Abstract
Little is known about the physiology of a common fluid ingestion pattern—sequential swallowing. This study investigated sequential swallowing biomechanics in healthy adults. Archival normative videofluoroscopic swallow studies were analyzed for hyolaryngeal complex (HLC) patterning and biomechanical measures from the first 2 swallows of a 90-mL thin liquid sequential swallow task. The effects of age, sex, HLC type, and swallow order were explored. Eighty-eight participants were included in the primary analyses as they performed sequential swallows. HLC Type I (airway opens, epiglottis approaches baseline) and Type II (airway remains closed, epiglottis remains inverted) most commonly occurred (47% each), followed by Type III (mixed, 6%). Age was significantly associated with Type II and longer hypopharyngeal transit, total pharyngeal transit (TPT), swallow reaction time (SRT), and duration to maximum hyoid elevation. Males demonstrated significantly greater maximum hyoid displacement (Hmax) and longer duration of maximum hyoid displacement. Significantly larger maximum hyoid-to-larynx approximation was linked to the first swallow, while the subsequent swallow had significantly longer oropharyngeal transit, TPT, and SRT. Secondary analyses included an additional 91 participants who performed a series of discrete swallows for the same swallow task. Type II had significantly greater Hmax than Type I and series of discrete swallows. Sequential swallowing biomechanics differ from discrete swallows, and normal variance exists among healthy adults. In vulnerable populations, sequential swallowing may challenge swallow coordination and airway protection. Normative data allow comparison to dysphagic populations. Systematic efforts are needed to further standardize a definition for sequential swallowing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sequential swallowing is a frequently used fluid ingestion pattern that allows humans to consume liquids quickly and efficiently [1,2,3]. Sequential swallows have been deemed more challenging than discrete (single) swallows. This generalization is commonly applied in dysphagia practice and research, with the idea of keeping the patient safe. Sequential swallowing is believed to place increased demands on the swallowing motor complex primarily because it must accommodate the coordination, magnitude, and quicker rate of various structural movements in a continuous and cyclical manner [4]. Compared to discrete swallows, sequential swallowing is associated with varying uncontrolled ingested volumes per swallow that is impacted by the motor response of the hyolaryngeal complex (HLC) [5] and increased breath drive that often results in changes to normal respiratory-swallow patterning [3, 6]. Thus, tasks involving sequential swallows have been mainly employed as a dysphagia screening tool to identify aspiration risk, such as the 3-oz (90 mL) water swallow challenge [7,8,9].
Nevertheless, such high demands on the swallowing system raise specific concerns for disordered or vulnerable populations. For example, the common occurrence of inspiration surrounding sequential swallows has been documented, including before, during, and after a set of sequential swallows [3, 6, 10]. Healthy adults may not experience adverse effects as a result of changes to respiratory-swallow patterning [6]. However, in patients with dysphagia, such as those treated for oropharyngeal cancer or neurogenic conditions, perturbation of respiratory-swallow patterning may lead to an increased predisposition to penetration or aspiration [11,12,13]. This may require these patients to develop better respiratory-swallow coordination or control.
Given these perceived challenges, clinicians may unintentionally favor the use of discrete rather than sequential swallow tasks during swallow assessments, providing instructions, such as ‘take a sip.’ This may be common when there are concerns for airway invasion. Bennett et al. [14] reported individuals who completed uninstructed and uncontrolled liquid drinking tasks had a higher mean sip volume (19 mL) than those instructed to take discrete sips (6–7 mL). Although controlling and standardizing the bolus (ingested material) has merit for diagnostics and research, unregulated volumes are necessary to evaluate variations in liquid swallowing behavior and obtain a more comprehensive understanding of swallowing physiology [2, 14].
However, sequential swallowing physiology in healthy adults remains poorly understood. With the few studies that have investigated normal sequential swallowing, evidence suggests that physiological differences exist between discrete and sequential swallowing [1, 2, 4,5,6, 15, 16]. A primary example is the taxonomy that has been used to characterize the latter. Early research by Martin et al. [17] concluded that sequential swallows were best characterized by a pattern of sustained HLC excursion and prolonged apnea. Subsequent studies found greater variability in HLC patterning. Chi-Fishman and Sonies [4, 15] reported HLC movement predominately moves in a consistent ‘rise and partial fall’ pattern during sequential swallowing, where the laryngeal vestibule remains closed or slightly opens (more prominent) during partial laryngeal descent [4, 15]. This paradigm was later supported by Susa et al. [18].
Daniels et al. [2, 15, 16] further extended the evidence, specifying different HLC types judged between swallows, which has been supported more recently by Tsushima et al. [19]. Particularly, Daniels et al. [2, 5, 16] indicated HLC Type I occurs when the HLC ‘lowers’ and epiglottis returns to its ‘upright’ position, causing the laryngeal vestibule to open. Type II occurs when the HLC remains ‘partially elevated’ and epiglottis remains inverted, resulting in the laryngeal vestibule remaining closed. Less common, but also possible, are instances when both HLC types are used interchangeably [2, 5]. In its current form, Type I’s description lacks clear parameters for understanding the extent of HLC lowering, making it challenging to determine whether the HLC and pharyngeal cavity returns to rest, which are distinguishing features of discrete swallows [4].
