Abstract
Background
Postoperative pancreatic fistula (POPF) is one of the major complications after laparoscopic gastrectomy (LG). We investigated the impact of the anatomical location of the pancreas, especially in relation to the suprapancreatic lymph nodes, on the incidence of POPF after LG.
Methods
We retrospectively reviewed the preoperative computed tomography (CT) images of 246 patients who underwent LG with the suprapancreatic lymph node dissection between November 2008 and November 2015. The length between the levels of the pancreatic body surface and the root of the common hepatic artery (LPC) was measured on a CT image with an axial view. A receiver operating characteristics (ROC) curve analysis was performed to determine the cutoff LPC value. A multivariate analysis was performed to determine the predictive factors for POPF.
Results
POPF occurred in 11 patients (4.5 %). The median LPC was significantly longer in the patients with POPF than in those without (26 mm vs. 21 mm, p = 0.026). The ROC curve analysis revealed that the optimal cutoff LPC value for predicting POPF was 25 mm. The POPF rate was significantly higher in the long LPC group than in the short LPC group (10 vs. 1.3 %, p = 0.002). A multivariate analysis demonstrated that a long LPC (p = 0.018) and dissection of the lymph nodes along the distal splenic artery (p = 0.042) were independent predictors of POPF. The amylase level in the drainage fluid on postoperative day 1 was significantly higher in the long LPC group than in the short LPC group.
Conclusions
The LPC is a simple and reliable predictor of POPF after LG. Surgeons should take the anatomical location of the pancreas into consideration when performing LG with suprapancreatic lymph node dissection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since its introduction, laparoscopic gastrectomy (LG) has widely prevailed as the treatment for early gastric cancers with a relatively low risk of lymph node metastasis, especially in Japan and Korea [1]. A number of trials have demonstrated several advantages of LG over open gastrectomy (OG), including better cosmetic results, less pain, earlier recovery of bowel activities, shorter hospital stay, and the early improvement in postoperative quality of life [2–4]. Recently, the indications for LG have been extended to advanced gastric cancer, despite a lack of solid evidence regarding the long-term oncological outcomes [5, 6]. At the present time, the Japanese guidelines allow for the performance of LG as an investigational treatment for early gastric cancer [7].
LG with radical lymphadenectomy involves the dissection of the suprapancreatic lymph nodes. Suprapancreatic lymph node dissection is associated with a high degree of technical difficulty and may cause postoperative pancreatic fistula (POPF). Once POPF develops, it sometimes contributes to other major complications, including bleeding, anastomotic leakage, and intra-abdominal abscess. Although the incidence of POPF after LG is reportedly comparable with that after OG [8], several investigators have suggested that POPF may occur more frequently after LG [9, 10]. During suprapancreatic lymph node dissection, it is necessary to reach over the pancreatic body to remove the lymph nodes; in order to do this, it is necessary to compress and retract the pancreatic body. These procedures have the potential to result in POPF. We therefore hypothesized that the anatomical location of the pancreas, especially in relation to the suprapancreatic lymph nodes, might be associated with the incidence of POPF after LG. In the present study, we evaluated the anatomical location of the pancreas on a computed tomography (CT) image with an axial view and investigated its impact on the incidence of POPF in patients who underwent LG.
Materials and methods
Study design
Between November 2008 and November 2015, 295 patients with gastric cancer underwent LG with lymphadenectomy at Nara Medical University Hospital. The medical records and operative reports of all patients were retrospectively reviewed. Then, the following patients were excluded: patients for whose medical records did not include a measurement of the total amylase concentration in the drainage fluid (d-AMY; n = 13); patients in whom no suprapancreatic lymph nodes were dissected (n = 28); and patients who underwent conversion to open surgery (n = 8). After excluding the above-mentioned patients, 246 patients remained and were analyzed in the present study. Written informed consent was obtained from all patients.
The following procedures were performed: laparoscopy-assisted distal gastrectomy (LADG; n = 156), laparoscopy-assisted pylorus-preserving gastrectomy (LAPPG; n = 27), laparoscopy-assisted total gastrectomy (LATG; n = 32), laparoscopy-assisted proximal gastrectomy (LAPG; n = 16), and laparoscopy-assisted pylorus-preserving nearly total gastrectomy (LAPPNTG) [11] (n = 15). The clinical findings were confirmed on the basis of an upper gastrointestinal study, gastrointestinal endoscopy, endoscopic ultrasonography, and CT, according to the third edition of the Japanese classification of gastric carcinoma [12]. In our institution, the indications for LADG are a preoperatively diagnosed depth of tumor invasion of T1–T3 with N0 or N1 lymph node metastasis. In principle, the indication for LAPPG, LATG, LAPG, and LAPPNTG was the presence of a clinical T1N0 tumor.
