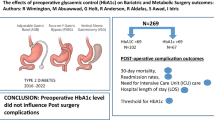
Abstract
Background
Bariatric surgery is the most effective treatment for morbidly obese type II diabetics. However, guidelines for perioperative glucose control are not well established. We examined management of perioperative glucose levels in diabetic patients undergoing bariatric surgery and determined the impact of optimal glucose control as defined by the American Society for Metabolic and Bariatric Surgery (ASMBS) on patient outcomes, including long-term diabetes resolution.
Methods
A single-institution, retrospective analysis of 155 morbidly obese diabetic patients who underwent laparoscopic gastric bypass (RYGB) or sleeve gastrectomy (LSG) from 2010 to 2014 was performed. Inpatient finger-stick glucose levels were extracted from the electronic health record and defined as optimal if all values were <180 mg/dl. Ninety-day and one-year outcomes, including diabetes resolution, medication management, mortality and total costs were compared for patients with and without optimal control.
Results
80 % (n = 124) of patients with type II diabetes underwent RYGB, while the remaining patients underwent LSG. Diabetes resolution at 1 year was 70.1 % (73.4 % for RYGB and 53.9 % for LSG, p = 0.191). Preoperatively, 72 % (n = 112) of patients were taking one or more oral antihyperglycemic agents, while only 50.3 % (n = 78) took an oral medication on discharge. 93 % of RYGB and 82 % of LSG patients, respectively, reduced their long-acting insulin dosage by greater than 50 % upon discharge (p = 0.251). Ninety-day and one-year outcomes including total costs were not improved by optimal perioperative glucose control. In total, 96.7 % of optimally controlled patients experienced diabetes resolution at 1 year compared to 53.2 % in the non-optimally controlled group (p < 0.001).
Conclusion
Bariatric surgery leads to significant resolution of type II diabetes and a prompt improvement in glucose tolerance in the perioperative period. Optimal glucose control as defined by the ASMBS was not associated with improved postoperative outcomes in our patient population but was highly predictive of long-term diabetes resolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity and diabetes have been called “twin epidemics.” Recent United States census data and Centers for Disease Control estimates indicate that approximately 14.5 % of Americans are severely obese (BMI > 35), and nearly 10 % are diabetic [1, 2]. Worldwide, 80–90 % of patients diagnosed with type II diabetes (DMII) are overweight or obese [3]. Observational studies and randomized controlled trials have demonstrated that bariatric surgery is the most effective and durable therapy for DMII treatment in obese individuals. In a systematic review and meta-analysis, Buchwald et al. [4] reported 77 % diabetes resolution in patients undergoing bariatric surgery with the highest rate of resolution resulting from biliopancreatic diversion/duodenal switch (BPDDS, 98 %), followed by Roux-en-Y gastric bypass (RYGB, 84 %) and laparoscopic adjustable gastric banding (48 %). Laparoscopic sleeve gastrectomy (LSG) is also an effective therapy for diabetes, with resolution rates ranging from 47 to 81 % [5, 6]. Moreover, improvements in glycemic control for type II diabetics are rapid, taking place in the days to months following bariatric surgery prior to any significant weight loss [7].
Despite the immediate onset and effectiveness of bariatric surgery in treating diabetes, many questions remain about the perioperative management of diabetic patients undergoing bariatric surgery. Dosing diabetic medications and insulin in the postoperative period, while balancing the risks of hypo and hyperglycemia is particularly challenging. In 2013, a joint clinical practice guideline published by the American Society for Metabolic and Bariatric Surgery (ASMBS), the American Association of Clinical Endocrinologists, and the Obesity Society made a series of recommendations for perioperative glucose management in diabetic patients undergoing bariatric surgery [8]. However, all recommendations are classified as Grade D with no direct evidence to support the published guidelines. There is a clear need for a more standardized glucose management protocol in diabetic bariatric surgery patients. Additionally, the role of perioperative glucose control on patient outcomes, short- and long-term diabetes resolution must be better characterized.
The objectives of this study were to characterize postoperative glycemic control and medication/insulin management in a cohort of Roux-en-Y gastric bypass and sleeve gastrectomy patients with DMII. Further, we sought to quantify how often ASMBS recommendations were met and whether outcomes and costs were affected by optimal versus non-optimal glucose control postoperatively.
Materials and methods
Study population
A retrospective analysis of 325 morbidly obese patients undergoing bariatric surgery at the University of Wisconsin Medical and Surgical Weight Management Program from 2010 to 2014 was performed. Adjustable gastric banding and revisional bariatric surgery cases were excluded. Patients were considered to have a diagnosis of DMII if preoperative HgBA1C measured >6.5 mg/dL or if oral antihyperglycemic agents and/or insulin were present on the initial visit medications list [9].