Poor consensus regarding the different HLC patterns, in the already limited literature, precludes accurate quantification of sequential swallowing physiology. This issue may be due to the lack of a standardized operational definition for sequential swallowing and other methodological differences between studies, such as small sample sizes and varying instrumental assessment procedures and tools used. An overview of the study design characteristics of the normal sequential swallowing literature can be found in Supplementary Table 1. Further, few studies have investigated influential factors of sequential swallowing, which unfortunately limits the ability to contextualize sequential swallowing physiology. Preliminary data suggest that older adults have been associated with Type II [2], longer swallow apnea [10], increased total swallow duration and pharyngoesophageal segment (PES) restriction [20], less frequent sips per swallow [21], and longer caudally directed anterior tongue movement duration [22]. In addition, sex-based influences on sequential swallowing have been found. Females have been linked to having longer durations of oral clearance, maximum anterosuperior hyoid excursion (interval between the start of hyoid movement toward maximum excursion and this structure returning to rest), and PES opening (interval between the first moment the PES opens and the first moment it closes) [4]. Contrary, males have shown to display greater submental maximal amplitudes, total distance, and forward peak velocity during sequential swallows [15].
These are important foundational studies, but there is a dearth of comprehensive, reproducible, and replicated evidence for comparison. This makes clinical translation of the evidence challenging. The aforementioned gaps in the evidence necessitate further exploration of normal sequential swallowing. To address these gaps, we examined the HLC types and biomechanics (temporal and kinematic) of sequential swallowing in healthy adults using a validated and standardized swallow assessment approach. Additionally, we mapped the effects of age and sex on HLC patterning and the influence of age, sex, HLC type, and swallow order on sequential swallowing biomechanics. We hypothesized that healthy adults would demonstrate variability in HLC patterning and biomechanics, and they would be influenced by the respective aforementioned variables, suggestive of having flexibility in motor strategy.
Methods
Participants
Participant data were obtained from an archival normative dataset from a study originally authorized by the Medical University of South Carolina Institutional Review Board (Protocol #: 00011566). This original dataset included 195 healthy adults (109 females, 86 males) between the ages of 21–89 years [M(SD) = 47(17.4) years], all of whom provided signed informed consent. Inclusion characteristics were: (a) ≥ 21 years, (b) normal cognition (determined by personnel from the original study or a score of ≥ 26 on the Montreal Cognitive Assessment [23]), (c) no history or complaints of dysphagia, and (d) able to consume unrestricted solids and liquids. Participants who had a history of or presented with these traits were excluded: (a) neurological disorder, (b) respiratory disease, (c) head and neck cancer, (d) hiatal hernia > 2 cm, (e) barium sulfate allergy, (f) pregnant or suspected pregnancy, and (g) head and anterior neck surgery.
Modified Barium Swallow Study (MBSS) Procedures
Each MBSS from the original study was completed under continuous fluoroscopy at 30 frames per second and recorded digitally (Digital Swallow Workstation, Model 7100, Kay Electronics Corp.; TIMS Medical). MBSS procedures were standardized across participants, following the validated Modified Barium Swallow Impairment Profile (MBSImP) protocol [24, 25]. We extracted the thin liquid sequential swallow task captured in the lateral plane. Varibar barium sulfate (Bracco, E-Z-EM, Inc.) thin liquid (40% weight/volume [w/v], < 15 centipoises [cps], International Dysphagia Diet Standardization Initiative level 0) was used. Participants were given approximately 90 mL of thin liquid by cup and instructed, ‘Drink this in your usual manner until I tell you to stop.’ Therefore, the liquid volumes (uncontrolled per swallow) were self-administered via cup sips to simulate natural liquid ingestion.
Data Collection
HLC patterning and biomechanical sequential swallowing events were analyzed on Swallowtail™ (Belldev Medical, Arlington Heights, IL), an integrated and semi-automated MBSS analysis software. The software uses the well-published MBSS timing and displacement analysis method developed by Leonard and Kendall [26,27,28,29,30]. These measures are defined in Supplementary Table 2. Image calibration to a 1.9 cm coin was applied. The second through fourth cervical vertebrae were measured semi-automatically to align the image axes for certain displacement measures, such as maximum hyoid displacement, thereby increasing measurement accuracy and accounting for head movement between frames.
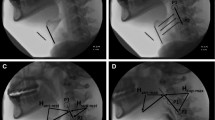
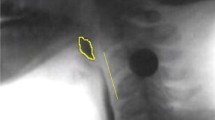
We defined sequential swallowing a priori as demonstrating two or more consecutive swallows where the HLC did not return to rest, and the pharynx lacked complete patency between swallows. This definition was derived based on the aforementioned compilation of potential HLC patterning characteristics found in the works of Chi-Fishman and Sonies [4, 15] and Daniels et al. [2, 5, 16]. In addition, these characteristics were appraised relative to distinguishing characteristics of discrete swallows [4] to generate a priori operational definitions for the HLC types used in our study (modelled after Daniels et al. [2, 5, 16]), which are detailed in Table 1 and captured in images (Figs. 1, 2, 3). HLC types were identified and coded according to the following characteristics: HLC movement, epiglottic position, and arytenoid-epiglottic closure integrity.