The factors that were investigated included the patients’ age, gender, presence of cardiovascular disease, diabetes mellitus and chronic renal failure, American Society of Anesthesiologists score, body mass index (BMI), and the clinical tumor stage. The surgical findings included the type of resection, the use of combined organ resection, the extent of lymph node dissection, the duration of the operation, the amount of operative blood loss, and the use of perioperative transfusion. We also investigated the incidence of postoperative complications. Postoperative complications were defined as complications which occurred within 30 days of surgery or during the period of hospitalization and were graded according to the Clavien–Dindo classification system [13].
Evaluation of the anatomical characteristics of the pancreas
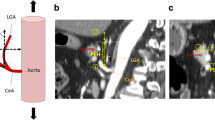
We reviewed the preoperative abdominal CT images of all of the patients. We first identified the top of the pancreatic body surface and the root of the common hepatic artery (CHA) on a CT image with an axial view. We then measured the length between the levels of the two points (LPC; Fig. 1A). When these two points were not located on the same slice, the root of the CHA was marked and the length between the levels of the two points was measured (Fig. 1B). We also measured the length between the levels of the top of the pancreatic body surface and the abdominal aorta (LPA) and the maximal thickness of the pancreatic body.
Measurement of the length between the levels of the pancreatic body surface and the root of the common hepatic artery (LPC) and the abdominal aorta (LPA). A Top of the pancreatic body surface and the root of the common hepatic artery (CHA) were identified on a CT image with an axial view. The LPC and LPA were then measured. B In cases where the top of the pancreatic body surface and the root of the CHA were not located on the same slice, the root of the CHA was marked, and the LPC was then measured
Surgical procedures
The type of gastrectomy and the extent of lymph node dissection were decided according to the Japanese gastric cancer treatment guideline version 3 [7]. Five or six ports were used. Lymph node dissection was generally carried out with the use of ultrasonically activated coagulating shears (SonoSurg; Olympus, Tokyo, Japan). In LADG and LAPPG, patients with T1 and N0 tumors underwent D1 plus lymph node dissection. In some patients, the lymph nodes along the proximal splenic artery (no. 11p) were also dissected. Patients with T2–T3 or N1 tumors underwent LADG with D2 lymph node dissection. In LATG, LAPG, and LAPPNTG, D1 plus lymph node dissection was principally performed. In some patients, the dissection of lymph nodes along the distal splenic artery (no. 11d) was also performed based on the surgeon’s decision. Regarding the suprapancreatic lymph nodes, the lymph nodes along the common hepatic artery (no. 8a) and at the base of the celiac artery (no. 9) were dissected in all 246 patients. The lymph nodes along the proximal splenic artery (no. 11p) and along the distal splenic artery (no. 11d) were dissected in 219 (89 %) and 9 (3.7 %) patients, respectively.
In LADG and LAPPG, a closed drain was routinely placed in the nearby suprapancreatic area. In LATG, LAPG, and LAPPNTG, a closed drain was placed around the dorsal side of the esophagojejunostomy through the space of the suprapancreatic area. On postoperative day (POD) 1, the drain fluid was analyzed to determine the d-AMY level.
Definition of POPF
POPF was diagnosed based on the definition of the International Study Group for Pancreatic Fistula (ISGPF) with some modifications [14] because the drain was removed before POD 3 in many of the patients. Briefly, POPF was diagnosed when the patients fulfilled the following criteria: (1) a d-AMY level on POD 1 of more than three times the upper normal serum value (normal serum value: 44–132 U/l) and (2) no evidence of anastomotic leakage. The severity of POPF was graded, according to the ISGPF definition, as follows: grade A, ‘transient fistula’ (no clinical impact); grade B (requires a change in management or adjustment of the clinical pathway); and grade C (requires a major change in clinical management or deviation from the normal clinical pathway) [14]. In the present study, grades B and C were regarded as clinically significant POPF.
Statistical analysis
The categorical variables are presented as numbers and percentages. Groups were compared using the Chi-squared test or Fisher’s exact test. Continuous variables are expressed as medians and ranges, and the medians were compared using the Mann–Whitney U test. To evaluate the sensitivity and specificity of the LPC and LPA for detecting the development of POPF, receiver operating characteristics (ROC) curves were calculated, and the Youden index was estimated to determine the optimal cutoff values [15]. All variables with a p value of <0.1 in the univariate analysis were entered into a multivariate analysis. The multivariate analysis used a logistic regression model to investigate the factors associated with the incidence of POPF. p values of <0.05 were considered to indicate statistical significance. The statistical analyses were performed using the SPSS software program (version 19.0; SPSS, Chicago, IL).
