Abstract
Background
Controversy exists regarding type 2 diabetes (T2D) remission rates after bariatric surgery (BS) due to heterogeneity in its definition and patients' baseline features. We evaluate T2D remission using recent criteria, according to preoperative characteristics and insulin therapy (IT).
Methods
We performed a retrospective study from a cohort of 657 BS from a single center (2006–2011), of which 141 (57.4 % women) had T2D. We evaluated anthropometric and glucose metabolism parameters before surgery and at 1-year follow-up. T2D remission was defined according to 2009 consensus criteria: HbA1c <6 %, fasting glucose (FG) <100 mg/dL, and absence of pharmacologic treatment. We analyzed diabetes remission according to previous treatment.
Results
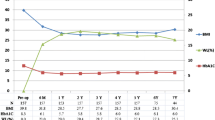
Preoperative characteristic were (mean ± SD): age 53.9 ± 9.8 years, BMI 43.7 ± 5.6 kg/m2, T2D duration 7.4 ± 7.6 years, FG 160.0 ± 54.6 mg/dL, HbA1c 7.6 ± 1.6 %. Fifty-six (39.7 %) individuals had IT. At 1-year follow-up, 74 patients (52.5 %) had diabetes remission. Percentage weight loss (%WL) and percentage excess weight loss (%EWL) were associated to remission (35.5 ± 8.1 vs. 30.2 ± 9.5 %, p = 0.001; 73.6 ± 18.4 vs. 66.3 ± 22.8 %, p = 0.037, respectively). Duration of diabetes, age, and female sex were associated to nonremission: 10.3 ± 9.4 vs. 4.7 ± 3.8 years, p < 0.001; 55.1 ± 9.3 vs. 51.2 ± 9.9 years, p = 0.017; 58.9 vs. 33.3 %, p = 0.004, respectively. Prior treatment revealed differences in remission rates: 67.1 % in case of oral therapy (OT) vs. 30.4 % in IT, p < 0.001. OR for T2D remission in patients with previous IT, compared to those with only OT, were 0.157–0.327 (p < 0.05), adjusting by different models.
Conclusions
Consensus criteria reveal lower T2D remission rates after BS than previously reported. Prior insulin use is a main setback for remission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing prevalence of both obesity and type 2 diabetes (T2D) has prompted the need for more effective prevention and treatment strategies. Bariatric surgery (BS), which was designed to achieve a substantial and durable weight loss, has proved to be successful for diabetes remission [1]. Its impact has been recently reviewed [2] and several position statements now include BS in T2D treatment algorithms [3–5].
Numerous publications in the past recent years have described a wide variety of diabetes remission rates, mainly due to differences in criteria used for its definition and heterogeneity of basal characteristics of study populations [2, 6, 7]. In an attempt to overcome this controversy, a consensus group of experts in pediatric and adult endocrinology, diabetes education, transplantation, metabolism, bariatric/metabolic surgery, and hematology–oncology proposed a definition for diabetes remission [8]. Consequently, remission rates have been communicated to be lower [9, 10].
Diabetes remission is explained by several mechanisms, both weight-dependent and independent [11, 12], and several preoperative factors have been identified as possible predictors [13–16]. Diabetes duration, insulin resistance (measured homeostasis model assessment [HOMA]), C-peptide levels, and weight loss have been the most explored ones, but stratification of patients according to previous hypoglycemic treatment has not been thoroughly examined. The aim of this study is to evaluate diabetes remission according to standardized criteria after three bariatric procedures and to identify the differences according to previous insulin use.
Materials and Methods
Study Population
We conducted a retrospective study of a cohort of 657 bariatric surgeries performed in a single center during the years 2006–2011. A total of 141 individuals (81 women; mean age 53.0 years, range 22–73) had medication-controlled T2D, defined according to current ADA guidelines [17]. Cases that suggested late autoimmune diabetes, a history of type 1 diabetes, and diabetes secondary to a specific disease were excluded.
Information was obtained from medical charts before surgery and at 1-year follow-up. Data collected included: hypoglycemic treatment used, duration of diabetes, anthropometric characteristics (height; weight; body mass index [BMI], calculated as weight (kilograms) / height2 (square meters); percentage weight loss [%WL]; and percentage excess weight loss [%EWL] [18]), and glucose metabolism parameters (fasting glucose [FG] and glycosylated hemoglobin [HbA1c]). All patients signed a written informed consent prior to surgery in which it was specified that clinical and analytical data collected before the bariatric procedure and during follow-up could be potentially used in an anonymous way for investigation and publication. This study was approved by the Ethics Committee of the Hospital Clinico San Carlos and was in compliance with the Helsinki Declaration.