Hyolaryngeal complex (HLC) Type I. Yellow marker = Tracing of the hyoid/epiglottis. Blue arrow = Open laryngeal vestibule. Red arrow = Closed laryngeal vestibule. Green marker = Tracing of pharyngeal patency. A The rest position frame. B The frame of maximum pharyngeal patency between swallows (less patent than A). C The hyoid position frame during the end of the first swallow. D The HLC is slightly lower than C, the epiglottis is returning to its baseline position, and the laryngeal vestibule is open
Hyolaryngeal complex (HLC) Type II. Yellow marker = Tracing of the hyoid/epiglottis. Blue arrow = Open laryngeal vestibule. Red arrow = Closed laryngeal vestibule. Green marker = Tracing of pharyngeal patency. A The rest position frame. B The frame of maximum pharyngeal patency between swallows (less patent than A). C The hyoid position frame during the end of the first swallow. D The HLC remains relatively elevated with minor recoil than C, sustained epiglottic inversion, and the laryngeal vestibule is closed
Hyolaryngeal complex (HLC) Type III. Yellow marker = Tracing of the hyoid/epiglottis. Blue arrow = Open laryngeal vestibule. Red arrow = Closed laryngeal vestibule. Green marker = Tracing of pharyngeal patency. A The rest position frame. B The frame of maximum pharyngeal patency between swallows (less patent than A). C The hyoid position frame during the end of the first swallow. D The HLC is slightly lower than C, sustained epiglottic inversion, and the laryngeal vestibule is open
Also defined a priori was the status of pharyngeal patency relative to HLC descent between swallows compared to the rest position. This criterion was intentionally used as a proxy to differentiate sequential from discrete swallows as previous evidence implies that a completely ‘off’ or deactivated state of the HLC and oropharyngeal valving is not representative of the motor strategies likely to be used during sequential swallowing [4]. In cases when participants drank consecutively but where the HLC and pharynx completely returned to their rest positions, the images were not considered ‘true’ sequential swallowing. This pattern more accurately represents the deactivation of structural movements and was coded separately as ‘series of discrete swallows.’
Across all MBSS files, only the first 2 swallows were analyzed. This decision was made for two reasons. First, the MBSImP protocol considers a successful sequential swallow as having a minimum of two consecutive swallows but does not specify to participants how many swallows they must perform. Therefore, this swallow task often elicits a variable number of swallows. Analyzing the first 2 swallows promotes consistency. As such, the number of swallows within each set of sequential swallows could be compared equally, thereby reducing the potential confounding of individuals who had greater than two swallows that could skew the effects of interest. Secondly, some individuals may naturally perform only two swallows during this task.
Rater 1 (KRA), blinded to participant demographics (age, sex), analyzed all images and reanalyzed 10% of the images after 1 week to determine intra-rater reliability for HLC types and biomechanical measures. Rater 2 (AMB) independently examined the same set of swallows to verify inter-rater reliability. Any major rating disputes beyond three videofluoroscopic frames for B1 (bolus head passing the posterior nasal spine) were addressed through consensus. Both raters underwent a reliability training regimen, including the completion of a standardized set of tutorial videos and protocol handouts provided by one of the senior authors (AM), who is highly trained and published using the selected MBSS analysis method. Inter-judge training sessions between raters were held to ensure consistency and competency in the MBSS analysis method. Any disputes were resolved by the senior authors.
Statistical Analyses
Primary Analyses
Frequencies for HLC types were identified. Descriptive analysis, including means, standard deviations, medians, ranges, and 95% confidence intervals (CI) were collected for each biomechanical swallowing measure and distributed based on age, sex, HLC type, and swallow order. A chi-square test for independence was employed during the primary analyses to compare the differences between HLC types, age, and sex. Cramer’s V was applied to identify the magnitude of effect for any significant differences found with the chi-square test for independence. Primary analyses of the biomechanical measures were conducted using generalized estimating equations (GEE) to examine the effects of age, sex, HLC type, and swallow order. For each GEE model, all variables were controlled among each other. An exchangeable condition was applied for the working correlation matrix for each GEE analysis. Statistical significance was determined by an alpha of 0.05. Post hoc pairwise comparisons with Bonferroni corrections were used to determine the simple effects. Kendall’s tau was used to assess correlations between biomechanical measures and age as a continuous variable.
Secondary Analyses
Secondary analyses were employed to map the differences between sequential swallowing and series of discrete swallows. Identical statistical procedures as described for the primary analyses were applied, except series of discrete swallows were added to each model.
Reliability
For reliability, Cohen’s kappa was executed for HLC patterning and interpreted as follows: ≤ 0.0 (no agreement), 0.01–0.20 (slight), 0.21–0.40 (fair), 0.41–0.60 (moderate), 0.61–0.80 (substantial), and 0.81–1.0 (near perfect or perfect) [31]. An intraclass correlation coefficient (ICC) analysis was conducted for the biomechanical measures. Two-way random-effect models were applied to generate a single measure ICC and an average measure ICC for intra- and inter-rater reliability, respectively. ICC ranges were interpreted based on the following criteria: < 0.50 (poor), 0.50–0.75 (moderate), 0.75–0.90 (good), and > 0.90 (excellent) [32]. Statistical Package for the Social Science (SPSS, version 28) was utilized for all statistical analyses.
