Abstract
Background
Postoperative pancreatic fistula (POPF)—often caused by pancreatic injury during dissection of the peripancreatic lymph nodes—is a serious complication after gastric cancer surgery. We defined protruding pancreatic tissue on the anterior side of the pancreas head as “process of the pancreas head” (PPH) and investigated whether PPH is a predictable risk factor for POPF after laparoscopic gastrectomy.
Methods
We reviewed 255 patients who underwent laparoscopic total or distal gastrectomy for gastric cancer. The perioperative outcomes of 142 patients operated in the study’s early phase were investigated to evaluate the risk factors for POPF. To evaluate whether preoperative identification of PPH by computed tomography (CT) and intraoperative prediction of pancreas head outline could reduce the risk of POPF, the outcomes of 113 patients operated in the late phase were assessed.
Results
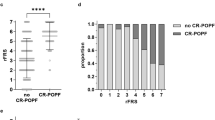
Of the 142 early-phase patients, PPH was identified intraoperatively in 38 patients (26.8 %). A total of 13 patients (9.1 %) developed POPF > grade 2. PPH was identified as a risk factor for POPF (P < 0.01). In early-phase patients with PPH, the POPF rate was 21.0 %; in the late phase, it decreased to 4.3 %. Further, the POPF rate in early-phase patients with BMI > 25 and PPH was 42.6 %, decreasing to 0 % in the late-phase patients.
Conclusions
The presence of PPH is a risk factor for POPF after laparoscopic gastrectomy for gastric cancer. Identifying PPH using preoperative CT images and predicting the shape of the pancreas head during infrapyloric lymph node dissection are valuable in preventing POPF following laparoscopic gastric cancer surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative pancreatic fistula (POPF) is a serious and fatal complication after radical gastrectomy for gastric cancer. Past studies have shown that POPF occurs in 0.3–7.2 % of patients undergoing gastrectomy; the risk factors for POPF include male sex, high body mass index (BMI), older age, and longer operative time [1–5]. One cause of POPF is thought to be pancreatic injury during dissection of the peripancreatic lymph nodes that directly face the pancreas, such as the infrapyloric lymph nodes.
Currently, laparoscopic gastrectomy is widely performed in patients with gastric cancer; in Japan, its validity is presently under investigation in a randomized controlled study [6]. Recent advances in laparoscopic surgery have increased the availability of high-definition images that enable a clearer understanding of the anatomy related to infrapyloric lymphadenectomy [7, 8]. Some authors have reported that the lower invasiveness of laparoscopic gastrectomy compared with conventional surgery may decrease postoperative complications [9, 10]; however, other reports have observed that POPF remains the main complication after laparoscopic radical gastrectomy [11–13]. During laparoscopic surgery, where the surgeon has to identify the pancreatic border from the monitor image without tactile sensation, preventing pancreatic injury is difficult due to the fragile nature of the pancreas combined with its rough and irregular surface. Past anatomical exploration has found structures projecting from the pancreas, such as the omental tuberosity, which projects from the right end of the superior border above the level of the lesser curvature of the stomach, and the uncinate process, which is the left part of the pancreas head and projects upwards and to the left behind the superior mesenteric vein.
We have often encountered protruding pancreatic tissue on the anterior surface of the pancreas head, which lies along the right gastroepiploic vein (RGEV) and right gastroepiploic artery (RGEA), and we defined this tissue as the “process of the pancreas head” (PPH). Because the PPH is covered with the mesoduodenum adjoining the pylorus, which includes adipose tissue and infrapyloric lymph nodes, the outline of the pancreas head surface is unclear before lymphadenectomy. Previously, we inadvertently injured the PPH perioperatively, which directly faced the lymph nodes that were to be dissected; the resulting irreversible damage to the pancreas was considered to be the probable cause of the subsequent POPF.
