Abstract
Background
Laparoscopic gastrectomy is more frequently associated with postoperative pancreatic fistula than is open gastrectomy. We assumed that compression of the pancreas with various devices to obtain a proper operative view is associated with the higher incidence of PF in LG and that the extent of the compression differs depending on the anatomical position of the pancreas. The present study aimed to elucidate the correlation between the anatomical position of the pancreas and PF after LG for gastric cancer.
Methods
Patients who underwent LG for gastric cancer from 2005 to 2019 were retrospectively reviewed. Two anatomical parameters representing the height of the slope looking down the celiac artery from the top of the pancreas (P-A length) and the steepness of the slope (UP-CA angle) were measured in computed tomography sagittal projections. The correlation between PF and (1) P-A length, (2) UP-CA angle, and (3) other clinicopathological factors was analyzed using a logistic regression model.
Results
Among 3485 patients, grade ≥ II PF was observed in 140 (4.0%) patients. The UP-CA angle [odds ratio (OR), 2.472; 95% confidence interval (CI), 1.725–3.543; P < 0.001], a high BMI (OR 2.339; 95% CI 1.634–3.348; P < 0.001), and male sex (OR 2.602; 95% CI 1.590–4.257; P < 0.001) were independently correlated with grade ≥ II PF.
Conclusions
The present study identified a significant correlation between anatomical position of the pancreas and PF after LG. High BMI and male sex were also significantly correlated with PF after LG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have reported the advantages, such as less intraoperative bleeding, less pain, and a shorter postoperative hospital stay, of laparoscopic gastrectomy (LG) for gastric cancer compared with open gastrectomy [1,2,3]. Furthermore, the results of randomized controlled trials (RCTs) have established LG as a standard treatment option for stage I gastric cancer [4, 5]. Some drawbacks of LG have been reported, such as a longer operation time as well as greater difficulty in patients with a high amount of body fat. One of the limitations of LG is the relatively high incidence of postoperative pancreatic fistula (PF). Although an RCT with well-fit patients showed non-inferiority of LG to open gastrectomy with respect to the incidence of PF, real-world evidence from a Japanese nationwide survey showed that LG for gastric cancer was more frequently accompanied by postoperative PF compared with open gastrectomy [6, 7].
In standard surgery for gastric cancer, suprapancreatic lymph node dissection is mandatory, and the pancreas must be pulled caudally to gain access to the area. In LG, the celiac artery and its branches have to be visualized over the pancreas with a laparoscope inserted in the umbilicus. We previously conducted a pilot study that aimed to prove the correlation between the anatomical position of the pancreas and PF after laparoscopic distal gastrectomy (LDG). In the pilot study, PF of grade I or above, defined as “a drainage fluid amylase level of ≥ 3 times the upper limit of institutional normal on or after postoperative day 3” was used as a surrogate outcome. This was because the number of events of PF of grade II or above that required pharmacological, surgical, endoscopic, or radiological treatment was too small to be used as an outcome in multivariate logistic regression analysis. Another surrogate outcome used in the pilot study was postoperative overall complications of grade II or above, which included complications irrelevant to PF. Although the pilot study revealed that the anatomical position of the pancreas may be an independent predictor of PF in patients undergoing LDG for gastric cancer, whether pancreas position correlates with clinically significant PF was still unclear. Furthermore, the pilot study targeted only the patients who underwent LDG. Therefore, applicability of the pilot study results to other types of gastrectomy or influence of the type of gastrectomy on the occurrence of PF when the standardized procedure of LG in our institution was used was unclear [8].
Therefore, this study was performed to elucidate the correlation between the anatomical position of the pancreas and PF of grade II or above that required any treatment in patients who underwent LG for all types of gastrectomy.
Material and methods
Patient data
Patients who underwent LG for gastric cancer at the Cancer Institute Hospital from January 2005 to December 2019 were retrospectively reviewed. Patient data were retrieved from the patients’ hospital records and our institutional database. Patients in whom intraoperative conversion to open gastrectomy was performed for either an oncological reason or because of a technical problem were excluded from the analyses. In addition, patients who underwent simultaneous surgery for gastric cancer and another disease or splenectomy for gastric cancer were also excluded from the subsequent analyses. This is because the need for splenectomy is formally considered an indication for open gastrectomy in our institution, and only a few splenectomies were performed probationally during the introductory period of LG.