Results
Eligible Participants
Of the original sample, 179 participants were eligible for statistical analyses as 16 participants were excluded due to poor MBSS video quality. The 88 participants who performed sequential swallows and were included in the primary analyses consisted of 45 females and 43 males between 21 and 82 years [M(SD) = 46(17.3) years]. This meant a total of 176 swallows (88 sets of sequential swallows) that were analyzed for the primary analyses. Demographics for these participants are presented below based on three age groups, well aligned with those used by Chi-Fishman and Sonies [14]:
-
21–39 years (younger adults), N = 35, 18 males, 17 females, M(SD) = 28(4) years,
-
40–59 years (middle-aged adults), N = 27, 16 males, 11 females, M(SD) = 47.7(6) years, and
-
≥ 60 years (older adults), N = 26, 17 females, 9 males, M(SD) = 67.4(6.4) years.
The remaining 91 participants who performed a series of discrete swallows comprised of 55 females and 36 males between 22 and 89 years [M(SD) = 48.6(17.6) years]. Therefore, a total of 182 swallows (91 sets of sequential swallows) were included in the secondary analyses. These participants were also stratified into the same age groups:
-
Younger adults, N = 30, 16 females, 14 males, M(SD) = 28.6(4.7) years,
-
Middle-aged adults, N = 35, 20 females, 15 males, M(SD) = 50(6.1) years, and
-
Older adults, N = 26, 19 females, 7 males, M(SD) = 69.9(9.4) years.
Reliability
Intra-rater reliability was perfect for determining HLC patterning [κ = 1.0, p < 0.001, 95% CI (1.0, 1.0)] and excellent for biomechanical measures [ICC = 0.97, p < 0.001, 95% CI (0.93, 0.99)]. Similarly, near perfect inter-rater reliability was found for HLC patterning [κ = 0.92, p < 0.001, 95% CI (0.77, 1.1)] and excellent for biomechanical measures [ICC = 0.93, p < 0.001, 95% CI (0.88, 0.97)].
Primary Analyses of HLC Patterning
All three HLC types were present. Types I and II were most frequently observed (N = 41 each), followed by Type III (N = 6). Frequencies for HLC type can be found in Table 2. HLC type significantly differed between age groups, χ2 (4, N = 88) = 10.8, p = 0.029. Specifically, Type I and III were most common in the youngest group (N = 19 and 5, respectively), while the oldest group primarily used Type II (N = 17). However, a small effect size was noted, φc = 0.029. HLC type did not differ by sex, χ2 (2, N = 88) = 3.2, p = 0.20.
Primary Analyses of Biomechanical Measures
A descriptive overview of the aggregate normative reference values for temporal and kinematic sequential swallowing data is displayed in Table 3 and further categorized by age, sex, age and sex, HLC type, and swallow order (Supplementary Tables 3–7). Associations between biomechanical measures and the aforementioned influential factors are presented subsequently. Table 4 summarizes the main effects found for each biomechanical measure relative to each predictor variable. All temporal values are listed in seconds (s), and kinematic measures are noted in cm.
Age
When age was analyzed as a continuous variable, correlations were found between age and hypopharyngeal transit (HPT), total pharyngeal transit (TPT), swallow reaction time (SRT), duration to maximum hyoid elevation (Hdur), duration of maximum hyoid displacement (Hm), airway closure duration (AEdur), PES opening duration (PESdur), and maximum PES opening (PESmax). Table 5 provides an overview of the statistical output and relationship strength associated with the correlations identified. Comparisons between age groups revealed significant main effects for HPT, TPT, SRT, and Hdur. Longer HPT was found for the oldest group than the youngest group, Wald χ2 (2, N = 176) = 21.64, mean difference (MD) = 0.15, p < 0.001. Middle-aged adults also had the same trend in HPT compared to the youngest group, MD = 0.086, p = 0.041. The oldest group exhibited longer TPT compared to the youngest group, Wald χ2 (2, N = 176) = 21.4, MD = 0.25, p < 0.001. Middle-aged adults also presented with longer TPT than the youngest group, MD = 0.16, p = 0.022. The oldest group compared to the youngest group displayed longer SRT, Wald χ2 (2, N = 176) = 10.2, MD = 0.14, p = 0.022. Hdur was longer for the oldest group compared to the youngest group, Wald χ2 (2, N = 176) = 12.9, MD = 0.089, p = 0.002.
Sex
Significant main effects were found between sex and Hm and maximum hyoid displacement (Hmax). For Hm, males displayed longer intervals than females, Wald χ2 (1, N = 176) = 4.2, MD = 0.045, p = 0.040. Males also exhibited greater Hmax than females, Wald χ2 (1, N = 176) = 6.9, MD = 0.48, p = 0.009.
HLC Type
A significant main effect was observed between HLC type and Hmax, Wald χ2 (2, N = 176) = 7.0, p = 0.030. However, post hoc analysis revealed no significant simple effects.
Swallow Order
Significant main effects were found between swallow order and oropharyngeal transit (OPT), TPT, SRT, and maximum hyoid-to-larynx approximation (HL). Swallow two presented with longer OPT [Wald χ2 (1, N = 176) = 25.75, MD = 0.13, p < 0.001], TPT [Wald χ2 (1, N = 176) = 23.7, MD = 0.14, p < 0.001], and SRT [Wald χ2 (1, N = 176) = 64.7, MD = 0.22, p < 0.001] than swallow one. Conversely, HL was larger for the first swallow than the second swallow, Wald χ2 (1, N = 176) = 7.6, MD = 0.066, p = 0.006.
