Abstract
Introduction
Bariatric surgery has been shown to lead to significant improvement in glucose homeostasis, resulting in greater rates of type 2 diabetes mellitus (T2DM) remission. While there is substantial evidence of the benefits of bariatric/metabolic surgery in obese diabetic patients on oral therapy (O-T2D), more evidence is necessary in the case of insulin-treated type 2 diabetes (I-T2D) patients and the selection of surgical procedure.
Methods
Analysis of the Ontario Bariatric Registry data was performed, comparing outcomes of Roux-en-Y-gastric bypass (RYGB) and sleeve gastrectomy (SG) on insulin-treated versus non-insulin-treated T2DM patients. We compared weight loss, medication use and remission rates during a 3-year follow up.
Results
A total of 3668 diabetic Bariatric Registry patients underwent surgery from Jan 2010 to Feb 2017, across 7 Bariatric Centers of Excellence in Ontario. Of these 2872 were O-T2D and 1187 were I-T2D. Weight loss was similar between the two groups at 3 years; with mean %WL of 30.1% for the insulin group vs. 28.3% non-insulin (p = 0.0673). At 3 years, 11.3% of the non-insulin and 59.6% of the insulin-dependent group were using anti-diabetic medication (p < 0.0001). Among insulin-dependent patients, RYGB showed greater reduction in insulin use with 26.5 and 40% compared to SG at 3 years. O-T2D patients experienced more complete diabetes remission, with 66.5 vs. 18.5% (p < 0.0001) at 3 years. Complete remission for I-T2D patients was higher in the RYGB group than SG (p < 0.0001) at years 1 and 2 (8.5 vs. 5.4% and 24.4 vs. 21.1%). The same trend was found regardless of insulin use; complete remission higher for RYGB at 1 and 2 years [50.7 vs. 39.8% (p < 0.0001), and 54.6 vs. 49.1% (p < 0.0001)].
Conclusion
While both RYGB and SG procedures provide effective treatment for I-T2D patients in terms of weight loss and diabetes, incidence of complete remission for insulin-dependent patients is higher with RYGB in earlier years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The World Health Organization estimated the number of adult diabetics had reached 422 million in 2014, compared to 108 million in 1980, an increase of over 290% [1]. Obesity and associated type 2 diabetes mellitus (T2DM) have reached epidemic proportions worldwide. Bariatric surgery is considered the most effective treatment for obesity in terms of weight loss and comorbidity resolution, and has been proven superior to medical management for the treatment of diabetes [2]. While data suggests that bariatric procedures with malabsorptive and restrictive elements provide superior results of weight loss and resolution of obesity-related comorbidities, sleeve gastrectomy (SG) has gained popularity among bariatric surgeons in the last few years. While SG was initially used to treat super-obese or high-risk patients as the first stage to Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion (BPD) surgery [3,4,5], it has recently increased in use as a stand-alone bariatric procedure. SG has become the second most performed bariatric procedure globally, increasing in use from 0% to 37% from 2003 to 2013 [6].
As sleeve gastrectomy gained in practice, the effectiveness of this procedure on weight loss and diabetes remission began to be scrutinized. Studies have compared the effectiveness of SG to RYGB in terms of diabetes remission, and found comparable results. A study comparing metabolic syndrome of severely obese T2DM subjects following SG and RYGB surgery found equivalent resolution rates, with 84.6% of both cohorts achieving resolution of T2DM at one year (p = 0.618) [7]. Another study comparing RYGB and SG in obese patients found a remission rate of 22 and 21.5% respectively one and 2 years post-surgery [8].
While the effect of RYGB and SG on diabetes has been explored, more research is required specifically on the outcomes of insulin-dependent patients. Of the studies comparing insulin and non-insulin-dependent patients’ outcomes; one which found similar weight loss results at 2 years (39.56 ± 14.7 vs. 40.36 ± 24.4 kg), however the I-T2D patients had significant improvement in HbA1c levels and medication use (anti-hyperglycemic, anti-hypertensive and lipid-lowering medications) (p < 0.0001) whereas the type 1 diabetes mellitus (T1DM) group only experienced a decrease in anti-hypertensive medication use [9]. Another study matching type I and type II diabetic patients found greater weight loss (77.1 vs. 68.3%, p = 0.14) and remission and improvement rates with the T2DM groups versus the T1DM (55 and 45% vs. 0 and 90% respectively) [10]. A study examining remission rates following RYBG revealed a similar remission rate of 49% for I-T2D patients, and found that those I-T2D patients that achieved remission had a greater percent excess weight loss (%EWL) [11].