Clinical classification of the depth of the primary tumor (cT) and lymph node metastasis (cN) were determined by preoperative evaluations, namely upper gastrointestinal endoscopy, barium radiography, endoscopic ultrasonography, and computed tomography (CT). All tumors were histopathologically diagnosed as adenocarcinoma or signet ring cell carcinoma. Clinical stage cT1N0, cT2N0, or cT1N1 was generally considered an indication for LG in accordance with the Japanese gastric cancer treatment guidelines [9]. Some patients who had more advanced tumors underwent LG as part of an RCT comparing long-term survival between laparoscopic and open distal gastrectomy (UMIN000003420) or as part of two other single-institution prospective studies (UMIN000029317, UMIN000036621) [10]. Regarding lymphadenectomy, patients with cT1N0 cancer underwent D1 + , while D2 was performed for other patients.
Evaluation of preoperative general conditions and postoperative complications
Information on patient age, sex, body mass index (BMI), type of gastrectomy and reconstruction, operation time, intraoperative blood loss, and postoperative complications was extracted from the patient records and our institutional database. The severity of postoperative complications was graded using the Japan Clinical Oncology Group Postoperative Complications (JCOG PC) criteria, which provide detailed grading criteria for each postoperative complication in accordance with the general grading rules of the Clavien–Dindo classification [11, 12]. In the JCOG PC criteria, grade I PF is defined as “a drainage fluid amylase level on or after postoperative day 3 of ≥ 3 times the upper limit of the institutional reference range but without the need for intervention”. Therefore, PF was defined as “a drainage fluid amylase level of ≥ 396 IU on postoperative day 3” in this study, given that the upper limit of the reference range in our institution is 132 IU. In patients in whom the drainage fluid amylase level was not measured on or after postoperative day 3, the diagnosis of PF was made by comprehensive evaluation of symptoms and imaging findings.
Measurement of pancreas-related anatomical parameters
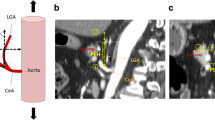
The anatomical position of the pancreas was measured according to the method established by Kumagai et al. [8]. “The height of the slope” looking down the root of the celiac artery from the top of the pancreas was evaluated by measuring the maximum length of the vertical line between the pancreas body surface and the aorta (P-A length), and “the steepness of the slope” was evaluated by measuring the angle between a line drawn from the upper border of the pancreas to the root of the celiac artery and the aorta (UP-CA angle) in sagittal projections of preoperative CT scans (Fig. 1).
Anatomical position of the pancreas and measurement of anatomical parameters related to the pancreas in sagittal projections on computed tomography scans. P, pancreas; C.A., celiac artery; P-A length, maximum length of the vertical line between the surface of the pancreas and the aorta; UP-CA angle, angle produced by the meeting of a line drawn from the upper border of the pancreas to the root of the celiac artery and the aorta
Surgery
Surgery was performed or assisted by one of five laparoscopic experts qualified by the Japan Society for Endoscopic Surgery or equivalent. The first port for the camera was placed on the umbilicus using the open technique, and 10–12 mmHg of capnoperitoneum was induced. A 10-mm 30° or 45° oblique rigid laparoscope was used, and four ports (each 5–12 mm) were placed in the right and left hypochondrium, and the right and left lumbar regions, respectively.
The port in the right lumbar region was placed on a line between the port in the right hypochondrium and the umbilicus, and most of the suprapancreatic lymphadenectomy was performed through this port using an energy device held in the operator’s right hand. Dissection procedures were mostly performed using a Sonicision™ 39 cm cordless ultrasonic dissector (Medtronic plc, Dublin, Ireland), HARMONIC ACE® + Shears (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA), or THUNDERBEAT energy device (Olympus Corp., Tokyo, Japan).
The operators stood on each side of the patient, and the scopist stood between the patient’s legs. The operation was performed in accordance with the systematized procedure established by Hiki et al. [13]. During approximately the first two-thirds of the study period, the assistant applied traction or compression on the pancreas using gauze or sponges during the suprapancreatic lymph node dissection.
After completion of lymphadenectomy, the stomach or esophagus was dissected with endostaplers, and reconstruction was performed as described previously [14].
Statistical analysis
All data are presented as the median (range) or as the number of patients. Statistical analyses were performed as described above for each analysis using SPSS, ver. 11.0 (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0.05. Univariate and multivariate logistic regression analyses were performed to evaluate factors that might be correlated with PF. Quartile values were used to determine the cut-off value for each factor or parameter in the logistic regression analysis. Lower quartiles were used for the albumin and prealbumin concentrations, and upper quartiles were used for the operation period, age, BMI, P-A length, and UP-CA angle. Factors or parameters with a P-value of < 0.1 in the univariate analysis were selected as covariables in the multivariate analysis. Multivariate logistic regression analysis with backward elimination for variable selection with α = 0.10 was performed, and the variance inflation factor (VIF) was used to measure the degree of multicollinearity among the variables.