Secondary Analyses of HLC Patterning
Table 6 shows the frequencies of HLC type associated with age and sex. HLC type did not differ by age group, χ2 (6, N = 179) = 12.4, p = 0.053, and sex, χ2 (3, N = 179) = 4.8, p = 0.185.
Secondary Analyses of Biomechanical Measures
Significant differences were found between Types I and III, series of discrete swallows, and Hmax, Wald χ2 (3, N = 358) = 11.5, p = 0.009. Specifically, Type II resulted in more extensive Hmax than Type I, MD = 0.62, p = 0.015, and series of discrete swallows, MD = 0.47, p = 0.045. Supplementary Table 8 provides an overview of the descriptive statistics and main effects associated with series of discrete swallows relative to each biomechanical measure.
Discussion
We investigated sequential swallowing biomechanics in a large cohort of healthy adults. Various influential factors were explored, including age, sex, HLC type, and swallow order, giving greater context to this common swallowing behavior.
HLC Patterning
Types I–III were present, meaning airway opening and closure were normal variants of the motor strategy used during sequential swallowing in healthy adults. Types I and II were most common and equally distributed. Our findings appear to corroborate the trends found by Daniels et al. [2], despite the differences in swallow tasks employed. However, the extent of HLC lowering and pharyngeal deactivation for Type I as described by Daniels et al. [2] remains equivocal. Notably, it is unclear whether these components returned to rest, which would better be defined as a series of discrete swallows.
Other studies reported a Type I predominance [5, 6, 18, 19] but were mainly comprised of younger and middle-aged adults and were limited in power due to small sample sizes (N = 15, [5]; N = 21, [18]; N = 12, [19]). Dozier et al. [6] had the only comparably sized cohort (N = 70) to our study. However, they did not appear to separate Type I users from those who performed a series of discrete swallows in their analysis, despite observing the latter frequently. We elected to control for this behavior by excluding it from the primary analyses as it may better represent a drinking behavior instead of a sequential swallowing pattern. Nonetheless, the high cases of participants who demonstrated this behavior in both studies may be due to the overlapping swallow task instructions. Participants were asked to drink in their typical manner, except our study also informed participants to continue drinking until told to stop. The former may be interpreted more loosely than the latter. Perhaps, another consideration is sequential swallows and series of discrete swallows are both common responses to successive drinking tasks.
Biomechanical Measures
There is a scarcity of prior studies exploring normal sequential swallowing biomechanics. Chi-Fishman and Sonies [4] documented pharyngeal transit clearance, or the interval between bolus head arrival at the jaw angle, to bolus clearance into the PES, which is similarly defined to the term we used (TPT). The authors recorded this interval across two 150 mL sequential, thin liquid swallow cup drinking conditions [head neutral (HN) and head tilt-back (HT) positions], which yielded similar biomechanical measures [4]. The mean TPT for the HN position in our study, however, was longer (MD = 0.38 s) than the values presented by Chi-Fishman and Sonies [4]. In their subsequent work [14], they documented lower mean Hmax amplitudes than our study (MD for 1st swallow = 0.04 and 2nd swallow = 0.81 cm). Nevertheless, comparable results were found for PESdur despite the head position conditions applied by Chi-Fishman and Sonies [4] (MD for HN = 0.06 and HT = 0.08 s). Methodological differences likely account for these conflicting results, such as different operational definitions, employing a rapid ingestion rate component, and administering boluses via a cup with the rim cut off [4, 15].
Inspection of our aggregate data revealed wide ranges in sequential swallowing biomechanics and that healthy adults accommodate their swallowing motor strategy to increased task demands. This further demonstrates the functional variability within the swallowing system. However, this may be impaired in those with dysphagia. Thus, sequential swallowing tasks may be clinically useful for challenging the system. Given most evidence has assessed the application of sequential swallowing as a screening tool to detect patients at risk of aspiration, our data extend the clinical utility of this task beyond a screening tool toward more quantitative, comprehensive, and sensitive assessment. Using these data, clinicians could also further quantify components of the MBSImP, which is widely utilized by clinicians globally [24, 25, 33, 34].
Influential Factors
Age
Our findings appear to replicate those found by Daniels et al. [2], revealing a significant, albeit small, age effect on HLC patterning. This may suggest normal protective changes in HLC movement as people age. However, having a cohort composed of only healthy adults may have attenuated this effect, given the dipropionate cluster of Type I and II users compared to the far fewer Type III users. The motor control literature may provide further insight, particularly the notion that older adults may have a poorer ability to modulate timely cessation of muscle activity due to decreased regulation of cortical inhibition [35]. Additionally, functional compensation to increased task demands due to an overactivation of cortical recruitment is a normal observation in older adults [36]. Aging muscles also tend to display longer burst activity than in younger adults as muscle movement times increase for fast velocity movements [37]. Thus, older adults may be more prone to maintaining airway closure during sequential swallows.