Definition of Diabetes Remission
Diabetes remission was defined according to the 2009 consensus document [8]: HbA1c <6 % and FG <100 mg/dL, in the absence of active pharmacologic treatment. Previously [2], diabetes remission was defined as being off diabetes medications with normal FG (<100 mg/dL) or an HbA1c level <6 %.
Surgical Procedures
For the purpose of analysis simplification, surgeries performed were divided into three subgroups: Roux-en-Y gastric bypass (RYGB) (52 patients, 36.9 %), biliopancreatic diversion (BPD) (72 patients, 51.1 %), and sleeve gastrectomy (SG) (17 patients, 12.1 %). Eligibility for each of them varied according to the patients' previous diabetes medical history and comorbidities, and was evaluated by the treating physician (endocrinologist and/or surgeon). All surgeries were performed laparoscopically by the same team in a single center. RYGB consisted of the creation of a small vertical gastric pouch of approximately 15–20 mL, a 150-cm Roux limb, and a 75–100-cm biliopancreatic limb. BPD included two types of procedures: a classic duodenal switch (17 cases) and a single-anastomosis duodeno-ileal bypass with sleeve gastrectomy or SADI-S (55 patients). Classic duodenal switch was performed as described by Hess [19]; basically, a sleeve gastric resection was followed by a douodeno-ileal bypass with a 250-cm alimentary limb and a 75–100-cm common channel. SADI-S was performed as described previously [20]; it consisted of a one-loop duodenal switch in which after the sleeve gastrectomy (performed over a 54Fr [18 mm] bougie) and the duodenal division, the proximal duodenal stump was end-to-side anastomosed to the ileum at 250 cm form the ileocecal valve, thus creating a long biliopancreatic channel and a 250-cm common + alimentary limb. Because SADI-S is a simplified duodenal switch that has proved to behave in the same way as the classic duodenal switch, both techniques were considered under the BPD group for statistical analyses. SG involved a greater curvature gastric resection, from 4 cm proximal to the pylorus, to the pericardial fat. In this case, the stomach was calibrated with a 42Fr (14 mm) bougie.
Statistical Analysis
Descriptive results were expressed as mean ± standard deviation and (range) for continuous variables. Categorical variables were summarized as frequencies and percentages. Preoperative features were examined for their influence on diabetes remission using analysis of variance and chi-square tests (Fisher's exact test as required). Additionally, logistic regression analyses were conducted for each baseline characteristic to determine the predictors of T2D remission as well as the relationship between insulin therapy and remission after adjustment by these variables. The p values were two-sided and statistical significance was considered when p < 0.05. All statistical analyses were performed using SPSS version 19.0 (IBM SPSS Statistics Inc., Chicago, IL, USA).
Results
Baseline Analysis
Baseline characteristics were: BMI 43.7 ± 5.6 (29.8–61.0) kg/m2, duration of T2D 7.4 ± 7.6 (0.5–40) years, FG 160.0 ± 54.6 (77.0–431.0) mg/dL, and HbA1c 7.6 ± 1.6 (4.9–16.3 %. A total of 56 (39.7 %) individuals were on insulin therapy. There were certain differences in patients' baseline characteristics according to the three types of procedures performed. Duration of T2D and preoperative FG and HbA1c levels were higher in those patients who underwent BPD (9.7 ± 9.0 years, 169.0 ± 48.9 mg/dL, and 7.8 ± 1.5 %, respectively), in comparison to those who underwent RYGB (4.7 ± 4.2 years, 143.0 ± 37.6 mg/dL, and 7.1 ± 1.3 %, respectively) (p < 0.05 in all cases). Patients who underwent SG had the highest preoperative values of FG (174.8 ± 99.4 mg/dL) and HbA1c (8.2 ± 2.7 %), and it was more frequent for patients in this group to have prior insulin therapy (nine of 17 patients, 52.9 %). There were no significant differences regarding age, sex, and preoperative BMI across the three types of BS, although mean preoperative BMI was higher in patients who underwent SG (45.8 ± 6.4 kg/m2).