Results
Postoperative complications
The overall rate of postoperative complications was 19.9 %. POPF occurred in 11 patients (4.5 %), including grade B (n = 10) and grade C (n = 1). Grade II, IIIa, and IIIb complications occurred in eight patients, two patients, and one patient, respectively. No mortalities were observed.
Risk factors for POPF
The associations between the patient characteristics, the perioperative data, and POPF are shown in Table 1. POPF only occurred in male patients. The median amount of operative blood loss was significantly greater in the patients with POPF than in those without (p = 0.037). POPF tended to occur more frequently in the patients who underwent the dissection of station no. 11d than in those who did not (p = 0.055).
Table 2 shows the anatomical characteristics of the pancreas and POPF. The median LPC and LPA of all patients were 22 mm (range 6–40 mm) and 39 mm (range 12–56 mm), respectively. The median LPC (p = 0.026) and LPA (p < 0.001) were significantly longer in the patients with POPF than in those without. There was no significant difference between the groups in the thickness of the pancreatic body. Using the incidence of POPF as an endpoint, the areas under the curve for the LPC and LPA were found to be 0.699 and 0.803, respectively (Fig. 2). When the LPC was 25 mm, the Youden index was maximal, with a sensitivity of 81.8 % and a specificity of 65.5 %. When the LPA was 43 mm, the Youden index was maximal, with a sensitivity of 81.8 % and a specificity of 68.5 %. Therefore, the cutoff values of the LPC and LPA were set at 25 mm and 43 mm, respectively. POPF occurred more frequently in the patients with an LPC of ≥25 mm (p = 0.002) and an LPA of ≥43 mm (p = 0.001; Table 2).
The multivariate analysis demonstrated that the LPC (p = 0.018) and the dissection of station no. 11d (p = 0.042) were independent risk factors for POPF (Table 3). The LPA was also identified as an independent predictor of POPF according to another multivariate analysis.
The influence of the cranial–caudal relationship between the pancreas and the suprapancreatic lymph nodes on the incidence of POPF
We further investigated the cranial–caudal relationship between the pancreas and the suprapancreatic lymph nodes, and evaluated its influence on the incidence of POPF. The top of the pancreatic body surface and the root of the CHA were located on the same CT slice in 87 (35.4 %) patients (group A) (Fig. 1A); these two points were located on different slices in 159 (64.6 %) patients (group B; Fig. 1B). Among the group B patients, the root of the CHA was located cranial to the pancreatic body in 155 patients and caudal to the pancreatic body in four patients. Overall, there was no difference in the POPF rates of the patients in groups A and B (5.7 vs. 3.8 %, p = 0.474). However, the incidence of POPF was significantly higher in the group A patients with an LPC of ≥25 mm than in other patients. The POPF rate was 2.1 % in the group B patients with an LPC of <25 mm, 0 % in the group A patients with an LPC of <25 mm, 6.3 % in the group B patients with an LPC of ≥25 mm, and 18.5 % in the group A patients with an LPC of ≥25 mm (p = 0.001).
The relationships between the LPC and the clinical and perioperative data
We finally investigated the relationship of the LPC with the clinical and perioperative findings (Table 4). The patients with an LPC of ≥25 mm were significantly more likely to have a BMI of ≥25 kg/m2 (p = 0.027), a longer duration operation (p = 0.03), and a greater amount of operative blood loss (p = 0.004) in comparison with those with an LPC of <25 mm. The median d-AMY level on POD 1 was significantly higher in the patients with an LPC of ≥25 mm than in those with an LPC of <25 mm (p = 0.003). In addition, a significant association was found between the LPC and the LPA (p < 0.001).