In this study, we reviewed the characteristics of patients with early gastric cancer who underwent laparoscopic gastrectomy from December 2011 to December 2014. In the early phase of the study, we investigated whether the presence of PPH was a risk factor for POPF. In the late phase of the study, we aimed to reduce the risk of POPF in patients with PPH by preoperative identification of PPH using computed tomography (CT) and intraoperative prediction of pancreatic surface outlines.
Materials and methods
Patients in the early phase of the study
A total of 255 patients with clinically suspected T1 gastric cancer underwent laparoscopic distal gastrectomy or total gastrectomy from December 2011 to December 2014 in Toranomon Hospital (Tokyo, Japan). In the early phase of this study, from December 2011 to June 2013, the perioperative and pathological records of 142 patients, including information on PPH's presence, age, sex, BMI, operative procedure, operative time, amount of blood loss, number of harvested lymph nodes, drain amylase concentration on postoperative days 1 and 3, stage of carcinoma, and morbidity, were assessed with regard to patient characteristics and statistically analyzed using the Chi-square test to identify the risk factors for POPF.
Definition of POPF
POPF was classified according to the Clavien–Dindo classification. Patients who had grade 1 POPF, as diagnosed from the concentration of drain amylase (higher than three times the upper limit), were excluded from the assessment of the morbidity rate. Patients who needed pharmacological intervention, such as somatostatin analog therapy, based on high drain amylase concentrations and those with postoperative CT findings of peripancreatic fluid collections were classified as having grade 2 pancreatic fistulas; those who needed surgical intervention, such as drain insertion, were classified as grade 3; and those who needed intensive care unit treatment were classified as grade 4.
Definition and anatomical features of PPH
The presence of PPH was evaluated by viewing individual operative video records that showed the protrusion of the exposed pancreas head along the RGEV and RGEA during lymphadenectomy. While the presence or absence of PPH as well as PPH height differed between individuals, the anatomical relationship between PPH and the gastroepiploic vessels was the same in all the patients with PPH. Figure 1 shows the anatomical schema of PPH and the vessels with and without the assistant’s upward traction of the infrapyloric part of stomach. The RGEV descends along the right side of the PPH to its origin that connects to Henle’s gastrocolic trunk at the lower right portion of the PPH. The right side of the PPH sometimes forms a deep groove in which the RGEV descends from the pylorus. The root of the RGEA branches from the gastroduodenal artery at the dorsal side of the PPH and ascends to the top of the PPH. Upward traction provided by assistants enhances the visibility of the PPH during infrapyloric lymphadenectomy.
a Process of the pancreas head (PPH) is protruding pancreatic tissue on the anterior surface of the pancreas head that lies on the right gastroepiploic vein and artery. b The PPH is lifted by the assistant providing upward traction during infrapyloric lymphadenectomy. IPLN infrapyloric lymph node, RGEA right gastroepiploic artery, GDA gastroduodenal artery, RGEV right gastroepiploic vein, ASPDV anterior superior pancreaticoduodenal vein, ARCV accessory right colonic vein, SMV superior mesenteric vein
Identification of PPH's presence from CT images
In the early phase of this study, we retrospectively reviewed the shape of the pancreas in the preoperative CT image to determine whether PPH's presence can be identified preoperatively. In the late phase of this study, we began to preoperatively identify the presence of PPH, recognized as protruding pancreatic tissue lying on the right gastroepiploic vessels in the horizontal plane of the CT, as shown in Fig. 2. Preoperative identification of the PPH allowed us to predict the outline of the pancreatic surface during the operation.