Aging influenced several biomechanical measures. PESmax was negatively correlated with aging, suggesting older adults were more likely to have decreased PESmax values. These results are consistent with those found by Cock et al. [20], who identified more reduced PES opening in older adults during a rapid 150 mL sequential, thin liquid swallowing task via cup. HPT, TPT, SRT, Hdur, Hm, AEdur, and PESdur were positively correlated with age. Thus, as age increased, these values also increased. We further explored these interactions through GEE modeling, which revealed apparent between-group differences in biomechanical measures across the youngest group and oldest group, particularly for temporal measures (i.e., HPT, TPT, SRT, and Hdur). Comparisons to previous study findings can only be made for Hdur as this measure is equivalently defined to ‘start-to-max duration’ which trended toward a significant increase with age. Similar to prior evidence [38], our findings support the natural decline of oropharyngeal sensory function given only temporal measures were influenced by age. However, age-related changes may also be linked to sarcopenia, in which motoric oropharyngeal response, strength, force, and endurance decrease due to a natural loss of skeletal muscle mass [39]. Although several age-related anatomical and physiological changes in swallowing have been documented in studies utilizing discrete swallow tasks [40], growing evidence, including in our study, suggests there are functional and robust variations in motor swallowing response as people age.
Sex
While most biomechanical measures were not impacted by sex, males displayed greater Hmax and Hm than females. The findings related to Hmax corroborate prior evidence, but the directionality of results for Hm differed between sexes [4]. The aforementioned procedural differences between studies may explain the conflicting results. Further, we recognize that we did not use an anatomic scalar, which has recently shown to neutralize significant anatomical sex-based differences in displacement measures during discrete swallows [41]. However, ultrasound has captured greater Hmax in males compared to females during sequential swallowing [15]. We also wanted to maintain the integrity of the selected MBSS analysis method, which applies an absolute reference. This allows for equal comparison across the aggregate MBSS images and accounts for head movement across frames, which is commonly observed during sequential swallowing (e.g., HT position). Further, understanding an anatomic scalar’s sensitivity to capturing the effects of age and other conditions (i.e., sequential swallowing), arguably more clinically meaningful predictors of change in swallowing function, remains equivocal. For instance, there is inconsistent evidence related to age effects on Hmax when comparing results found by Brates et al. [42] and Mancopes et al. [43], who both used an anatomic scalar. To date, we are unaware of other studies that have used this approach to assess age effects on normal swallowing and none for sequential swallowing. Ultimately, until further large-scale studies can prove otherwise, there is value in both analysis methods.
HLC Type
Our study may be the first of its kind to probe associations between HLC patterning and biomechanical measures. Results from the primary analyses suggest the only significant difference between HLC types was in Hmax; however, a confident conclusion cannot be drawn as the post hoc analysis failed to show significant differences. As such, healthy adults’ physiological motor response to increased swallowing task demand appears flexible and robust despite many individuals who open their airways between swallows. Akin to a prior study of the same cohort suggests that Penetration-Aspiration Scale (PAS) scores are predictably benign during sequential swallowing in healthy adults, despite airway invasion is more common for sequential than discrete swallows [44]. Still, the small number of participants exhibiting Type III (N = 6) as compared to Types I and II (N = 41 each) and the conservative multiple comparison tests could have contributed to the nonsignificant post hoc.
Secondary analyses also showed that Hmax was the only biomechanical measure significantly different between sequential swallows and series of discrete swallows, with Type II yielding larger Hmax than Type I and series of discrete swallows. Although bolus volume was uncontrolled, sequential swallowing is often associated with larger volumes ingested per swallow and in total, particularly for Type II than Type I [5]. Characteristics of Type I and series of discrete swallows are similar, except the HLC partially returns to rest in the former, whereas it returns completely in the latter. This may explain why Hmax had a similar trend across these behaviors. Several studies have also reported that hyoid displacement is sensitive to bolus volume, with larger amounts resulting in increased movement [29, 45,46,47]. Perhaps, the increased Hmax seen with Type II was related to the presumed larger boluses ingested during sequential swallowing versus series of discrete swallows. These findings indicate that healthy adults generate greater HLC displacement during sequential swallowing, likely contributing to more complete airway closure throughout the set of sequential swallows. Clinicians assessing patients with difficulty managing smaller boluses should consider trialing sequential swallow tasks as it may yield a more robust HLC response, and it may serve as a stress test for airway protection.
Swallow Order
Swallow order effects were identified for OPT, TPT, SRT, and HL. More prolonged temporal measures (OPT, TPT, SRT) were noted for the second swallow than the first swallow, possibly due to a refractory period between the first and subsequent swallows during sequential swallowing. This phenomenon has been observed in esophageal peristalsis when performing successive swallows, during which any subsequent muscle contractions may exhibit a poorer response to ongoing excitatory stimuli due to neural and peripheral inhibition [48, 49]. Conversely, kinematics (HL) may appear more prominent for the first swallow, which could be explained by the HLC traveling a greater distance needed to achieve maximum displacement for the first swallow, whereas the HLC does not return to rest for subsequent swallows and only partial displacement must be achieved. Only Chi-Fishman and Sonies [15] previously explored swallow order, indicating significantly larger Hmax for subsequent swallows compared to the first swallows. In contrast, we found no difference. However, the comparisons made in the prior study [15] were based on unbalanced swallow groups within each set of sequential swallows, precisely 53 first swallows and 265 subsequent swallows. The authors also applied an arbitrary between-swallow cycle cutoff rate to define sequential swallowing [4, 15].
