%). Mean tumor size was 34.9 mm (range 10–100 mm).
Results
The procedures included 11 (37.9%) major hepatectomies and 21 (62.1%) minor resections (one patient was submitted to repeat laparoscopic liver resection) . There were three conversions to open. Mean blood loss was 210 ml (range 0–700 ml). Mean operative time was 115 min (range 45–210 min). There were no deaths and no reoperations for complications. No port-site metastases occurred in patients with malignant lesions.
Conclusions
Laparoscopic liver resections, including major hepatectomies, are feasible and safe. Major and posterior resections are difficult, though, and conventional surgery remains an option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liver resections are demanding procedures, and it might seem that laparoscopic approach was not even to think of. Gagner et al. [11] reported the first liver resection in 1992, a wedge resection. We performed a first entirely laparoscopic left lobe resection in 1995 without publishing the case. The first published resection was by Azagra et al. [1] in 1996. Since then, increasing numbers of reports of small series have been published, showing feasibility and safety. Concerns about laparoscopy have risen because of potential technical shortcomings, safety issues (hemorrhage and gas embolism), and even ethical aspects (conventional surgery has been proven effective in treating liver malignancies and increasing patients’ survival).
The purpose of this article is to prospectively evaluate the results of a homogeneous, single-center series. Intraoperative and postoperative complications and late outcome are the main endpoints.
Patients and Methods
Laparoscopic liver resections were prospectively evaluated from 1995 to January 2004.
Indications
Indications for surgery included benign lesions, hepatocellular carcinomas (HCCs) in cirrhotic and noncirrhotic patients, and liver metastases.
Resection of benign lesions was considered because of pain or compression, suspicion of liver cell adenoma (because of the well-known potential for bleeding and malignant transformation [2, 11, 19]), and in cases of uncertain nature of the tumor. Indications in HCC patients were Child Pugh class A and absence of signs of severe portal hypertension. Indications for liver metastases were synchronous or metachronous liver tumors from colorectal or other malignancies.
Patients with complicated cirrhosis or with cardiac or respiratory failure were excluded. Neither prior open liver surgery nor conventional upper abdominal tract surgery was considered a contraindication for a laparoscopic approach.
Patients
We enrolled 31 patients, 16 men and 15 women, of mean age 57 years, ranging from 33 to 77 years. Twenty patients (64.5%) were ASA I; 11 (35.5%) were ASA II. Eighteen patients had a liver malignancy and 13 a benign condition. In the malignancy group three patients had a hepatocellular carcinoma (HCC): among them, two had Child Pugh A liver cirrhosis, were asymptomatic, and had their lesion discovered during a screening program; the third patient was symptomatic and underwent an ultrasound investigation because of persistent diarrhea. Metastatic liver disease was encountered in 15 patients: 11 had a primary colorectal cancer (10 metachronous lesions), one had small bowel adenocarcinoma, one had breast cancer, one gallbladder cancer, and one had an ovarian granulosa tumor. The only symptomatic patient was the one affected by gallbladder cancer complaining of chronic pain in the right upper quadrant. The remaining 14 patients had lesions identified during oncological follow-up or preoperative workup (one case).
Among patients operated on for benign liver conditions there were five cases of focal nodular hyperplasia (FNH), three cases of liver cell adenoma (LCA), two cases of hepatic polycystosis (APLD), two liver haemangioma, one Caroli’ s disease of the left lobe submitted first to a left lobectomy and 1 year later to a S4 resection for recurrence. In this group indications for surgery were pain and compression in seven cases (53.8%: two FNH, two LCA, one hemangioma, two APLD). Two patients underwent resection because of lesions detected during follow-up of previous malignancies (one FNH, one liver haemangioma); the patient affected by Caroli’s disease complained of recurrent cholangitis. The remaining three patients were operated on for tumors of undetermined nature with atypical appearance (two FNH, one LCA) and detected incidentally on abdominal ultrasound.