Changes After BS, Diabetes Remission, and Predictors of Remission
Table 1 shows different baseline and 12-month follow-up characteristics according to diabetes remission and prior use of diabetes medications. According to the 2009 consensus definition [8], complete remission of diabetes was achieved in 74 patients (52.5 %) at 1 year after BS, while according to previous definition [2], 96 patients (68.1 %) achieved remission (p < 0.001). Preoperative BMI did not influence the rate of remission, but greater percentages of weight loss (%WL) and excess weight loss (%EWL) were observed in the remission group: 35.5 ± 8.1 vs. 30.2 ± 9.5 %, p = 0.001, and 73.6 ± 18.4 % vs. 66.3 ± 22.8 %, p = 0.037, respectively. On the other hand, duration of diabetes, age, and female sex were associated to nonremission; in comparison to those in remission, nonremitters had a longer preoperative duration of diabetes (10.3 ± 9.4 vs. 4.7 ± 3.8 years, p < 0.001), were older (55.1 ± 9.3 vs. 51.2 ± 9.9, p = 0.017), and were more frequently women (58.9 vs. 33.3 %, p = 0.004). Preoperative FG and HbA1c levels were lower in those who were under remission status at 1 year follow-up.
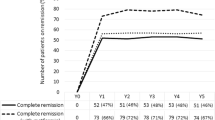
Diabetes remission differed according to previous hypoglycemic treatment. Based on the 2009 consensus criteria [8], complete remission was achieved in 57 out of the 85 patients (67.1 %) who were treated with oral agents while only in 17 out of the 56 (30.4 %) who required insulin (p < 0.001), and according to previous definition [2], remission was achieved in 73 out of 85 patients (85.9 %) who were on oral agents vs. in 23 of 56 (41.1 %) who were on insulin (p < 0.001) (Fig. 1). Differences in diabetes duration were maintained when stratifying according to the type of preoperative therapy; in both groups, duration of the disease was longer in the nonremitters (6.4 ± 6.7 vs. 3.8 ± 3.2 in the oral treatment group, p = 0.039, and 13.0 ± 10.2 vs. 7.8 ± 4.1 in the insulin group, p = 0.010). Greater prevalence of female sex among the nonremitters was maintained in the group with prior oral medication (44.7 vs. 18.4 %, p = 0.010), but no significance was observed in the insulin group (76.5 vs. 59 %, p = 0.167). Age was not different when diabetes remission was stratified according to prior insulin use.
Significant differences in the %WL and %EWL, stratified by remission status, were observed in those patients who had received insulin prior to BS. In those who had only been treated with oral agents, however, the significance of this difference was lost for the %EWL, and only a trend for the same finding was found.
Preoperative FG and HbA1c levels, stratified by remission status, were lower in those patients with former oral therapy (136.6 ± 30.0 mg/dL and 6.9 ± 1.2 % in those with remission vs. 153.0 ± 39.0 mg/dL and 7.5 ± 1.4 % in the nonremission category, p = 0.036 in both cases); however, this difference was not observed in those with prior insulin treatment.
Crude odds ratios (OR) and 95 % confidence intervals (CI) of preoperative characteristics for prediction of T2D remission are shown in Table 2. Additionally, Table 3 shows OR and 95 % CI for preoperative insulin therapy and T2D remission, adjusted for different models. OR for T2D remission in patients with prior insulin therapy, in comparison to those with only oral medications, were set between 0.157 and 0.327, with differences being statistically significant. In the adjusted models, OR for diabetes duration and T2D remission maintained statistical significance (OR 0.880, 95 % CI 0.804–0.964, p = 0.006) as well as female sex (OR 0.274, 95 % CI 0.115–0.652, p = 0.003) and %EWL (OR 1.041, 95 % CI 1.017–1.067, p = 0.001).
Regarding the three different bariatric procedures performed, at 1 year follow-up, patients who underwent BPD had greater %WL and %EWL (p < 0.001 in both cases), and 12-month HbA1c levels were lower (p < 0.001). Also, T2D remission rates were higher in this group (39 out of 72 patients, 54.2%), in comparison to RYGB (28 out of 52 patients, 53.8 %) and SG (seven out of 17 patients, 41.2 %), although we did not find significant differences in remission rates according to the type of BS performed (p = 0.609).
Discussion
The fact that T2D can be reversed after BS has been frequently reported [21], but due to the lack of a consistent definition of diabetes remission, rates have varied widely across studies and may have probably even been overestimated [2, 22, 23]. Since the consensus criteria proposed by Buse et al. [8], remission rates have been reported to be lower [9, 10, 24, 25], as it was expected by using more stringent cutoff values to establish the definition. In this retrospective study, we report overall diabetes remission rates in a closer range to papers that used these latter criteria, corroborating the importance of uniformity in defining diabetes remission.