When comparing RYGB to SG for improvement in metabolic syndrome, Vidal et al. (2008) found that insulin use prior to surgery was correlated to a lower rate of remission [7]. Another study that stratified patients by T2DM severity (by the treatment required to maintain glycemic control of HbA1c ≤9%) showed that increasing severity negatively affects weight loss [12]. At the mean follow up of 20 months, patients with the least severe disease of impaired fasting glucose experiencing the greater weight loss with a mean EWL of 73% compared with 65% for diet controlled; 57% for oral agent users; and 59% for I-T2D (p = 0.01) [12]. Similar results were shown by Ramos-Levi et al. (2013) with insulin treatment pre-surgery affecting remission rates, with 67.1% oral therapy (O-T2D) vs. 30.4% I-T2D patients achieving remission (p < 0.001) [13]. Although studies comparing post-surgery T2DM outcomes have been increasing, few report longer follow-up periods or compare the response of those with insulin dependence. To reduce this gap in the literature, our study assesses the response of I-T2D compared to O-T2D patients after bariatric surgery.
Methods
Data from the Ontario Bariatric Registry from Jan. 2010 to Feb. 2017 was analyzed, comparing weight loss, use of anti-diabetes medication and remission rates between insulin and non-insulin-dependent patients during a 3-year follow up following bariatric surgery. We further compared Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Descriptive statistics are used for all related variables.
The Ontario Bariatric Registry is maintained by the Ontario Bariatric Network, collecting clinical information on all patients undergoing surgery in all seven Bariatric Centers of Excellence in Ontario. The Registry has research ethics approval at all participating sites, and consent is obtained from all patients. Charts are reviewed at each centre and de-identified data is entered at each site by data clerks into an electronic data capture system (iDatafax). Follow-up procedures and outcomes are entered at several intervals: 3 and 6 months, and 1, 2 and 3 years. Due to the nature of data collection, the rate of follow-up is therefore based on the amount of participants who consent to participate, and is contingent on diligent, up to date data entry at each site (Table 1). At the time of our analysis, 12,750 patients in the Bariatric Registry had had surgery. The cohort for this sub-study included all diabetic patients who had RYGB or SG surgery. This group was comprised of 3668 diabetic patients, of which 2481 were on non-insulin treatment and 1187 received insulin; 3090 underwent RYGB and 578 SG. For the purpose of this study patients were divided in 4 groups according to the surgical procedure and their insulin dependence (Table 2).
The insulin group (I-T2D) does not exclude patients who were also taking oral anti-diabetic medications along insulin at baseline. The non-insulin group (O-T2D) includes patients who were receiving only oral anti-diabetic medications or not taking any diabetic medication at baseline. The total number of patients in this study at each visit is shown in Table 1. Rate of follow up for our cohort was 71% at 6 months, 55.8% at 1 year, 27.2% at 2 years, 12.6% at 3 years, and is comparable to the entire Registry population, which is 72.2, 62.2, 37.2 and 23.2% respectively.
Data analysis
We conducted a secondary analysis of the Ontario Bariatric Registry on diabetic patients who had Roux-en-Y gastric bypass and sleeve gastrectomy. All data is aggregated data from project initiation to February 2017. Descriptive statistics were generated for all three outcomes weight loss; medication use; and rate of remission. We examined the rate of weight loss, medication use and remission amongst the sub-groups of insulin-dependent versus non-insulin diabetic patients and then by surgical procedure: RYGB vs. SG, to analyze any variation.
For anthropometrics and lab, paired t test is done to compare two visits at a time. Only patients with non-missing values in both visits are included in the test.
For weight loss, we examined outcomes at 3, 6 months, 1, 2 and 3 years post-surgery. Percent weight loss is percent of total weight lost (%WL), calculated using the formula:
And percent excess weight loss (%EWL), we calculated using the formula:
One-way analysis of variance, ANOVA statistical test, was used to compare the surgical groups as class effect was used to calculate p-values in excess weight loss and percent weight loss.
Medication use is collected in the Bariatric Registry in categories. For this sub-study, we defined the number of patients who continued using either insulin ± oral anti-diabetic medication, or only anti-diabetic medication at each visit.