Discussion
In the present study, we evaluated risk factors for POPF after LG, focusing on the anatomical location of the pancreas, especially on its relationship with the suprapancreatic lymph nodes. The incidence of POPF after LG is reported to range from 1.7 to 7.2 % [6, 8–10, 16, 17]. In the present study, the POPF rate was 4.5 %. Some authors have compared the incidence of POPF between LG and OG. Obama et al. reported that the POPF rate after LG was 7.2 %, while that after OG was 2.1 % [9]. Fujita et al. [10] reported that the POPF rate after LG was 5.3 %, while that after OG was 0 %. Although there were no significant differences between the groups in the rate of POPF, the d-AMY level after LG was significantly higher than that after OG in both studies. This indicates that potential risk of POPF development may be higher after LG than after OG. Previous studies have shown various risk factors for POPF in LG, including male gender, age, high BMI, operative time, and the number of retrieved lymph nodes; these risk factors are similar to those reported in OG [16–19]. However, the risk factors for POPF after LG may differ from those after OG. In the present study, the anatomical relationship between the pancreas and the suprapancreatic lymph nodes was evaluated on a CT image with an axial view in the patients who underwent LG. We first identified the top of the pancreatic body surface and the root of the CHA, as a landmark of the suprapancreatic lymph nodes. Then, the length between the levels of these two points (LPC) was measured. We found that the incidence of POPF was significantly higher in the patients with an LPC of ≥25 mm than in those with an LPC of <25 mm. Importantly, the multivariate analysis demonstrated that a long LPC was an independent predictor of POPF after LG. We further measured the LPA, as another marker of the anatomical location of the pancreas, which could be obtained more easily. We observed a significant correlation between the LPC and the LPA. A long LPA was also an independent predictor of POPF in another multivariate analysis. These findings indicate that the LPC and LPA can predict POPF after LG.
The present study clearly demonstrated that the patients with a long LPC had a greater degree of pancreatic damage than those with a short LPC. The disadvantages of LG, in comparison with OG, include the limited mobility of long straight forceps and inconvenient surgical positioning [20]. In addition to thermal injuries which occur in association with the use of energized devices, the compression and mobilization of the pancreas may cause parenchymal damage to the pancreas, potentially resulting in POPF [9, 10, 21]. In order to create a better surgical field and dissect the posterior side of the suprapancreatic lymph nodes, some degree of pancreatic body compression may be unavoidable [9]. In the present study, we found that the d-AMY level on POD 1 was significantly higher in the patients with a long LPC than in those with a short LPC. These findings indicate that the patients in whom the pancreatic body was located more ventral to the suprapancreatic lymph nodes might suffer more excessive pancreatic damage during suprapancreatic lymph node dissection from the operating surgeon’s and assistant’s forceps.
In the present study, the impact of the cranial–caudal relationship between the pancreas and the suprapancreatic lymph nodes on the incidence of POPF was also evaluated. Among the long LPC group, the patients in whom the top of the pancreatic body surface and the root of the CHA were located on the same CT slice were found to have a significantly higher POPF rate than the other patients. These results suggest that the potential for damage to the pancreas may be highest if the LPC is long and in cases in which the two points are located on the same slice.
In addition, the present study showed that the operations of the patients in the long LPC group were more complicated than those of the patients in the short LPC group. In the long LPC group, the operation time was significantly longer and the amount of operative blood loss was significantly greater than in the short LPC group. Furthermore, the patients in the long LPC group more frequently had a BMI of ≥25 kg/m2 than the short LPC group. In obese patients, the borderline between the upper edge of the pancreas and the fat tissue is not clear, and bleeding and lymph leakage can easily occur from the cut edge of the fat tissue. Thus, suprapancreatic lymph node dissection is sometimes technically difficult, and the chance of injuring the pancreas may be increased in obese patients [22]. Indeed, previous studies have identified a high BMI as the risk factor for POPF after LG [16, 17]. These results suggest that the LPC may reflect the likelihood of surgical difficulties during suprapancreatic lymph node dissection and that a long LPC therefore increases the risk of POPF, although the precise mechanisms for this remain unclear.
Given the higher rate of POPF, some measures to prevent this complication are required in patients with a long LPC. One measure may be to insert an additional port for the operating surgeon’s right hand at a level close to the upper edge of the pancreatic body, during suprapancreatic lymph node dissection. The additional port might reduce the compression and thermal damage caused to the pancreas by the surgeon’s instruments. In addition, an increase in the pneumoperitoneum pressure and the extreme rotation of the operative table may improve the surgical view of the suprapancreatic lymph node area [23]. It is, of course, important to exercise the utmost care in order to avoid injury to the pancreas [16, 18]. Another measure to prevent POPF may be the use of a surgical robot. Surgical robots have been developed to overcome some of the disadvantages of the laparoscopic surgery [21, 24]. Surgical robots provide a greater degree of freedom with articulating surgical instruments and filter the tremor of the surgeon’s hands. Suda et al. [21] demonstrated no cases of POPF in 88 robotic gastrectomies and found that the incidence of POPF was significantly higher in LG than in robotic gastrectomy. Seo et al. [20] also showed that POPF occurred more frequently after LG than after robotic gastrectomy and that LG was an independent risk factor for POPF. The use of surgical robots may therefore reduce the damage and injury of the pancreas and prevent POPF after gastrectomy. Further trials are needed to clarify whether the use of surgical robots can reduce POPF after gastrectomy.