Operative technique
All procedures were completed laparoscopically. Preoperatively, patients adhered to a two-week Optifast meal replacement diet. RYGB was performed in a standard technique with the formation of a 20–30-ml gastric pouch, a stapled antecolic, antegastric gastrojejunostomy and a stapled jejunojejunostomy with a 100–150-cm Roux limb. LSG was performed over a 36–44 Fr Bougie, with biosynthetic staple-line reinforcement (Bio-A, Gore, Flagstaff, AZ), and omentoplasty.
Patient characteristics
Demographic data including gender, age, and race as well underlying obesity-related comorbidities were obtained from the electronic health record. The number of oral antihyperglycemic agents and insulin regimen (long and short acting) was noted during four time points: during initial visit history and physical, upon discharge after bariatric procedure, at 90 days (±14 days) postoperatively, and at one year postoperatively (±3 months). Intermediate-acting insulin (NPH) was converted unit per unit to long-acting insulin when administered daily with addition of a 20 % correction factor when given twice daily [10]. For patients on a variable insulin sliding scale as an outpatient, the mean number of units of insulin administered could not be estimated and was not included in our analysis. Diabetes resolution was defined as HgBA1C < 6.5 and absence of antihyperglycemic medications in the patient medication log was calculated at one year.
Perioperative data
American Society of Metabolic and Bariatric Surgery (ASMBS) recommendations for perioperative blood glucose management following bariatric surgery include the following: (1) discontinuation of insulin secretagogues (sulfonylureas and meglitinides), (2) continuation of metformin and incretins until clinical resolution of DMII, (3) using a combination of short- and long-acting insulin to achieve a glycemic target of 140–180 mg/dL in non-intensive care unit patients, (4) use of regular intravenous insulin in intensive care unit patients, and (5) endocrinology consults for difficult to control diabetic patients [8]. A patient was considered to have optimal glucose control if all point-of-care (POC) glucose readings from the immediate postoperative period to discharge were <180 mg/dL. Hypoglycemia was defined as any blood glucose level <70 mg/dL [11]. Total number of short- and long-acting insulin units administered for the duration of the inpatient stay was determined from the medication administration report.
Glucose management protocol
All diabetic patients were placed on a short-acting insulin sliding scale postoperatively with finger-stick glucose checks performed every 6 h. Patients on long-acting insulin preoperatively were continued on long-acting insulin postoperatively if blood sugars were persistently elevated. Long-acting insulin dose was reduced by 50 % or greater and titrated according to total units of short-acting insulin administered on the sliding scale. Oral antihyperglycemic medications were held during inpatient hospitalization. However, metformin was continued on discharge unless patients were euglycemic and did not require any sliding scale insulin throughout their hospital stay.
Ninety-day outcomes
Wound complications encompassing superficial, deep, or organ space surgical site infections, leak demonstrated by extravasation on abdominal computed tomography (CT) or found on reoperation (exploratory laparotomy or laparoscopy), bleeding requiring transfusion of one or more units of packed red blood cells postoperatively, reoperation, and mortality at 90 days were reported. Other ninety-day complications were based on variables established by the National Surgical Quality Improvement Program [12] and included deep vein thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular accident, acute renal failure, pneumonia, and urinary infection.
One-year outcomes and cost
Length of stay following bariatric surgery, readmission rates, and emergency department visits within ninety days post-op were recorded for patients with optimal versus non-optimal perioperative glucose control. Surgical complications assessed at one year included marginal ulcer or anastomotic stricture requiring dilation (in bypass patients with gastrojejunostomy less than 10 mm in diameter) found on upper endoscopy, surgical revision, and mortality. One-year facility costs were obtained from the UW information technology division and were classified as inpatient, outpatient, ED, and total.
Statistical analyses
Fischer’s exact tests were used to compare categorical variables. Student’s t tests were used to compare the means, and Wilcoxon’s rank sum tests were used for the comparison of medians for continuous variables (length of stay or follow-up interval). p values were two-sided and considered significant if they were ≤ 0.05. SAS version 9.3 (SAS Institute, Cary, NC) was used for all analyses.
The study protocol was approved by the Health Sciences Institutional Review Board of the University of Wisconsin.