It is beneficial to understand potential inter-swallow biomechanical differences that may explain the complexity of sequential swallowing, especially when applying this task to vulnerable patients who have difficulty closing their airways. Differences in biomechanical measures between swallows may be problematic for persons with dysphagia. Depending on the type of biomechanical measure impacted, they may experience more significant motor response burden at different points during a set of sequential swallows. Additionally, motor control relevant to swallow order may be distinct from one another, where the first swallow (voluntary) appears to be mediated by the cortex while subcortical brainstem involvement predominates during the subsequent swallows (involuntary) [50].
Limitations
First, only a single swallow trial was performed. This factor restricts the overall picture of the swallowing mechanism within each individual, and by extension, the cohort’s performance. Thus, individual variation in sequential swallowing may have been missed and should be considered for future studies. Similar to all other previous studies reviewed, an inherent limitation is that volume per swallow was uncontrolled, but this may more accurately reflect natural sequential swallowing. Measuring volume on an MBSS may result in inaccurate results since images are captured two-dimensionally, whereas volume is a three-dimensional measure. Also, only cup sips were assessed, which has been associated with larger total volume ingested in a shorter period compared to straw sips [51]. Another consideration is the MBSImP does not explicitly instruct individuals to ingest the bolus using sequential swallows, so participants may have interpreted the instructions differently. If the intention is to elicit sequential swallowing, future studies should carefully consider the instructions provided to participants. Potentially using the instructions from the Yale Swallow Protocol [8] but excluding the component regarding the rate of ingestion may be advantageous as this validated tool specifies consuming 3-oz of water using sequential swallows. Further, only the first 2 swallows of each set of sequential swallows were analyzed. This does not account for the average total number of swallows in a set of sequential swallows in healthy adults, possibly impacting how the oropharyngeal musculature responds from swallow initiation to termination. Lastly, age groups were unbalanced, with more participants in the youngest group than the two older groups.
Conclusions
Limited research currently exists regarding sequential swallowing in healthy adults. We contribute a sizeable normative dataset for a standardized sequential, thin liquid swallowing task. There was variability in HLC patterning and sequential swallowing biomechanics. Thus, shying away from using sequential swallows in assessments may yield an incomplete understanding of the patient’s swallowing mechanism. Therefore, clinicians should consider using this swallow task to test the complex motor coordination of the swallowing system. Comparison of patient data to the established norms could improve diagnostic accuracy and the development of targeted treatment plans. Further, several factors influenced sequential swallowing, including age (HLC type, HPT, TPT, SRT, Hdur), sex (Hmax, Hm), HLC patterning (Hmax, only when controlling for series of discrete swallows), and swallow order (OPT, TPT, SRT, HL). These factors help to contextualize the normative data.
Future Considerations
A microanalysis of sequential swallowing characteristics is challenging without a clearly defined operational definition, which is lacking in the literature. Our work intends to begin the discussion about developing a robust and standardized definition for sequential swallowing. Based on our study, we recommend focusing on the physiological response of the pharynx during liquid ingestion instead of the drinking behavior (e.g., drinking rapidly). Researchers and clinicians are encouraged to consider the proposed characteristics that distinguish sequential from a series of discrete swallows: the former should involve at least two successive swallows, the HLC does not return to rest between swallows, and the pharynx lacks complete patency between swallows. Subsequent swallows performed to clear pharyngeal residue represent an alternative compensatory motor strategy and should also be discerned from ‘true’ sequential swallowing. Future research should consider applying a patient cohort for comparison to normative data. Building the normative database for sequential swallowing would also benefit from including all components of each set of sequential swallows. Lastly, the effects of volume, viscosity, and other sequential swallowing ingestion conditions should be considered and may generate new insights that further inform the clinical utility of this swallow task.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Chi-Fishman G, Stone M, McCall GN. Lingual action in normal sequential swallowing. J Speech Lang Hear Res. 1998;41(4):771–85. https://doi.org/10.1044/jslhr.4104.771.
Daniels SK, Corey DM, Hadskey LD, et al. Mechanism of sequential swallowing during straw drinking in healthy young and older adults. J Speech Lang Hear Res. 2004;47(1):33–45. https://doi.org/10.1044/1092-4388(2004/004).
Lederle A, Hoit JD, Barkmeier-Kraemer J. Effects of sequential swallowing on drive to breathe in young, healthy adults. Dysphagia. 2012;27(2):221–7. https://doi.org/10.1007/s00455-011-9357-x.
Chi-Fishman G, Sonies BC. Motor strategy in rapid sequential swallowing: new insights. J Speech Lang Hear Res. 2000;43(6):1481–92. https://doi.org/10.1044/jslhr.4306.1481.
Daniels SK, Foundas AL. Swallowing physiology of sequential straw drinking. Dysphagia. 2001;16(3):176–82. https://doi.org/10.1007/s00455-001-0061-0.
Dozier TS, Brodsky MB, Michel Y, Walters BC Jr, Martin-Harris B. Coordination of swallowing and respiration in normal sequential cup swallows. Laryngoscope. 2006;116(8):1489–93. https://doi.org/10.1097/01.mlg.0000227724.61801.b4.