Results
Patients analyzed
During the study period, LG for gastric cancer was attempted in a total of 3842 patients at the Cancer Institute Hospital. Intraoperative conversion to open gastrectomy was performed in 77 patients and these patients were excluded from the subsequent analyses. Simultaneous surgery for gastric cancer and other diseases was performed in 268 patients, and splenectomy for gastric cancer was performed in 12 patients; these patients were also excluded from the subsequent analyses. Ultimately, 3485 patients who underwent LG were analyzed.
Clinicopathological parameters and surgical data
Table 1 summarizes the patients’ characteristics and the anatomical parameters related to the pancreas. Notably, the anatomical position of the pancreas varied widely. Table 2 is a summary of surgical and histopathological data. Because the indication for LG was limited to cT1N0 gastric cancer in the introductory period of LG, D1 + dissection accounted for more than 80% of the surgeries.
Postoperative complications
Table 3 shows the postoperative complications classified as grade II or above in the JCOG PC criteria. Grade ≥ II PF was observed in 140 patients (4.0%) and grade ≥ III PF was observed in 62 patients (1.8%).
Factors associated with PF
According to the univariate analysis, sex (male), BMI (≥ 24.7 kg/m2), prealbumin level (≥ 23.3 mg/dL), P-A length (≥ 46 mm), and UP-CA angle (≥ 95°) were significantly associated with grade ≥ II and grade ≥ III PF after LG (Table 4). Factors with a P-value of < 0.1 were selected as covariables in the multivariate logistic regression analysis, which identified sex, BMI, and UP-CA angle as independent predictors of a higher incidence of postoperative grade ≥ II and grade ≥ III PF after LG (Table 5). The VIFs in the multivariate logistic regression analysis for grade ≥ II PF were 1.19 for sex, 1.21 for BMI, and 1.06 for UP-CA angle. The VIFs for grade ≥ III PF were 1.18 for sex, 1.22 for BMI, and 1.08 for UP-CA angle.
Discussion
PF is a common complication after gastrectomy. The reported incidence of grade ≥ II PF in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) criteria in a Japanese RCT (JCOG0912) was 0.4% after both open and laparoscopy-assisted distal gastrectomy. However, comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy using the Japanese National Clinical Database, which prospectively accumulates data from various types of hospitals, revealed a significant difference in the incidence of Grade B or Grade C PF according to the International Study Group of Pancreatic Fistula (ISGPF) criteria between laparoscopy-assisted distal gastrectomy (2.2%) and open distal gastrectomy (1.0%) [6]. The difference between LG and open gastrectomy in how the celiac artery and its branches are visualized possibly affected the difficulty in handling the pancreas, resulting in a higher incidence of PF in LG.
Surgical difficulty can vary between patients despite a similar BMI. Additionally, some patients develop postoperative PF while others do not, despite using a similar procedure. We hypothesized that the amount of traction applied to the pancreas to expose the celiac artery and its branches differs depending on the anatomical position of the pancreas and that difference affects the occurrence of postoperative PF. Our pilot study, which focused on LDG, revealed significant correlations between P-A length and grade I or more severe PF. This study elucidated the correlation between the anatomical position of the pancreas and the occurrence of postoperative PF of grade II or above that required any treatment after LG with a much larger patient population than that of the previous study. The present study also provided new findings. The anatomical position of the pancreas in the sagittal direction (UP-CA angle) is more important than that in the transverse direction (P-A length) in predicting postoperative PF after LG. VIF analyses showed no multicollinearity among the factors related to PF, suggesting that the results of the multivariate logistic regression analyses are reliable. Furthermore, the present study revealed that type of gastrectomy did not affect the occurrence of PF.
The question then arises as to what we can do to avoid postoperative PF in LG for patients with a large UP-CA angle, in whom more traction or compression of the pancreas tends to be applied compared with patients with a small UP-CA angle. A 45° oblique laparoscope can be proactively used to obtain a better surgical view during suprapancreatic lymph node dissection. If the camera port is placed cranial to the umbilicus, the celiac artery and its branches can be visualized with less traction on the pancreas. Additionally, a flexible scope may provide better exposure of the celiac artery and its branches when looking down at these structures over the pancreas compared with a rigid laparoscope. Another promising development is robot-assisted surgery. Robotic gastrectomy facilitates an approach to the lymph nodes along the common hepatic or splenic artery without compressing the pancreas by the use of multiflexible forceps, and the use of robotic technology has shown a trend toward better outcomes in PF compared with LG [15, 16]. Although robotic gastrectomy is still under development, its great advantages are expected to overcome the flaws of LG even in patients with a difficult body shape.