Results
Cohort characteristics
155 patients were diagnosed with DMII preoperatively. The mean preoperative body mass index was 47.2 kg/m2 (Table 1). 80 % (n = 124) underwent RYGB and 20 % (n = 31) had LSG performed. 84.5 % of the cohort had a diagnosis of hypertension, 66.5 % had obstructive sleep apnea, and 63.2 % had hyperlipidemia.
Utilization of oral antihyperglycemic medications
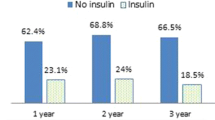
Preoperatively, 51.6 % of patients were on one oral antihyperglycemic medication, 14.2 % were on two, and 6.4 % were on three or more medications (Fig. 1). Of the 43 patients (27.7 %) on no oral medications preoperatively, 22 were on insulin, while 21 were managed by diet and lifestyle changes alone. At discharge and 90 days postoperatively, approximately 50 % took no oral medications while the remaining 50 % took only one antidiabetic medication. At one year postoperatively, 85.2 % were taking no oral medications, 14.2 % took one medication and 0.6 % were taking two medications. Of the patients taking no oral medications at the one-year time point, 7.6 % (n = 10) were insulin dependent.
Utilization of insulin
The mean total daily units of insulin required decreased from 26.4 U preoperatively to 10.4 U on discharge for long-acting insulin and from 19.3 to 1.7 U for short-acting insulin (Fig. 2a). 36.1 % of insulin-dependent patients were taking long-acting insulin, and 34.2 % were taking short-acting insulin preoperatively (Fig. 2b). At discharge and 90 days, approximately 30 % of patients were taking long- and short-acting insulin. There was a greater than 50 % reduction in long-acting insulin dose at discharge for 93.3 % RYGB patients and 81.8 % LSG patients. Similarly, 90.6 % RYGB patients and 100 % LSG patients experienced a greater than 50 % reduction in short-acting insulin dose (Table 2). At one-year follow-up, 9.0 % required long-acting insulin with a mean of 3.7 U daily and 8.4 % remained on short-acting insulin with a mean of 1.7 U daily.
Inpatient glycemic control for gastric bypass vs. sleeve gastrectomy patients (Table 2)
Optimal glucose control (<180 mg/dL) was achieved in 36.3 % (n = 45) patients undergoing laparoscopic RYGB and 48.4 % (n = 15) LSG patients. There were nine episodes of hypoglycemia (<70 mg/dL) in seven patients. All were self-limited episodes that resolved with either observation or oral glucose administration. The overall incidence of hypoglycemia was low in both groups, 4.0 % for RYGB and 6.5 % for LSG.
Adherence to ASMBS recommendations (Table 2)
Secretagogues were discontinued postoperatively in 97.6 % of RYGB patients and 96.8 % of LSG patients. Metformin and incretins were continued in 75 % of bypass and 60 % of sleeve patients. A combination of short- and long-acting insulin use was 35.4 % for RYGB and 43.8 % for LSG. Of note, endocrinology consultations were not obtained for difficult to control diabetics. Our institutional experience has demonstrated that medical consultants are often not accustomed to the rapid rate of diabetes resolution in bariatric surgery patients and may be overly aggressive with glucose correction.
Surgical outcomes and costs for patients with non-optimal vs. optimal glycemic control (Table 3)
Patient follow-up at 90 days was 100 %, while follow-up at one year was 58 %. There were no differences in ninety-day outcomes between patients with optimal and non-optimal glucose control in the perioperative period. At one-year postoperatively, excess weight loss was identical for both cohorts at 52 %. Fifty patients had HgBA1C checked at one year postoperatively. The mean HgBA1C decreased from 7.4 to 6.1. 70.1 % of patients met criteria for resolution of DMII (73.4 % of RYGB patients versus 53.9 % of LSG patients). Diabetes resolution at 1 year was statistically significant and better in patients with optimal perioperative glucose control (96.7 %) compared to those with non-optimal control (53.2 %, p < 0.001). One-year inpatient, outpatient, emergency room, and total costs for optimally and non-optimally glucose controlled patients were similar.
Discussion
Our data indicate that the effect of bariatric surgery on diabetes control is rapid and significant. Over 90 % of RYGB patients and 80 % of LSG patients had a greater than 50 % decrease in long-acting insulin requirements upon discharge following surgery. Despite the decrease in antihyperglycemic medication needs, relatively few patients achieved “optimal” glycemic control in the post-op period as defined by the ASMBS with approximately one third of RYGB patients and half of LSG patients achieving the ASMBS target of < 180 mg/dL during their hospital stay. Optimal glycemic control was not associated with improved ninety-day or one-year outcomes or costs. However, optimal perioperative glucose control was a strong marker for diabetes resolution at one year. Nearly, all patients with preoperative glucose control had DMII resolution at one year, while only half of the non-optimally controlled patients experienced resolution.