Another source of disparity in remission rates across different publications is the heterogeneity of patients' preoperative characteristics. Age has been associated to lower remission rates, presumably because of both greater deterioration of pancreatic reserve and an assumed progression of disease [16]. The longer preoperative duration of T2D, which has been used as an estimate of disease severity, proved to significantly reduce the chances of diabetes remission [26, 27], and this finding has been consistent in subsequent reports [6, 7, 10, 28], including ours.
On the other hand, the influence of baseline BMI has been more controversial. BS in patients with a BMI >35 kg/m2 determined a profound weight loss and amelioration of associated comorbidities, including diabetes [2, 23]. More recently published papers, however, indicated that BS can safely and effectively achieve this endpoint in patients with only mild obesity (BMI 30–35 kg/m2) [29]. Also, Mingrone et al. [6] and Schauer et al. [7] reported that preoperative BMI did not predict control of diabetes after surgery. However, the first study accepted patients with BMI >35 kg/m2 and reported remission rates of 75 and 95 % after RYGB and BPD, respectively, while the second one included patients with a BMI starting at 27 up to 43 kg/m2 and reported lower diabetes remission rates (42 % after RYGB and 37 % after SG). It is undeniable that severity of diabetes is influenced by BMI, making the relationship between BMI and diabetes remission complex.
In our study, baseline BMI did not appear to influence the effect of BS on T2D remission, supporting the argument that indications for this type of surgery could be broadened and viewed as “primarily metabolic,” rather than merely bariatric [29]. However, a fact that did influence diabetes remission was %WL and %EWL. This is consistent with major previous publications, as the association between overweight and diabetes prevalence is unquestionable [21].
Our study goes one step further in identifying potential predictors of diabetes remission after BS by stratifying patients according to their previous hypoglycemic treatment. Requiring prior insulin therapy may be considered as a surrogate of preoperative disease severity [27], so it would play an essential role in determining diabetes remission, presumably due to deterioration of pancreatic beta-cells [30]. In our study, in addition to a shorter duration of diagnosis of diabetes, we identified the absence of insulin therapy as another independent predictor of remission at 1-year follow-up: 67.1 % of patients with oral medication exclusively achieved remission 1 year after BS vs. 30.4 % in those with insulin. Insulin therapy status had the lowest OR for achieving T2D remission and, furthermore, it remained a significant predictor after adjustment for age, gender, diabetes duration, and other preoperative characteristics. This would allow us to infer that the presence of previous insulin treatment should be taken into account to estimate the possibilities of T2D remission after BS, regardless of diabetes duration.
Nevertheless, it is worth noting that only patients with medication-controlled T2D were included in our study; it is possible that if there had been more cases with diet-controlled T2D, a greater beneficial effect of BS may have been observed.
Few reports have suggested that previous treatment with insulin determines lower remission rates [27, 31, 32], but, to our knowledge, only one has clearly stratified outcomes according to it [10]. We emphasize the need to establish these differences when comparing remission outcomes because chronicity is a characteristic feature of diabetes, and knowledge of the factors related to its severity may be useful to determine how much improvement can be potentially expected after a specific bariatric procedure. If a greater severity (estimated by longer disease duration and insulin therapy) is associated to less favorable outcomes, the current role of surgery in the algorithms of management of diabetes could be argued [4, 33].
In our study, duration of diabetes was a significant predictor of remission even after stratifying patients according to their previous pharmacologic treatment and after adjusting for age and gender, in agreement with previous publications [10, 28]. However, this stratification uncovered that age was not different according to remission status, which could be relevant when setting cutoff values for eligibility to surgery. %WL and %EWL were found to be different according to the previous treatment, which corroborates the importance of weight loss to improve any degree of hyperglycemia [21]. It may be noted that significance decreased in the group treated with oral medication exclusively, a fact that could be attributed to the existence of weight-independent mechanisms involved in diabetes resolution [11, 34].
Another remarkable feature of our study, which distinguishes it from previous ones, is that it evaluated the outcomes after three different types of BS, from restrictive to malabsortive procedures. We did not find differences in T2D remission rates according to the type of BS performed, in a similar way to previous studies [7, 25], although in contrast to other published data [9, 12]. However, this should be interpreted with caution because patients' baseline characteristics differed in relevant aspects such as diabetes duration, prior insulin therapy, and preoperative FG and HbA1c. On the other hand, differences observed in %WL and %EWL across the different BS were consistent with previous publications [12, 21]. A reason for not obtaining significant statistical results when comparing the types of surgery and remission rates may have been the small number of SG performed. This was probably due to the fact that current trends have not usually considered SG as a primary “metabolic” procedure, in contrast to RYGB and BPD techniques, and our study reflects this surgical tendency over the last years in different bariatric centers around the world. However, it has sometimes been used as a “first-step” procedure in patients with severe morbid obesity, and recent publications have started to regard SG as a valid option of treatment for obese patients with T2D [35, 36] with promising results. Overall comparisons should, therefore, be revaluated when a significant increase in the number of SG performed is achieved.