Most patients presented with a single lesion (84.4%). Multiple lesions were found in five cases, but were in the same lobe. The overall distribution of the lesions according Couinaud’s classification [6] is shown in Fig. 1. The tumor mean size was 4.1 cm (range: 1.0–10.0 cm) for benign lesions and 3.1 cm (range: 1.5–7.5 cm) for malignancies.
Surgical technique
The procedures were performed by two surgeons (JLD, WTG), both expert in advanced laparoscopic surgery. Operations were as follows: left hepatectomy for resection of segments II, III IV; right hepatectomy for resection of segments V, VI, VII, VIII; left lateral segmentectomy for segments II, III; right lateral segmentectomy for segments VI, VII; segmental resection for single segments; and wedge resection in case of nonanatomical resection of less than one segment [6, 13].
The patient was placed supine, with legs spread, in a 10° Trendelenburg position and under general anesthesia. The surgeon was standing between the legs of the patient; the first assistant was placed on the patient’s right side, and the second assistant on the left. Pneumoperitoneum was created and five or more ports were used. Intraabdominal pressure was <12 mmHg to reduce the risk of gas embolism.
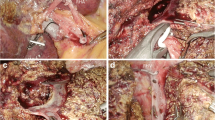
The liver was checked visually on its surface and a laparoscopic ultrasound was carried out to confirm the number of lesions and their position in relation to the main hepatic structures. A Pringle’s maneuver was carried out, mainly in case of major resections, with continuous pedicle clamping.
Parenchymal dissection was done with monopolar scissors for the glissonian surface. The ultrasonic dissector (Dissectron, Satelec Medical, France), the harmonic scalpel (Ultracision, Ethicon Endosurgery, Johnson & Johnson, USA), and the Ligasure Vessel Sealing System (Valleylab, Tyco, UK) were used to complete the procedure. The resection was done laparoscopically by transparenchymal dissection and elective clipping or sealing (Ligasure) of vessels and bile structures as in open resection.
Once the resection was completed, the surgical field was checked for bleeding or biliary leaks and, if needed, further hemostasis was obtained with nonabsorbable sutures. In all cases the resected raw surface was sealed with fibrin glue (Tissucol, Immunofrance, France).
The specimen was then extracted inside a bag through a Pfannenstiel type or other short incision with or without crushing technique according to the preoperative diagnosis.
Right hepatectomy
After cholecystectomy and section of the right triangular and falciform ligaments, the anterior aspect of the inferior vena cava (IVC) was dissected (monopolar scissors and Ligasure), with progressive lifting of the inferior face of the liver. The accessory suprahepatic veins, when encountered were ligated with Ligasure, until the right hepatic vein was reached and controlled with vascular stapler or Ligasure. We consider this maneuver essential because it allows the verticalization of the future line of transaction. The glissonian surface was incised with monopolar scissors on the right side of the projection of the middle suprahepatic vein. Parenchymal transection was carried out with a combination of LIGASURE and ultrasonic dissector, down to the right portal structures, which were controlled electively (Weblink: www.e-laparoscopy.com).
Studied criteria
We evaluated type and details of the operative procedure, early postoperative course including complications, blood transfusion and reoperations, postoperative return to normal activities and hospital stay, and late outcome of the patients. Perioperative mortality and morbidity were assessed within 30 days after the procedure. All patients had radiological investigations at follow up. Statistical analysis included the χ2 test and Student t-test when appropriate. A statistical test difference ≤0.05 was considered significant.
Results
Intraoperative results
We performed 11 major hepatectomies (six right hepatectomies, four left hepatectomies, one left lateral segmentectomy with associated resection of S1) and 21 minor hepatectomies (Table 1).
The procedure was done entirely laparoscopically in 29 patients (90.6%). Conversion was needed in three cases after the resection started.
Portal triad clamping was used in 15 patients (46.9%) and was always continuous; its mean duration was 40 min (range 25–62 min). Mean blood loss was 210 ml (range near 0–700 ml), higher for patients undergoing major resections (313 vs 128 ml, Student t-test p = 0.05). Intraoperative ultrasound was used in 79.3% of cases.