Remission of diabetes mellitus is categorized into partial and complete remission. Complete remission included all patients who were off all anti-diabetic medications for a year or more with an HbA1c level of ≤6.0% [14, 15]. Partial remission included all patients who were off of all anti-diabetic medications for a year or greater with an HbA1c of >6.0 to ≤6.5% [14, 15]. Chi square statistics was calculated for contingency tables of partial vs. complete remission.
All data analyses were conducted in SAS version 9.4. Statistical significance was set at p < 0.01.
Results
Principal outcomes analyzed in this study at 3 years after surgical procedure for I-T2D patients are shown in Table 3.
Diabetes remission
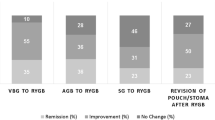
During the study period, each of the two surgical procedures showed evidence of improving glucose profile (Table 4). Resolution of diabetes was reported using current definitions for complete and partial diabetes remission [14, 15]. In this study, O-T2D patients experienced more complete remission than I-T2D patients with a statistical association (p < 0.0001) at each visit (Fig. 1). Remission rates for O-T2D patients were 62.4, 68.8, and 66.5%; and for I-T2D patients 23.1, 24 and 18.5% at 1; 2; and 3 years respectively. No statistic correlation was found during follow-up in regards to partial remission between these groups of patients (Fig. 2).
Roux-en-Y gastric bypass showed slightly higher numbers of complete remission among insulin (I-T2D) patients than sleeve gastrectomy with 24.1 vs. 17.2% at 1 year; and 24.4 vs. 21.1% at 2 years, for RYGB and SG respectively (p < 0.0001) (Fig. 3). At 3 years, statistical analysis was not possible due to the few I-T2D patients in the SG group (Table 4). Partial remission was slightly higher for the RYGB insulin-dependent group compared to SG, although there was no statistical correlation at 1 and 2 years (Fig. 4).
Independent of insulin use and comparing surgical procedures only, more complete remission was noticed after Roux-en-Y gastric bypass than sleeve gastrectomy at 1 year; 50.7 vs. 39.8% (p < 0.0001), and 54.6 vs. 49.1% (p < 0.0001) at 2 years (Fig. 5). This flipped at 3 years and SG reported 57.9% compared to 50.7% in the RYGB group (p = 0.0047) (Fig. 5). Partial remission was higher in the RYGB group but there was statistical correlation only at 2 years 8.5 vs. 3.8% SG (p = 0.0076) (Fig. 6).
Diabetes medication use
Tables 5 and 6 shows the use of diabetes medication in this cohort both at baseline and during follow-up. After surgery, the use of glucose-lowering medication dropped significantly in both O-T2D and I-T2D groups, independent of the type of surgery (RYGB or SG). In general, the number of patients who ceased diabetes medication increased in time, but a more drastic effect was noticeable after 6 months following surgery. O-T2D patients reached higher cessation numbers compared to I-T2D at each visit (Figs. 7, 8). 11.3% of the patients in the non-insulin groups and 59.6% in the insulin-dependent groups were still using anti-diabetic medications (p < 0.0001) at 3 years, a reduction of 86% (from 77 to 10.4%) was observed in the use of oral agents among O-T2D patients as opposed to I-T2D, in which use remained almost constant throughout follow-up. Reduction in insulin use was not correlated to procedure type; I-T2D patients experienced a reduction of 72.4% (from 100 to 27.4%). Of those initially assigned as O-T2D patients, 0.9% (n = 3) had started using insulin at 3 years.
Among I-T2D patients, Roux-en-Y gastric bypass showed more reduction in the requirement of medication use compared to sleeve gastrectomy at each yearly visit with statistical association at year 1 and 2 (p < 0.0001); no statistical significance could be calculated at 3 years due to limited number of patients in the SG group at this point of follow-up. The proportional percentage requiring medication in the RGYB group was approximately half that in the SG group. At 1 year 22.7% of I-T2D patients who underwent RYGB and 46.4% of I-T2D patients who underwent SG were still requiring insulin, and 26.5% (RYGB) and 40% (SG) at 3 years.
When data of both O-T2D and I-T2D groups was aggregated, as to compare only between procedures (RYGB and SG), a larger decrease in diabetes medication use was also evident (Fig. 9) in both groups, although higher in the Roux-en-Y gastric bypass group, no statistical correlation was found at 2 years (RYGB 6.9 vs. SG 17.9%, p = 0.0545), and 3 years (RYGB 9.0 vs. SG 10.5%, p = 0.2425).