The limitations of our study are mainly related to collecting data from clinical records and using retrospective analyses. Also, our observations are limited to a 12-month follow up, which in the majority of cases is when stabilization of weight loss occurs. Long-term studies are deemed necessary to evaluate durable diabetes remission rates defined by consensus criteria and according to prior hypoglycemic treatment and type of BS performed. We also suggest that overall improvement of patients should be evaluated, and not only remission rates, because a significant reduction of hypoglycemic treatment is surely achieved. For instance, in our series, after BS, more than half of the patients who were not strictly considered in remission of diabetes had an adequate glycemic control, according to ADA's target recommendations, with only diet or oral medications, and less than 10 % required insulin (data not shown).
In conclusion, we remark that what is important is to stratify patients according to their baseline characteristics in order to draw realistic conclusions and avoid confounding interpretations regarding the efficacy of BS for the treatment of T2D. Although standardized criteria may reveal lower T2D remission rates after BS than those previously reported in the literature, we encourage the generalization of their use to be able to overcome current controversies and make reliable comparisons. Moreover, patients' baseline preoperative characteristics, especially insulin status, should be taken into consideration by physicians in order to accurately estimate what to expect regarding diabetes remission after BS.
References
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–52.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and metaanalysis. Am J Med. 2009;122:248–56.
Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405.
American Diabetes Association. Standards of medical care in diabetes 2013 (position statement). Diabetes Care. 2013;36 suppl 1:S11–66.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011;28:628–42.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Schauer P, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Eng J Med. 2012;366:1567–76.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–5.
Pournaras DJ, Aasheim ET, Søvik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–3.
Blackstone R, Bunt JC, Cortés MC, et al. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis. 2012;8:548–55.
Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411.
Stefater MA, Wilson-Pérez HE, Chambers AP, et al. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622.
Hamza N, Abbas MH, Darwish A, et al. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7:691–6.
Dixon JB, Hur KY, Lee WJ, et al. Gastric bypass in Type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med. 2012 Dec 28. doi: 10.1111/dme.12107.
Lee WJ, Chong K, Ser KH, et al. C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes Surg. 2012;22:293–8.
Lee WJ, Chong K, Chen JC, et al. Predictors of diabetes remission after bariatric surgery in Asia. Asian J Surg. 2012;35:67–73.
American Diabetes Association. Diagnosis and classification of diabetes mellitus (position statement). Diabetes Care. 2013;36 suppl 1:S67–74.
Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13:59–60.
Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–82.
Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17:1614–8.
Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–11.
Vidal J, Ibarzabal A, Romero F, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. 2008;18:1077–82.
Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Ramos-Levi AM, Cabrerizo L, Matía P, et al. Which criteria should be used to define type 2 diabetes remission after bariatric surgery? BMC Surg. 2013 Mar 28;13:8. doi:10.1186/1471-2482-13-8.
Robert M, Ferrand-Gaillard C, Disse E, et al. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg 2013; Jan 25. doi:10.1007/s11695-013-0868-4.
Hall TC, Pellen MG, Sedman PC, et al. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20:1245–50.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–85.
Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20–6.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–8.
Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:1372–9.
Arterburn DE, Bogart A, Sherwood NE, et al. A Multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023–9.
Lecube A, Burguera B, Rubio MA. Breaking therapeutic inertia: should metabolic surgery be considered one more option for the treatment of type 2 diabetes mellitus? Endocrinol Nutr. 2012;59:281–3.
Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab. 2010;21:337–44.
Rizzello M, Abbatini F, Casella G, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20:50–5.
Abbatini F, Capoccia D, Casella G, et al. Long-term remission of type 2 diabetes in morbidly obese patients after sleeve gastrectomy. Surg Obes Relat Dis 2012 Sep 18. doi:pii: S1550-7289(12)00340-1.
Acknowledgments
This study was funded by Fundación Mutua Madrileña de Investigación Biomédica AP 89592011.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos-Levi, A., Sanchez-Pernaute, A., Matia, P. et al. Diagnosis of Diabetes Remission After Bariatic Surgery May be Jeopardized by Remission Criteria and Previous Hypoglycemic Treatment. OBES SURG 23, 1520–1526 (2013). https://doi.org/10.1007/s11695-013-0995-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0995-y