a PPH was evident on the preoperative computed tomography image as protruding pancreatic tissue along the right gastroepiploic vessels. b Horizontal schema. RGEV right gastroepiploic vein, SMV superior mesenteric vein, IPLN infrapyloric lymph node, RGEA right gastroepiploic artery, GDA gastroduodenal artery, ASPDV anterior superior pancreaticoduodenal vein
Infrapyloric lymph node dissection in patients with PPH
In patients with PPH, greater attention to infrapyloric lymph node dissection is needed to avoid pancreatic injury. According to our previous report of the procedure for laparoscopic gastrectomy [14], infrapyloric lymphadenectomy starts from division of the greater omentum to open the omental bursa. The division continues rightward beyond the right border of the omental bursa until the descending part of the duodenum is exposed. The outline of the PPH, which is covered with mesoduodenal adipose tissue, is still unclear at this point (Fig. 3a). The transverse mesocolon is then lifted and lowered to identify the RGEV, which is transected at the level of its confluence with the anterior superior pancreaticoduodenal vein (ASPDV) at the left right bottom of PPH (Fig. 3b). Once the RGEV is identified, the raised outline of the PPH along the left side of the RGEV should be predicted and confirmed. The pancreatic surface on the right side of RGEV is usually concave and covered with deep adipose tissue that includes the infrapyloric lymph nodes. This adipose tissue, including the lymph nodes, is carefully dissected from the anterior surface of the PPH and from the concave pancreas head on the other side (Fig. 3c). Keeping the dissection layer between the adipose and pancreatic tissue is important to avoid pancreatic injury. However, in certain cases, this layer is unclear, particularly when the pancreas is infiltrated with adipose tissue. By continuing dissection to the top of the PPH, the RGEA is identified and ligated with a clip (Fig. 3d). The IPA is subsequently ligated. Finally, the inferior wall of the duodenal bulb is skeletonized, the infrapyloric nodal region is removed en bloc with the gastric specimen, and the PPH is exposed in its natural appearance without the assistant’s upward traction (Fig. 3e).
Infrapyloric lymph node dissection in patients with PPH. a Infrapyloric lymph node dissection starts after taking down the mesocolon. The infrapyloric adipose tissue obscures the outline of the PPH. b The root of RGEV is transected with a clip at the right bottom of the PPH. c Infrapyloric adipose tissue is dissected at the anterior surface of the PPH and at the concave pancreas head on the right side of the RGEV. d The RGEA is identified at the top of the PPH. e The PPH is completely exposed in its natural appearance after infrapyloric lymph node dissection is completed. RGEV right gastroepiploic vein, RGEA right gastroepiploic artery
Patients in the late phase of the study
In the late phase of this study, from July 2013 to December 2014, 113 patients underwent laparoscopic gastrectomy with infrapyloric lymph node dissection after the presence of PPH was preoperatively evaluated using CT images. The operative outcomes of patients showing the presence of PPH from the early and late phases were compared to evaluate whether PPH prediction reduced the rate of POPF.
Results
Perioperative status and outcomes of the early-phase patients
The baseline and perioperative characteristics of the early-phase patients are shown in Table 1. A total of 142 patients (91 men, 51 women; mean age 63.7 ± 10.7 years) were examined. Their mean BMI was 23.0 ± 3.2 kg/m2. We identified PPH's presence based on visual confirmation of the exposed pancreas head in 38 (26.8 %) patients. PPH was highly elevated along with right gastroepiploic vessels by the assistant’s upward traction. The remaining 104 (73.2 %) patients had a smooth surface of the pancreas head after infrapyloric lymphadenectomy was completed, even though their pancreas heads sometimes seemed to be protruding during the assistant’s perioperative upward traction. In the retrospective review of CT images, the presence of PPH was recognized as protruding pancreatic tissue lying on the right gastroepiploic vessels in 31 of 38 patients. The presence of PPH could not be recognized in the remaining 7 patients owing to scarce visceral adipose tissue, which made it difficult to evaluate the outline of the pancreas head.
A total of 19 patients experienced postoperative morbidity, including POPF in 13 patients, anastomotic leakage in 1 patient, and pneumonia in 2 patients. Of the 13 patients with POPF, 7 had grade 2 POPF; 5, grade 3; and 1, grade 4 POPF. Two patients needed reoperation to repair POPF and control the subsequent abdominal bleeding. There was no surgery-related mortality.