Weight loss
Both %WL (Tables 7, 8) and %EWL (Table 9) were used to report analyzed weight loss. In this study insulin dependence, regardless of surgical procedure, did not affect weight loss (Table 8). Mean %WL for the insulin group was 30.1 vs. 28.3% for the non-insulin group at 3 years (p = 0.0673) with a similar trend during the entire follow-up: 30.1 vs. 30.9% at 1 year (p = 0.0580) and 30.8 vs. 30.5% (p = 0.6674) at 2 years (Fig. 10). I-T2D patients, however, presented slightly higher %EWL than their counterpart. A statistically significance was found at 2 years (p = 0.0050) and 3 years (p = 0.0020). Interestingly, the number of patients with %EWL >50% was higher among insulin groups during the entire follow-up except at 1 year (Table 9).
Roux-en-Y gastric bypass patients, either insulin or non-insulin-dependent, achieved significantly greater weight loss than sleeve gastrectomy patients (Fig. 11). Percent excess weight loss (%EWL) was significantly greater in the RYGB group at all years follow-up (p < 0.0001) (Table 10), with a mean at 3 years of 63.8% (RYGB) compared to 49.8% (SG). At 3 years, 70.7% of the RYGB group achieved a %EWL >50%, as opposed to 47.2% in the SG group (Table 9). Similarly, statistical association (p < 0.0001) was found for %WL from baseline to 2 years, favouring Roux-en-Y gastric bypass over sleeve gastrectomy (Fig. 11). Although this statistical significance was not present at 3 years (p = 0.0125), RYGB showed higher weight loss than SG, 29.3 ± 9.7 over 25.1 ± 8.7%.
Regarding insulin treated patients and type of surgery; I-T2D patients in Roux-en-Y gastric bypass had higher weight loss than in sleeve gastrectomy, with mean %EWL at 3 years of 68.2% compared to 56.8% (p < 0.0001). At that point 80.1% of RYGB patients vs. 60% of SG patients had a %EWL >50%. A same trend is found for %WL from baseline to 2 years (p < 0.0001). %WL was higher for those who underwent RYGB compared to SG. At 3 years Roux-en-Y gastric bypass still showed higher %WL (30.4 ± 10.7%) vs. (26.7 ± 10%) in the sleeve gastrectomy groups (Fig. 12), but no statistical correlation was calculated due to the number of patients in the SG group (Table 7).
Discussion
It is known that bariatric surgery induces weight loss and improves diabetes [16] and it is becoming more accepted that the procedures have underlying mechanisms, different than the mere changes in anatomy, which are generating these effects [17]. In the case of RYGB and SG, the rapid improvement of glucose concentrations, even before significant weight loss has been documented, suggesting alternate mechanisms [18] that are likely to be both endocrine and neuronal in nature, affecting energy balance and the control of metabolism. Remission after surgery has been linked to the type of procedure performed, the duration [19] and severity of T2DM at the time of surgery, the use of insulin prior to surgery [20], the length of follow-up, and the definitions of remission used by the authors [19].
Our results are consistent with studies demonstrating significant and sustained improvement in T2DM. At 3 years in our cohort, diabetics on oral medications experienced more complete diabetes remission than insulin-dependent patients (66.5 vs. 18.5% respectively). Partial remission was slightly higher for insulin-dependent patients who underwent RYGB than SG but there was no statistical correlation, 8.5 vs. 5.4% (p = 0.1984). Ramos-Levi et al. (2013) reported that insulin treatment pre-surgery affected remission rates negatively with 67.1% T2DM patients on oral medications vs. 30.4% insulin-dependent patients achieving remission at 1 year (p < 0.001) [13]. Kadera et al. reported a remission rate of 49% in insulin-dependent patients after RYGB, although remission was defined as cessation of diabetic medications with an HbA1c level of <7% [11]. The need of insulin post-surgery may be linked with a decreased pancreatic β-cell function [21] in non-remitters compared to those with diabetes remission. A validation study of the DiaRem score, one of the scoring systems created in an attempt to predict T2DM remission following bariatric surgery [22], found that for each point increase in the score (as use of insulin does), the probability of remission decreased nearly 18% [23].