The hepatic resection was carried out in association with another laparoscopic procedure 16 times, including 14 cholecystectomies with two explorations of the common bile duct (Caroli’s disease), one left colectomy, and, in one case, a radio frequency thermal ablation in order to treat residual tumor tissue identified at intraoperative ultrasound after completion of the hepatic resection. One tear of the inferior vena cava occurred during a laparoscopic resection after a previous right open hepatectomy, for a liver metastasis located in S1: The tear was repaired with nonabsorbable suture and did not require conversion. There were no signs suggesting gas embolism in any of the operated patients.
The resection margins in the group of malignancies were free from malignant tissue in 16 patients: two patients (6.3%), both with HCC and having a segmentectomy, had margin involvement: among them, one case was recognized intraoperatively and treated for completion with a radio frequency thermal ablation (R2 resection) and the other one was identified at pathological examination (R1 resection).
Mean duration of surgery was 115 min (range 45–210 min) and was longer for patients undergoing a major resection (on average 126 vs 110 min) but not statistically different (Student t-test, p = 0.44) from that of the minor resections. The mean operative time decreased in the last 10 cases to 89 min (range 45–180), whereas in the earlier cases it was 131 min (range 50–210 min), with a statistical relevance (Student t-test p = 0.02).
Early postoperative results
There were no deaths within 30 days after surgery. Twenty-six patients had an uneventful postoperative course. Five patients required blood transfusion; two were major hepatic resections (left hepatectomy and right hepatectomy), and three were minor resections of right-sided lesions; none of these patients needed reoperation. No postoperative bile leak occurred. One 74-year-old patient who underwent a left hepatectomy for metachronous colorectal metastasis developed a sepsis due to a Xantomonas maltophilia contamination of the central venous access; he recovered uneventfully on antibiotics. Close to his discharge from the hospital, this patient had an episode of acute upper gastrointestinal bleeding. Endoscopy discovered a nonresectable cancer of the middle third of the esophagus not detected at preoperative workup. Four patients developed postoperative fever, due to urinary tract infection in one case, to pulmonary infection in another case, and of unknown origin in two cases. All of them eventually did well.
Bowel movements resumed on average 2.5 days (range 1–9 days) after surgery. The mean time before resumption of normal activities (eating, moving without help, taking care of self-hygiene) was 4.1 days (range 2–9 days) and depended on the extent of resection. Patients submitted to major hepatectomies were considered self-sufficient after an average of 4.8 days (range 3–9 days) vs 3.7 days (range 2–5 days) for the group of minor resections (Student t-test p = 0.0316).
The mean overall hospital stay was 11 days after surgery (range 4–40 days) but was significantly longer (Student t-test p = 0.0135) for major hepatectomies rather than minor resections (16, range 4–40 days vs nine, range 4–15 days).
Late results
The mean follow-up was 20.3 months (range 5–63 months). Patients operated for benign lesions were submitted to physical and radiological follow-up 1, 6, and 12 months after the operation. They were all alive and recurrence free, except for one suffering from APLD presenting persistent asymptomatic cysts and another patient with Caroli’s disease (redo resection of S4 because of recurrence 1 year after the first procedure) . No late complications were registered.
All the patients with malignancies entered an oncological protocol of follow-up and were submitted to physical, biochemical, and radiological investigations. Among them, the patient operated for breast cancer metastasis developed bilateral metastatic disease of the liver 12 months after the laparoscopic procedure. She was put on a regimen of herceptine and navelbine and died 16 months after operation. The patient with gallbladder cancer died 6 months after the procedure because of diffuse progression of the disease. The patient with metastasis from adenocarcinoma of the small bowel was alive and disease-free 4 months after surgery. All the patients with HCC developed recurrent disease. One died because of liver failure 4 months after surgery; the two other patients were alive at 27 and 23 months, respectively. Among patients with colorectal liver metastases, two recurred in the liver after 12 and 15 months, respectively; one died, and the other is alive 23 months after initial surgery. The patient with both colorectal liver metastasis and primary tumor of the esophagus died 9 months after surgery. The seven remaining patients were alive and disease free at 5, 8, 8, 16, 25, 38, and 63 months, respectively. No port-site metastases occurred during the study.