The risk factors for POPF were investigated by univariate analysis (Table 2). The POPF rate was significantly higher in patients with BMI ≥ 25 kg/m2 than that in those with BMI < 25 kg/m2 (18.4 vs. 5.8 %, P = 0.047). The POPF rate was also significantly higher in patients with PPH than in those without PPH (21.1 vs. 4.8 %, P = 0.008). Moreover, the POPF rate was significantly higher in patients with an operative time longer than 300 min (15.9 vs. 2.7 %, P = 0.01). Age, sex, depth of tumor invasion, and the presence of lymph node metastases were not associated with the occurrence of POPF.
Operative outcomes of the late-phase patients with PPH
In the late phase of the study, a total of 113 patients underwent operation after identification of PPH's presence from preoperative CT images. The presence of PPH was identified in 19 patients; however, PPH's presence could not be identified in 4 patients because scant adipose tissue made it difficult to evaluate the outlines of the pancreatic surface.
Table 3 shows the perioperative characteristics and outcomes of the patients with PPH in the early and late phases of the study. The rate of POPF occurrence in the late-phase patients was 4.3 %, which was lower than but not statistically significantly different from that in the early-phase patients. Table 4 shows the perioperative characteristics of the patients with PPH as well as BMI > 25 in the early and late phases of the study. In this group, the rate of POPF significantly decreased from 42.6 % in the early-phase patients to 0 % (P = 0.016) in the late-phase patients.
Discussion
The dissection of lymph nodes lying in the infrapyloric area is an important surgical step in radical gastrectomy. Several studies have demonstrated that metastasis to the lymph nodes in the infrapyloric area is common [15, 16]. However, dissection of this area, which directly faces the anterior surface of the pancreas head, is associated with the risk of pancreatic injury and POPF. Further, BMI is strongly associated with visceral fat mass and is known as a risk factor for POPF [1, 2, 5].
From our clinical experience, the degree of operative difficulty differs for each patient with high BMI. The technical difficulties of infrapyloric lymph node dissection appear to be associated with operative morbidity, particularly the occurrence of pancreatic fistula that manifests as fluid collection in front of the pancreas head. In this study, we identified PPH as a risk factor for POPF, which is related to the pancreatic aspect of the difficulty involved in infrapyloric lymph node dissection. The presence of PPH could be intraoperatively identified in the patients with scant peripancreatic adipose tissue before infrapyloric lymph node dissection. In patients with high BMI, the deep adipose tissue covering the surface of PPH can lead to missed identification of its presence intraoperatively; therefore, the PPH can be injured during infrapyloric lymph node dissection. In such cases, the preoperative identification of PPH's presence using CT images can limit the risk of POPF, by paying attention to its presence during infrapyloric lymph node dissection.
According to our retrospective review of the preoperative CT images, the presence of PPH can be recognized as protruding pancreatic tissue lying on the right gastroepiploic vessels in the horizontal plane of the CT image. Although 5-mm slice CT images could reveal PPH, the 1-mm slices showed a finer image of PPH and the right gastroepiploic vessels. In CT images, it is difficult to evaluate PPH's presence in patients with little peripancreatic visceral adipose tissue. However, the risk of POPF is not high in patients with low BMI and scant visceral adipose. In contrast, the shape of the pancreas head can be easily evaluated from preoperative CT in patients with thick peripancreatic adipose tissue with high BMI, who were shown to be at a higher risk of POPF in this study; thus, predicting the presence of PPH was noted to be more useful for avoiding POPF patients with higher BMI.
In our study, during infrapyloric lymph node dissection, the mesoduodenal adipose tissue—along with the right gastroepiploic and infrapyloric vessels—was dissected from the surface of the pancreas head based on the prediction and/or identification of the presence of PPH. The dissection layer between the adipose tissue and pancreatic tissue is clear in patients with a smooth pancreatic surface. However, in patients with adipose infiltration of the pancreas, this layer is sometimes obscured, making it difficult to maintain the required plane of dissection; however, such infiltration can also be recognized from preoperative CT images as pancreas of diffuse low density.