Most studies do not compare T2DM remission rates associated with insulin dependence and surgical procedures. In our study Roux-en-Y gastric bypass offered higher rates of complete remission for insulin-dependent patients at 1 and 2 years (p < 0.0001). Other studies have also reported higher remission rates with RYGB, including a retrospective review by Pournaras et al. with rates of 41 and 26% after RYGB and SG, respectively at a median follow-up of 23 months (p < 0.001) [24] as well as the recent report of 5-year outcome data from the STAMPEDE trial [2] Another set of studies reported no statistical difference in T2DM remission when RYGB and SG were compared, among them studies by Robert et al. [25], Todkar et al. [26], Abbatini et al. [27], and Yip et al. [28] Jimenez et al. found that the likelihood of T2DM remission was greater in SG than in RYGB patients (hazards ratio = 1.9; p = 0.026) [29] and Fernandez-Soto in a recent prospective study (2017) found higher remission rates for SG than RYGB, but with no statistical significance [30].
Our study corroborates the effectiveness of both RYGB and SG to improve control of T2DM whether the patient is treated with oral medications or is requiring insulin. Consistent with recent STAMPEDE trail results [2], our findings show that at 3 years the use of glucose-lowering medications, including insulin, was significantly reduced in both insulin and non-insulin groups, as well as in the two surgical groups (RYGB and SG).
Nguyen et al. suggested that in order for bariatric surgery to be the most effective in resolving T2DM a residual pancreatic islet cell function must be present [31]. This may explain the fact that more medications were still used after 3 years within the insulin-dependent group than in the oral medication group. Schauer and colleagues [12] found that most patients who remained dependent on antidiabetic medications had either long-standing disease (>10 years) or severe disease (high insulin requirement) before surgery. It is noteworthy that 40% of insulin-dependent and 88% of oral anti-diabetes medication users in our cohort ceased using any diabetic medications at 3 years. Roux-en-Y gastric bypass offered insulin-dependent patients more reduction, with 26.5% patients in this group still using insulin at that point compared to 40% in the sleeve gastrectomy group. Very few studies have compared cessation of anti-diabetic medication use by bariatric procedure. The BOLD database compared Roux-en-Y gastric bypass to adjustable gastric band (AGB) and resulted in 62% cessation of insulin at year 1 with RYGB vs. 34% with AGB [20]. Schauer et al. did not compare procedures, but showed an 80% reduction in oral agent usage and 79% reduction in insulin usage after RYGB (65 to 13% and 27 to 6%, respectively, p < 0.001) [12]. Such reduction could have a major impact on patient’s quality of life and lead to cost savings.
Schauer et al. have suggested that an increasing severity of T2DM negatively affects net weight loss, independent of preoperative BMI. To explain this apparent resistance to weight loss they invoke an inherent metabolic difference. Additionally, the use of antidiabetic medications—both oral agents and insulin—are known to cause weight gain, which may also infer resistance in patients who remain on these agents after surgery [12]. Although insulin use would suggest an increased severity of diabetes, our study showed that insulin-dependent patients actually had a slightly higher %EWL than oral medication users with a positive correlation at 2 and 3 years.
When comparing insulin dependence and procedural type, our study showed that insulin-dependent patients who underwent RYGB experienced greater weight loss during the entire follow-up. At 2-years %EWL for insulin-dependent RYGB patients was 71% and SG 48.2% (p < 0.0001). In the BOLD database, Ardestani and colleagues reported a similar rate %EWL in diabetic patients undergoing RYGB of 62.8% at 12 months post-surgery [20]. Li P et al. [32] concluded in a meta-analysis that in bariatric surgery, Roux-en-Y gastric bypass is a more effective and reliable treatment for morbid obesity and for surgical treatment of poorly controlled T2DM.
In summary, for insulin-dependent patients, bariatric surgery offers favourable outcomes improving diabetes. In our study, at 3 years, 73.5% of the Roux-en-Y gastric bypass groups and 60% of the sleeve gastrectomy groups were able to stop insulin; complete T2DM remission was observed in 17.6 and 30%; and weight loss was elicit at a rate of 68.2 and 56.8% EWL, respectively. These data suggest that even in the case of a more severe presentation, as in insulin-dependent patients, remission can occur after bariatric surgery; and a portion of non-remitters no longer required insulin. RYGB appears to be superior to SG improving metabolic parameters and weight loss, especially at 2 years follow-up. Altogether, these outcomes have a bearing on the control of T2DM, which consequently improves quality of life and reduces associated costs. Very few studies have shown results in this subset of patients associated to these two procedures.