Discussion
This paper confirms feasibility, safety, and oncological adequacy of laparoscopic hepatic resections. No randomized study is yet available. Some recent comparative studies however, mainly based on historical matched controls [16, 18, 20, 21, 25], suggest that laparoscopy could be a better approach to liver resections because of reduced blood loss and reduced morbidity (especially in cirrhotic patients) and because of a shorter hospital stay. Our results are similar. The specific complication rate is low, there are no reoperations, and the specific short-term morbidity is low. Why should laparoscopy do better than open? The magnification through the endoscope might possibly allow a better, more precise surgical technique. Our mean hospital stay remains high, but the length of stay depends on various cultural and patient factors, especially in Europe.
Concerns about laparoscopic liver resections have risen regarding exposure and bleeding control in case of injury of major vessels. Therefore, the safest segments for laparoscopy are considered to be the anterior and lateral ones (from S2 to S6). Some authors [3, 5, 17] contraindicate laparoscopic resections of lesions located posterior and inferiorly (S1, S4b, S7, S8). Our series, like Hüscher’s [15], is one of the largest for the number of major and “difficult” hepatectomies attempted and the first containing two S1 laparoscopic segmentectomies.
We do believe that liver resections represent a challenge for laparoscopic surgery. Recently, a Chinese group published results using the Hand Port System (Smith & Nephew, Andover, MA) for posterior located lesions [14]. We don’t feel that this should be the solution. The Hand Port System still requires a large incision (6–8 cm), which could reduce the clinical benefits from a completely minimal-access procedure and; moreover, air leakage and fatigue in the inserted hand still represent a limit to this approach. More interesting and promising are the results of a recently published series from Japan [29] of posterior resections done by a fully thoracoscopic route, but further investigation is required.
Since the most frequent indication for a laparoscopic liver resection is malignancy, one should remember that the functional postoperative advantages of the laparoscopic approach are only a small part of the global aim of the procedure, which is the improvement of the overall survival. The initial concerns about laparoscopic oncological surgery (port-site metastases, tumor dissemination) , as demonstrated by recent publications on other types of digestive malignancies, are only the effect of an initial lack of experience in the very beginning of minimal access surgery. Today, with the adoption of extraction site protections and bags for the specimens, they can be avoided. No-touch technique, R0 resection, and tumor-free margins of >1 cm [13, 23, 29] remain mandatory. Two studies [13, 18] have reported results concerning overall and disease-free survival that were not different from those reported for open resections, but had a short follow-up.
The issue of the safety margin, even if challenged [4, 9, 10, 26, 27] remains a problem, as does the correct orientation of the transection line [13, 18]. The absence of direct organ palpation and the lack of the third dimension are still limits of laparoscopy (two cases of incomplete resection in our series, near to 7% in the literature [13, 18], 30–40% of free margins <1 cm reported in the literature) . Routine use of intraoperative contact ultrasound should be advocated, a tool that we have found mandatory for adequate location of lesions and for the avoidance of incidental injuries, especially when dealing with deeply located lesions. Probably the ultimate solution will be in new technologies, such as virtual reality and computer-guided surgery [22]. Intraoperative real-time 3D reconstruction of the surgical field will surely help. In case of malignancy, the specimen should be retrieved complete, not crushed, to allow assessment of the margins by the pathologist. This raises the question of a more or less large retrieval wound, potentially limiting the benefit of minimal access.
As far as benign lesions are concerned, the main objectives should be the absence of postoperative mortality, a low morbidity rate, the absence of heterologous blood transfusions, and a satisfactory late outcome. In the present series mortality was absent, and no major early (one case of fever of unknown origin) or late postoperative morbidity occurred. All the patients who had a blood transfusion received autologous blood.