A limitation of the present study is that PF may occur in the infrapyloric area, and this risk might differ from that of PF in the suprapancreatic area. However, the anatomical position of the pancreas represented by the UP-CA angle may also represent the difficulty of all procedures related to the pancreas. Further study is required to differentiate the risks of PF in these different areas. Another limitation of the present study is that it incorporated the patients included in our pilot study. The reason is the present study targeted all patients who underwent LG from 2005 (when LG was adopted in our institution) to the end of 2019. The exclusion of patients from the pilot study (who underwent LDG between 2013 and 2015) did not seem reasonable. There were also limitations in the selection of the factors for the logistic regression analyses. Factors such as the predominantly used energy device, surgeon volume, total duration of compression of the pancreas, and port arrangement may affect the development of PF; however, it was not feasible to incorporate all of these factors in the analyses. Therefore, factors generally known as risks for postoperative complications after gastrectomy and that are easily measured clinically were chosen as the covariates for the logistic regression analyses. Finally, the results of the present study were based on the standardized LG procedure in the Cancer Institute Hospital. Therefore, the conclusions may be valid only for patients operated in our hospital, and different factors may correlate with PF after LG in other hospitals using different methods.
In conclusion, the results of the present study elucidated the significant correlation of the anatomical position of the pancreas with PF after LG, particularly the anatomical position of the pancreas in the sagittal direction (UP-CA angle).
References
Kim MC, Kim KH, Kim HH et al (2005) Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol 91:90–94
Mochiki E, Nakabayashi T, Kamimura H et al (2002) Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg 26:1145–1149. https://doi.org/10.1007/s00268-002-6286-8
Noshiro H, Nagai E, Shimizu S et al (2005) Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 19:1592–1596
Katai H, Mizusawa J, Katayama H et al (2019) Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan clinical oncology group study JCOG1401. Gastric Cancer 22:999–1008
Katai H, Mizusawa J, Katayama H et al (2020) Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 5:142–151
Hiki N, Honda M, Etoh T et al (2018) Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer 21:162–170
Katai H, Mizusawa J, Katayama H et al (2017) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan clinical oncology group study JCOG0912. Gastric Cancer 20:699–708
Kumagai K, Hiki N, Nunobe S et al (2018) Impact of anatomical position of the pancreas on postoperative complications and drain amylase concentrations after laparoscopic distal gastrectomy for gastric cancer. Surg Endosc 32:3846–3854
Japanese gastric cancer association (2020) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric cancer
Inaki N, Etoh T, Ohyama T et al (2015) A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 39:2734–2741. https://doi.org/10.1007/s00268-015-3160-z
Katayama H, Kurokawa Y, Nakamura K et al (2016) Extended clavien-dindo classification of surgical complications: Japan clinical oncology group postoperative complications criteria. Surg Today 46:668–685
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Hiki N, Fukunaga T, Yamaguchi T et al (2008) The benefits of standardizing the operative procedure for the assistant in laparoscopy-assisted gastrectomy for gastric cancer. Langenbecks Arch Surg 393:963–971
Kumagai K, Hiki N, Nunobe S et al (2011) Different features of complications with Billroth-I and Roux-en-Y reconstruction after laparoscopy-assisted distal gastrectomy. J Gastrointest Surg 15:2145–2152
Suda K, Man IM, Ishida Y et al (2015) Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 29:673–685
Ojima T, Nakamura M, Nakamori M et al (2019) Robotic radical lymphadenectomy without touching the pancreas during gastrectomy for gastric cancer. Medicine (Baltimore) 98:e15091
Author information
Authors and Affiliations
Contributions
Study conception and design, KK, NH and SN. Acquisition of data, KK and XJ. Analysis and interpretation of data, KK, NI and MH. Drafting of manuscript, all authors.Critical revision of manuscript, all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All patients included in this study provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumagai, K., Nunobe, S., Hiki, N. et al. Anatomical Position of the Pancreas as a Risk Factor for Pancreatic Fistula after Laparoscopic Gastrectomy for Gastric Cancer. World J Surg 47, 1744–1751 (2023). https://doi.org/10.1007/s00268-023-06972-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-06972-z