As early as 1995, it was recognized that the effect of bariatric surgery on diabetes resolution was rapid with a ten-fold reduction of insulin doses in some patients one day following surgery [13]. In that respect, it is surprising that the postoperative glycemic response of patients following bariatric surgery has been relatively poorly characterized to date. Most guidelines for tight perioperative glucose control are based on research from other fields in general surgery where hyperglycemia is clearly associated with increased morbidity after surgery [14, 15].
While obese patients face a higher risk of developing complications such as wound infection after general surgery [16, 17], the same trend has not been observed in obese patients undergoing bariatric surgery [18]. According to our data and that of one other group, tight perioperative glucose control does not appear to improve overall 30-day postoperative outcomes [19]. One partial explanation for this clinical difference is that bariatric surgery itself is a better treatment for diabetes in obese patients than medical therapy with oral antihyperglycemic agents and insulin [20]. In this light, there is a need for a perioperative glucose management protocol that is specific to the bariatric population. Perhaps higher postoperative glucose levels should be tolerated in bariatric surgery patients and blood glucose should be used as more of a prognostic indicator for future resolution of diabetes.
While ASMBS guidelines have represented a helpful starting point for perioperative glucose control in bariatric surgery patients, further investigation is necessary to provide a more clinically relevant regimen. ASMBS recommendations regarding oral medication management are clear and were well implemented by our group. Sulfonylureas and meglitinides were discontinued in greater than 90 % of RYGB and LSG patients postoperatively, while metformin and incretin use was continued in 75 % of RYGB patients and 60 % of LSG patients. ASMBS recommendations for insulin use are less well defined, simply stating that a combination of long- and short-acting insulin should be used in the postoperative period. Our adherence to this criterion was low, as it was our practice to start long-acting insulin in only patients taking long-acting insulin preoperatively. While numerical parameters for insulin adjustment were not stated by the ASMBS, a recent study indicates that a 75 % dose reduction of short-acting insulin in gastric bypass patients is safe and that long-acting insulin can effectively be replaced with glimepiride [21]. Our data suggests that insulin-dependent patients experienced a mean 61 % decrease in short-acting insulin dose and a mean 91 % decrease in long-acting insulin dose.
The long-term implications of perioperative glucose control in bariatric surgery are also significant. Previous studies have demonstrated that patients with poorly controlled diabetes preoperatively (HgBA1c > 8.0) are less likely to achieve euglycemia in the postoperative period and have the lowest rates of diabetes resolution 18 months after bariatric surgery [22]. Another group found that gastric bypass patients placed on a strict protocol designed to achieve optimal perioperative glucose control experienced a higher rate of diabetes resolution at one year [19], though a small pilot randomized controlled trial performed by the same group was not able to demonstrate the same trend [23]. Our data also indicate that optimal glucose control in the postoperative period after bariatric surgery is associated with diabetes resolution at one year. Larger prospective trials would be necessary to confirm the association with optimal perioperative glucose control with long-term diabetes resolution.
Our study has several limitations. It is a retrospective study with a limited sample size. Long-term data were also restricted by a one-year follow-up rate of 58 %. Our comparison of short- and long-term complications among optimal and non-optimal glucose control patients was not statistically significant, which is not surprising given that the rate of major postoperative complications following bariatric surgery is less than 5 % [24, 25]. Another limitation is our definition of diabetes resolution using HgBA1C < 6.5 mg/dL as a marker for cure of diabetes. A 2009 consensus by the American Diabetes Association established more stringent criteria for diabetes resolution, defining partial remission as HgBA1C < 6.5 mg/dL and fasting glucose < 100-125 mg/dL and complete remission as HgBA1c < 6.0 mg/dL and fasting glucose < 100 mg/dL in the absence of pharmacologic therapy for one year [26]. Using the above criteria, our rates of diabetes resolution for RYGB and LSG would be lower. Lastly, mean units of short-acting insulin administered as an outpatient could not be calculated for patients on a variable insulin sliding scale as this information was not regularly charted by patients.
In conclusion, understanding the perioperative glucose response of obese, diabetic patients to bariatric surgery is clinically significant in terms of establishing more concrete protocols for managing postoperative hyperglycemia in this unique patient population and with regard to predicting long-term diabetes resolution. Further research needs to be performed on the optimal management of oral medications and insulin in bariatric surgery patients postoperatively. In particular, defining a safe and effective range for decrease in short- and long-acting insulin doses would be useful clinically. Lastly, larger scale, prospective studies are necessary to determine if the link between optimal perioperative glucose control and long-term diabetes resolution is durable.