The laparoscopic approach, however, should not change the management of benign liver lesions. In our cohort of patients, mainly affected by FNH, symptoms were present in 64.3% of cases. The asymptomatic patients had a resection for a lesion impossible to assess at preoperative workup. There were no cases of unjustified resection. We believe that the feasibility of laparoscopic liver resections should not modify the indications for surgery of benign, asymptomatic lesions [6, 8].
Laparoscopic liver resection is still in its beginnings. Further technologies must be developed before this procedure could become the “gold standard” for treatment of hepatic tumors. Its advantages could represent the best possible solution in case of benign lesions, as open surgery may seem a major procedure that, most of the time, is merely diagnostic. Moreover, for malignant tumors, if survival data are confirmed, the achievement of a safe, oncologically effective and less traumatizing surgery could represent an interesting option, even for redo hepatic surgery and for advanced chronic liver disease. At present, best candidates for a laparoscopic liver resection remain those with peripheral lesions requiring limited hepatectomies.
Reference
JS Azagra M Goergen E Gilbart et al. (1996) ArticleTitleLaparoscopic anatomical (hepatic) left lateral segmentectomy: technical aspects Surg Endosc 10 758–761
J Belghiti D Potiron Y Panis et al. (1993) ArticleTitleResection of presumed benign liver tumors Br J Surg 80 380–383 Occurrence Handle8472159
L Biertho A Waage M Gagner (2002) ArticleTitleLaparoscopic hepatectomy Ann Chir 127 IssueID3 164–170 Occurrence Handle10.1016/S0003-3944(01)00709-X Occurrence Handle11933628
MM Bilimoria Lanwers Gy DA Doherty et al. (2001) ArticleTitleUnderlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma Arch Surg 136 528–535 Occurrence Handle10.1001/archsurg.136.5.528 Occurrence Handle11343543
D Cherqui E Husson R Hammoud B Malassagne F Stephan S Bensaid N Rotman PL Fagniez (2000) ArticleTitleLaparoscopic liver resections: a feasibility study in 30 patients Ann Surg 232 IssueID6 753–762 Occurrence Handle10.1097/00000658-200012000-00004 Occurrence Handle11088070
D Couinaud (1957) The liver: anatomical and surgical studies Le foie: études anatomiques et chirurgicales; in French Masson Paris
B Descottes D Glineur F Lachachi et al. (2003) ArticleTitleLaparoscopic liver resection of benign liver tumors Surg Endosc 17 IssueID1 23–30 Occurrence Handle10.1007/s00464-002-9047-8 Occurrence Handle12364994
B Descottes F Lachachi M Sodji et al. (2000) ArticleTitleEarly experience with laparoscopic approach for solid liver tumors: initial 16 cases Ann Surg 232 IssueID5 641–645 Occurrence Handle10.1097/00000658-200011000-00004 Occurrence Handle11066134
D Elias A Cavalcanti JC Sabourin JP Pignon M Ducreux P Lauer (1998) ArticleTitleResults of 136 curative hepatectomies with a safety margin of less than 1 cm for colorectal metastasis J Surg Oncol 69 88–93 Occurrence Handle10.1002/(SICI)1096-9098(199810)69:2<88::AID-JSO8>3.0.CO;2-X Occurrence Handle9808511
JH Foster MM Berman (1994) ArticleTitleThe malignant transformation of liver cell adenomas Arch Surg 129 712–717 Occurrence Handle7517661
M Gagner M Rheault J Dubuc (1992) ArticleTitleLaparoscopic partial hepatectomy for liver tumor [abstract] Surg Endosc 6 97–98
JF Gigot D Glineur J Azagra et al. (2002) ArticleTitleLaparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study Ann Surg 236 IssueID1 90–97 Occurrence Handle10.1097/00000658-200207000-00014 Occurrence Handle12131090
NA Goldsmith RT Woodburne (1957) ArticleTitleThe surgical anatomy pertaining to liver resections Surg Gynecol Obstet 105 310–318 Occurrence Handle13467662