A limitation of this study is the attrition of the cohort partly explained by the nature of the source of the data in the Registry. As mentioned the rate of follow-up is based on the amount of participants who consent to participate, and is contingent on diligent, up to date data entry at each site; patients may also discontinue their visits. The attrition particularly affected the smaller insulin-dependent SG group. Another limitation is that a small number of patients (n = 5) who have had a previous bariatric surgery before and 24 who underwent a second bariatric surgery as part of a revision or repair during follow up, were not excluded: 14 no insulin/RYGB; 6 no insulin/SG; 7 insulin/RGYB; 2 insulin/SG. Of the insulin-dependent patients 3 continued with insulin up to 2 years and experienced no remission, the rest (6) received medications up to 6 months but there is no data to determine remission. At baseline; of the 20 non-insulin patients; 16 were using oral medications; 4 did not receive any; and all were without medications from 6 months thereafter, however no data on remission is available. Our study has also considerable strengths, such as the centralization of all the outcome measurements, the high degree of complete and standardized data-collection at each visit, and despite the attrition; the number of insulin-dependent patients in each cohort during a 2 year period allows us to explore the impact of bariatric surgery in these patients compared to diabetics on oral agents, and contributes to the literature in this regard. The Bariatric Registry will soon include follow-up to 5 years for all patients that should allow additional assessment of even longer-term outcomes and increased volume of patients, particularly for the sleeve gastrectomy group to support the results presented now.
Conclusions
Our study shows that bariatric surgery led to an improvement of T2DM, as demonstrated by the reduction of diabetes medication use and remission in all groups, even patients with more advance disease requiring insulin, although to a lesser rate compared to patients on oral medications. Insulin dependence did not affect weight loss. Compared to SG, RYGB appears to be more effective in improving metabolic parameters in insulin-dependent type two diabetic patients. Roux-en-Y gastric bypass should be the preferred surgical option for this group, it should also be recommended earlier in the course of treatment as the likelihood of achieving complete remission diminishes once insulin therapy becomes a necessity.
Abbreviations
- AGB:
-
Adjustable gastric banding
- BMI:
-
Body mass index
- BPD:
-
Biliopancreatic diversion
- HbA1c:
-
Glycated hemoglobin (A1C)
- I-T2D:
-
Insulin-treated type 2 diabetes
- O-T2D:
-
Oral-treated type 2 diabetes
- %WL:
-
Percent weight loss
- %EWL:
-
Percent excess weight loss
- SG:
-
Sleeve gastrectomy
- RCT:
-
Randomized control trial
- RYGB:
-
Roux-en-Y gastric bypass
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
References
World Health Organization (2016) Global report on diabetes. WHO Press, Geneva
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE (2017) Bariatric surgery vs. intensive medical therapy for diabetes -5-Year Outcomes. N Engl J Med 376(7):641–651
Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, Ramanathan R, Schauer P (2006) Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc 20:859–863
Regan JP, Inabnet WB, Gagner M, Pomp A (2003) Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 13:861–864
Silecchia G, Rizzello M, Casella G, Fioriti M, Soricelli E, Basso N (2009) Two-stage laparoscopic biliopancreatic diversion with duodenal switch as treatment of high-risk super-obese patients: analysis of complications. Surg Endosc 23:1032–1037
Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N (2015) Bariatric surgery worldwide 2013. Obes Surg 25(10):1822–1832
Vidal J, Ibarzabal A, Romero F, Delgado S, Momblán D, Flores L, Lacy A (2008) Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg 18(9):1077–1082
Bruno G, Gruden G, Barutta F, Cavallo Perin P, Morino M, Toppino M (2015) What is the impact of sleeve gastrectomy and gastric bypass on metabolic control of diabetes? A clinic-based cohort of Mediterranean diabetic patients. Surg Obes Relat Dis 11(5):1014–1019
Maraka S, Kudva YC, Kellogg TA, Collazo-Clavell ML, Mundi MS (2015) Bariatric surgery and diabetes: implications of type 1 vs. insulin-requiring type 2. Obesity 23(3):552–557
Robert M, Belanger P, Hould FS, Marceau S, Tchernof A, Biertho L (2015) Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis 11(4):798–805
Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ (2009) Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis 5(3):305–309
Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D (2003) Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238(4):467–484
Ramos-Levi A, Sanchez-Pernaute A, Matia P, Cabrerizo L, Barabash A, Hernandez C, Calle-Pascual A, Torres A, Rubio M (2013) Diagnosis of diabetes remission after bariatic surgery may be jeopardized by remission criteria and previous hypoglycemic treatment. Obes Surg 23(10):1520–1526
Buse JB, Caprio S, Cefalu WT et al (2009) How do we define cure of diabetes? Diabetes Care 32:2133–2135
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G (2016) Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care 39(6):861–877
Laferrère B (2012) Gut feelings about diabetes. Endocrinol Nutr 59:254–260
Hutch CR, Sandoval DA (2017) Physiological and molecular responses to bariatric surgery: markers or mechanisms underlying T2DM resolution? Ann N Y Acad Sci 1391(1):5–19
Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ (2012) All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 33(4):595–622
Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, Chand B, Schauer PR (2013) Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 258(4):628–636
Ardestani A, Rhoads D, Tavakkoli A (2015) Insulin cessation and diabetes remission after bariatric surgery in adults with insulin-treated type 2 diabetes. Diabetes Care 38(4):659–664
Kahn SE, Lachin JM, Zinman B et al (2011) Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 60:1552–1560
Min T, Barry JD, Stephens JW (2015) Predicting the resolution of type 2 diabetes after bariatric surgical procedures: a concise review. J Diabetes Metab 6:617
Honarmand K, Chetty K, Vanniyasingam T, Anvari M, Chetty VT (2017) Type 2 diabetes remission rates 1-year post-Roux-en-Y gastric bypass and validation of the DiaRem score: the Ontario Bariatric Network experience. Clin Obes 7(3):176–182
Pournaras DJ, Aasheim ET, Søvik TT, Andrews R, Mahon D, Welbourn R et al (2012) Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 99:100–103
Robert M, Ferrand-Gaillard C, Disse E, Espalieu P, Simon C, Laville M, Gouillat C, Thivolet C (2013) Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg 23(6):770–775
Todkar JS, Shah SS, Shah PS et al (2010) Long-term effects of laparoscopic sleeve gastrectomy in morbidly obese subjects with type 2 diabetes mellitus. Surg Obes Relat Dis 6:142–145
Abbatini F, Rizzello M, Casella G et al (2010) Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc 24:1005–1010
Yip S, Plank LD, Murphy R (2013) Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta-analysis of outcomes. Obes Surg 23(12):1994–2003
Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J (2012) Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg 256(6):1023–1029
Fernández-Soto ML, Martín-Leyva A, González-Jiménez A, García-Rubio J, Cózar-Ibáñez A, Zamora-Camacho FJ, Leyva-Martínez MS, Jiménez-Ríos JA, Escobar-Jiménez F (2017) Remission of type 2 diabetes mellitus after bariatric surgery–comparison between procedures. Endokrynol Pol 68(1):18–25
Nguyen KT, Billington CJ, Vella A et al (2015) Preserved insulin secretory capacity and weight loss are the predominant predictors of glycemic control in patients with type 2 diabetes randomized to Roux en-Y gastric bypass. Diabetes 64:3104–3110. doi:10.2337/db14-1870
Li P, Fu P, Chen J, Wang LH, Wang DR (2013) Laparoscopic Roux-en-Y gastric bypass vs. laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus: a meta-analysis of 16 recent studies. Hepatogastroenterology 60(121):132–137
Acknowledgments
The authors would like to acknowledge the data management team at the Population Health Research Institute (PHRI) who provide statistical analysis of the Bariatric Registry, and who assisted in the analysis for this study.
Funding
The Ontario Ministry of Health and Long-Term Care (MOHLTC) support the Ontario Bariatric Registry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Lemus, Karni, Hong, Gmora, Anvari and Ms. Breau have no conflict of interest or financial ties to disclose.
Additional information
Presented at the SAGES 2017 Annual Meeting, March 22–25, 2017, Houston, Texas.
Rights and permissions
About this article
Cite this article
Lemus, R., Karni, D., Hong, D. et al. The impact of bariatric surgery on insulin-treated type 2 diabetes patients. Surg Endosc 32, 990–1001 (2018). https://doi.org/10.1007/s00464-017-5777-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5777-5