References
Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA 311:806–814
Centers for Disease Control Diabetes Translation (2015) Maps in trends of diagnosed diabetes and obesity. Available at: http://www.cdc.gov/. January 2015; Accessed 13 Jan 2016
World Health Organization (2003) Obesity and overweight fact sheet. Available at: http://www.who.int/. 2003; Accessed 13 Jan 2016
Buchwald H, Yoav A, Eugene B, Michael JD, Walter P, Kyle F, Karen S (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1728
Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM (2011) Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 146:143–148
Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N (2010) Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc 24:1005–1010
Rubino F, Schauer PR, Kaplan LM, Cummings DE (2010) Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 61:393–411
Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S (2013) Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 9:159–191
American Diabetes Association (2016) Classification and diagnosis of diabetes. Standards of Medical Care in Diabetes. Diabetes Care 39(Suppl. 1):S13–S22. doi:10.2337/dc16-S005
O’Mara NB (2009) How to Switch Between Insulin Products. Available at: http://portal.mah.harvard.edu/cms/. October 2009; Accessed 4 Feb 2016
National Diabetes Information Clearinghouse (2008). Hypoglycemia. Available at: http://www.niddk.nih.gov/health-information/. October 2008; Accessed 9 Feb 2016
American College of Surgeons (2012). Surgeon Reference Information for National Surgical Quality Improvement Program (NSQIP). Available at: https://www.facs.org/quality-programs/acs-nsqip. March 2012; Accessed 10 Feb 2016
Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L (1995) Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350
Jackson RS, Amdur RL, White JC, Macsata RA (2012) Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg 214:68–80
Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO (2008) Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 248:585–591
Merkow RP, Bilimoria KY, McCarter MD, Bentrem DJ (2009) Effect of body mass index on short-term outcomes after colectomy for cancer. J Am Coll Surg 208:53–61
Regner JL, Mrdutt MM, Munoz-Maldonado Y (2015) Tailoring surgical approach for elective ventral hernia repair based on obesity and National Surgical Quality Improvement Program outcomes. Am J Surg 210:1024–1030
Lyons T, Neff KJ, Benn J, Chuah LL, le Roux CW, Gilchrist M (2014) Body mass index and diabetes status do not affect postoperative infection rates after bariatric surgery. Surg Obes Relat Dis 10:291–297
Fenske WK, Pournaras DJ, Aasheim ET, Miras AD, Scopinaro N, Scholtz S, le Roux CW (2011) Can a protocol for glycaemic control improve type 2 diabetes outcomes after gastric bypass? Obes Surg 22:90–96
Schauer PR, Bhatt DL, Kashyap SR (2014) Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med 370:2002–2013
Cruijsen M, Koehestani P, Huttjes S, Leenders K, Janssen I, de Boer H (2014) Perioperative glycaemic control in insulin-treated type 2 diabetes patients undergoing gastric bypass surgery. Neth J Med 72:202–209
Perna M, Romagnuolo J, Morgan K, Byrne TK, Baker M (2012) Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis 8:685–690
Chuah LL, Miras AD, Papamargaritis D, Jackson SN, Olbers T, le Roux CW (2015) Impact of perioperative management of glycemia in severely obese diabetic patients undergoing gastric bypass surgery. Surg Obes Relat Dis 11:578–584
Stenberg E, Szabo E, Agren G, Näslund E, Boman L, Bylund A, Hedenbro J, Laurenius A, Lundegårdh G, Lönroth H, Möller P, Sundbom M, Ottosson J, Näslund I, Scandinavian Obesity Surgery Registry Study Group (2014) Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Ann Surg 260:1040–1047
Longitudinal Assessment of Bariatric Surgery (LABS), Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, Pories W, Courcoulas A, McCloskey C, Mitchell J, Patterson E, Pomp A, Staten MA, Yanovski SZ, Thirlby R, Wolfe B (2009) Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361:445–454
Buse JB, Caprio S, Cefalu WT (2009) How do we define cure of diabetes? Diabetes Care 32:2133–2135
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jessica A. Zaman, Neil Shah, Glen E. Leverson, Jacob A. Greenberg and Luke Funk have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zaman, J.A., Shah, N., Leverson, G.E. et al. The effects of optimal perioperative glucose control on morbidly obese patients undergoing bariatric surgery. Surg Endosc 31, 1407–1413 (2017). https://doi.org/10.1007/s00464-016-5129-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5129-x