M Huang W Lee W Wang RJ Chen (2003) ArticleTitleHand assisted laparoscopic hepatectomy for solid liver tumors in the posterior portion of the right lobe Ann Surg 238 674–679 Occurrence Handle10.1097/01.sla.0000094301.21038.8d Occurrence Handle14578728
CG Hüscher MM Lirici S Chiodini (1998) ArticleTitleLaparoscopic liver resections Semin Laparosc Surg 5 IssueID3 204–210 Occurrence Handle9787208
H Kaneko T Sumito T Shita (1996) ArticleTitleLaparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series Surgery 120 468–475 Occurrence Handle8784399
A Laurent D Cherqui M Lesurtel F Brunetti C Tayar PL Fagniez (2003) ArticleTitleLaparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease Arch Surg 138 IssueID7 763–769 Occurrence Handle10.1001/archsurg.138.7.763 Occurrence Handle12860758
T Leese O Farges H Bismuth (1988) ArticleTitleLiver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit Ann Surg 208 558–564 Occurrence Handle3190282
M Lesurtel D Cherqui A Laurent C Tayar PL Fagniez (2003) ArticleTitleLaparoscopic versus open left lateral hepatic lobectomy: a case control study J Am Coll Surg 196 IssueID2 236–242 Occurrence Handle10.1016/S1072-7515(02)01622-8 Occurrence Handle12595052
T Mala B Edwin I Gladhaug E Fosse O Søreide A Bergan Mathisen ø (2002) ArticleTitleA comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases Surg Endosc 16 1059–1063 Occurrence Handle10.1007/s00464-001-9176-5 Occurrence Handle12165823
J Marescaux JM Clement V Tassetti C Kodel S Cotin Y Russier (1998) ArticleTitleVirtual reality applied to hepatic surgery simulation: the next revolution Ann Surg 228 627–634 Occurrence Handle10.1097/00000658-199811000-00001 Occurrence Handle9833800
S Masutani Y Sasaky S Imaoka et al. (1994) ArticleTitleThe prognostic significance of surgical margin in liver resection of hepatocellular carcinoma Arch Surg 129 1025–1030 Occurrence Handle7944931
Y Matanabe M Sato Y Abe et al. (1997) ArticleTitleLaparoscopic hepatic resection: a new and safe procedure by abdominal wall lifting method Hepatogastroenterology 44 143–147 Occurrence Handle9058133
Morino M, Morra I, Rosso E, Miglietta C, Garrone C (2003) Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc Oct 28 [Epub ahead of print]
T Ochiai T takayama K Inoue et al. (1999) ArticleTitleHepatic resection with and without surgical margins for hepatocellular carcinoma patients with impaired liver function Hepatogastroenterology 46 1885–1889 Occurrence Handle10430364
RT Poon Fan St IO Ng J Wong (2000) ArticleTitleSignificance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal Ann Surg 231 544–551 Occurrence Handle10.1097/00000658-200004000-00014 Occurrence Handle10749616
HG Rau E Buttler G Meyer HM Schardey FW Schildberg (1998) ArticleTitleLaparoscopic liver resection compared with conventional partial hepatectomy—a prospective analysis Hepatogastroenterology 45 IssueID24 2333–2338 Occurrence Handle9951918
K Shirabe K Tanekata T Gion (1997) ArticleTitleAnalysis of prognostic factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin Br J Surg 84 1077–1080 Occurrence Handle10.1046/j.1365-2168.1997.02743.x Occurrence Handle9278644
K Teramoto T Kawamura S Takamatsu N Noguchi N Nakamura S Arii (2003) ArticleTitleLaparoscopic and thoracoscopic partial hepatectomy for hepatocellular carcinoma World J Surg 27 IssueID10 1131–1136 Occurrence Handle10.1007/s00268-003-6936-5 Occurrence Handle12917768
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dulucq, J., Wintringer, P., Stabilini, C. et al. Laparoscopic liver resections: A single center experience . Surg Endosc 19, 886–891 (2005). https://doi.org/10.1007/s00464-004-2044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-2044-3