The extent of lymphadenectomy could directly affect the incidence of POPF. A previous report identified the dissection of the lymph nodes along the distal splenic artery as a risk factor for POPF after open TG [18]. Similarly, the present study found that the dissection of these lymph nodes was an independent risk factor for POPF, although no association between the dissection of the lymph nodes along the proximal splenic artery and the incidence of POPF was found. Surgeons should pay more attention to the incidence of POPF when dissecting these lymph nodes.
The present study is associated with some limitations. First, the present study was a retrospective analysis which used preoperative CT images. We could not evaluate the individual port position and surgical view under pneumoperitoneum. These factors may have affected the extent of the compression and mobilization of the pancreas during suprapancreatic lymph node dissection. Second, the present study did not measure the exact cranial–caudal length between the top of the pancreatic body surface and the root of the CHA. This could influence the incidence of POPF. A combined axial and sagittal view may allow us to evaluate the more precise relationship between the pancreas and the suprapancreatic lymph nodes. Nevertheless, our results have suggested that the LPC and LPA were easily obtained and that they were reliable predictors of POPF after LG. Further large-scale investigations are required to validate our results.
In conclusion, the present study demonstrated the significant involvement of the anatomical location of the pancreas in the incidence of POPF after LG. The LPC and LPA are easily obtained and were found to be reliable predictors of this complication. Surgeons should take the anatomical location of the pancreas into consideration when performing LG with the suprapancreatic lymph node dissection.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y (2002) A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131:S306–S311
Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM (2008) Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 248:721–727
Lee JH, Yom CK, Han HS (2009) Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 23:1759–1763
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A (1999) Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer 2:230–234
Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S (2015) A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 39:2734–2741
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K (2010) Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 13:238–244
Obama K, Okabe H, Hosogi H, Tanaka E, Itami A, Sakai Y (2011) Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications. Surgery 149:15–21
Fujita T, Ohta M, Ozaki Y, Takahashi Y, Miyazaki S, Harada T, Iino I, Kikuchi H, Hiramatsu Y, Kamiya K, Konno H (2015) Collateral thermal damage to the pancreas by ultrasonic instruments during lymph node dissection in laparoscopic gastrectomy. Asian J Endosc Surg 8:281–288
Takayama T, Matsumoto S, Wakatsuki K, Tanaka T, Migita K, Ito M, Nakajima Y (2014) Novel laparoscopic procedure for treating proximal early gastric cancer: laparoscopy-assisted pylorus-preserving nearly total gastrectomy. Surg Today 44:2332–2338
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Perkins NJ, Schisterman EF (2006) The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163:670–675
Jiang X, Hiki N, Nunobe S, Kumagai K, Nohara K, Sano T, Yamaguchi T (2012) Postoperative pancreatic fistula and the risk factors of laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Surg Oncol 19:115–121
Miyai H, Hara M, Hayakawa T, Takeyama H (2013) Establishment of a simple predictive scoring system for pancreatic fistula after laparoscopy-assisted gastrectomy. Dig Endosc 25:585–592
Katai H, Yoshimura K, Fukagawa T, Sano T, Sasako M (2005) Risk factors for pancreas-related abscess after total gastrectomy. Gastric Cancer 8:137–141
Miki Y, Tokunaga M, Bando E, Tanizawa Y, Kawamura T, Terashima M (2011) Evaluation of postoperative pancreatic fistula after total gastrectomy with D2 lymphadenectomy by ISGPF classification. J Gastrointest Surg 15:1969–1976
Seo HS, Shim JH, Jeon HM, Park CH, Song KY (2015) Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res 194:361–366
Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I (2015) Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 29:673–685
Kitano S (2009) What technique is suitable for laparoscopic suprapancreatic lymph node dissection? Gastric Cancer 12:67–68
Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M (2003) Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg 238:680–685
Boone J, Schipper ME, Moojen WA, Borel Rinkes IH, Cromheecke GJ, van Hillegersberg R (2009) Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 96:878–886
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kazuhiro Migita, Sohei Matsumoto, Kohei Wakatsuki, Masahiro Ito, Tomohiro Kunishige, Hiroshi Nakade, Mitsuhiro Nakatani, Mutsuko Kitano, and Yoshiyuki Nakajima have no competing interests or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Migita, K., Matsumoto, S., Wakatsuki, K. et al. The anatomical location of the pancreas is associated with the incidence of pancreatic fistula after laparoscopic gastrectomy. Surg Endosc 30, 5481–5489 (2016). https://doi.org/10.1007/s00464-016-4909-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4909-7