This study has some limitations. Other factors such as advances in individual operative technique might influence the improvement of operative outcomes in the late phase.
We have newly defined the anatomical structure of PPH in this study. During infrapyloric lymph node dissection in laparoscopic gastrectomy, the presence of PPH was shown to increase the risk of pancreatic injury, which is considered to cause POPF, particularly in patients with high BMI. However, preoperative CT imaging allowed the prediction of the presence of PPH, enabling the safe dissection of adipose tissue along the outline of the pancreatic surface while avoiding pancreatic injury.
References
Jiang X, Hiki N, Nunobe S et al (2012) Postoperative pancreatic fistula and the risk factors of laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Surg Oncol 19:115–121
Kung CH, Lindblad M, Nilsson M et al (2014) Postoperative pancreatic fistula formation according to ISGPF criteria after D2 gastrectomy in Western patients. Gastric Cancer 17:571–577
Park JM, Jin SH, Lee SR et al (2008) Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc 22:2133–2139
Degiuli M, Sasako M, Ponti A (2010) Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian gastric cancer study group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Sur 97:643–649
Yu HW, Jung DH, Son SY et al (2013) Risk factors of postoperative pancreatic fistula in curative gastric cancer surgery. J Gastric Cancer 13:179–184
Katai H (2015) Current status of a randomized controlled trial examining laparoscopic gastrectomy. Asian J Endosc Surg 8:125–129
Haruta S, Shinohara H, Ueno M et al (2015) Anatomical considerations of the infrapyloric artery and its associated lymph nodes during laparoscopic gastric cancer surgery. Gastric Cancer 18:876–880
Shinohara H, Haruta S, Ohkura Y et al (2015) Tracing dissectable layers of mesenteries overcomes embryological restrictions when performing infrapyloric lymphadenectomy in laparoscopic gastric cancer surgery. J Am Coll Surg 220:e81–e87
Lee WJ, Chan CP, Wang BY (2013) Recent advances in laparoscopic surgery. Asian J Endosc Surg 6:1–8
Kawamura H, Tanioka T, Tahara M et al (2013) Postoperative complication rates and invasiveness of laparoscopy-assisted distal gastrectomy and open distal gastrectomy based on American Society of Anethesiologists classification. Asian J Endosc Surg 6:170–176
Obama K, Okabe H, Hosogi H et al (2011) Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complication. Surgery 149:15–21
Jeong O, Ryu SY, Zhao XF et al (2013) Short-term surgical outcomes and operative risks of laparoscopic total gastrectomy (LTG) for gastric carcinoma: experience at a large-volume center. Surg Endosc 26:3418–3425
Haverkamp L, Weijs TJ, van der Sluis PC et al (2013) Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc 27:1509–1520
Shinohara H, Kurahashi Y, Kanaya S et al (2013) Topographic anatomy and laparoscopic technique for dissection of no. 6 infrapyloric lymph nodes in gastric cancer surgery. Gastric Cancer 16:615–620
Sasako M, McCulloch P, Kinoshita T, Maruyama K (1995) New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 82:346–351
Maruyama K, Gunven P, Okabayashi K et al (1989) Lymph node metastases of gastric cancer general pattern in 1931 patients. Ann Surg 210:596–602
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Nao Kobayashi, Hisashi Shinohara, and Shusuke Haruta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kobayashi, N., Shinohara, H., Haruta, S. et al. Process of Pancreas Head as a Risk Factor for Postoperative Pancreatic Fistula in Laparoscopic Gastric Cancer Surgery. World J Surg 40, 2194–2201 (2016). https://doi.org/10.1007/s00268-016-3536-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3536-8