Chen PC, Chuang CH, Leong CP, Guo SE, Hsin YJ. Systematic review and meta-analysis of the diagnostic accuracy of the water swallow test for screening aspiration in stroke patients. J Adv Nurs. 2016;72(11):2575–86. https://doi.org/10.1111/jan.13013.
Suiter DM, Sloggy J, Leder SB. Validation of the Yale Swallow Protocol: a prospective double-blinded videofluoroscopic study. Dysphagia. 2014;29(2):199–203. https://doi.org/10.1007/s00455-013-9488-3.
Ward M, Skelley-Ashford M, Brown K, Ashford J, Suiter D. Validation of the Yale Swallow Protocol in post-acute care: a prospective, double-blind, multirater study. Am J Speech Lang Pathol. 2020;29(4):1937–43. https://doi.org/10.1044/2020_AJSLP-19-00147.
Gürgör N, Arıcı Ş, Kurt Incesu T, Seçil Y, Tokuçoğlu F, Ertekin C. An electrophysiological study of the sequential water swallowing. J Electromyogr Kinesiol. 2013;23(3):619–26. https://doi.org/10.1016/j.jelekin.2012.12.003.
Brodsky MB, McFarland DH, Dozier TS, et al. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head Neck. 2010;32(4):481–9. https://doi.org/10.1002/hed.21209.
Garand KLF, Bhutada AM, Hopkins-Rossabi T, Mulekar MS, Carnaby G. Pilot study of respiratory-swallow coordination in amyotrophic lateral sclerosis. J Speech Lang Hear Res. 2022;65(8):2815–28. https://doi.org/10.1044/2022_JSLHR-21-00619.
Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM. Respiratory-swallowing coordination and swallowing safety in patients with Parkinson’s disease. Dysphagia. 2011;26:218–24. https://doi.org/10.1007/s00455-010-9289-x.
Bennett JW, Van Lieshout PH, Pelletier CA, Steele CM. Sip-sizing behaviors in natural drinking conditions compared to instructed experimental conditions. Dysphagia. 2009;24(2):152–8. https://doi.org/10.1007/s00455-008-9183-y.
Chi-Fishman G, Sonies BC. Kinematic strategies for hyoid movement in rapid sequential swallowing. J Speech Lang Hear Res. 2002;45(3):457–68. https://doi.org/10.1044/1092-4388(2002/036).
Murguia M, Corey DM, Daniels SK. Comparison of sequential swallowing in patients with acute stroke and healthy adults. Arch Phys Med Rehabil. 2009;90(11):1860–5. https://doi.org/10.1016/j.apmr.2009.05.014.
Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol. 1994;76(2):714–23. https://doi.org/10.1152/jappl.1994.76.2.714.
Susa C, Kagaya H, Saitoh E, et al. Classification of sequential swallowing types using videoendoscopy with high reproducibility and reliability. Am J Phys Med Rehabil. 2015;94(1):38–43. https://doi.org/10.1097/PHM.0000000000000144.
Tsushima C, Saitoh E, Baba M, et al. Hyoid movement and laryngeal penetration during sequential swallowing. J Med Dent Sci. 2009;56(3):113–21. https://doi.org/10.11480/jmds.560303.
Cock C, Omari TI, Burgstad CM, Thompson A, Doeltgen SH. Biomechanical correlates of sequential drinking behavior in aging. Neurogastroenterol Motil. 2021;33(1):e13945. https://doi.org/10.1111/nmo.13945.
Steele CM, Van Lieshout PH. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia. 2004;19(3):192–206. https://doi.org/10.1007/s00455-004-0006-5.
Steele CM, Van Lieshout P. Tongue movements during water swallowing in healthy young and older adults. J Speech Lang Hear Res. 2009;52(5):1255–67. https://doi.org/10.1044/1092-4388(2009/08-0131).
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment [published correction appears in J Am Geriatr Soc 67(9)]. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImP: establishing a standard. Dysphagia. 2008;23(4):392–405. https://doi.org/10.1007/s00455-008-9185-9.
Northern Speech Services (2020) Modified Barium Swallow Impairment Profile. https://www.mbsimp.com.
Kendall KA, Leonard RJ. Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg. 2001;127(10):1224–9. https://doi.org/10.1001/archotol.127.10.1224.
Kendall KA, McKenzie S, Leonard RJ, Gonçalves MI, Walker A. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(2):74–83. https://doi.org/10.1007/s004550010004.
Kendall KA, Leonard RJ, McKenzie SW. Accommodation to changes in bolus viscosity in normal deglutition: a videofluoroscopic study. Ann Otol Rhinol Laryngol. 2001;110(11):1059–65. https://doi.org/10.1177/000348940111001113.
Leonard R, Kendall KA, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19(2):133–41. https://doi.org/10.1007/s00455-003-0508-6.
Leonard R, Kendall K. Dysphagia assessment and treatment planning: a team approach. 4th ed. San Diego: Plural Publishing Inc; 2019.
Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. https://doi.org/10.1177/001316446002000104.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research [published correction appears in J Chiropr Med 16(4):346]. J Chiropr Med. 2017;15(2):155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Clain AE, Alkhuwaiter M, Davidson K, Martin-Harris B. Structural validity, internal consistency, and rater reliability of the Modified Barium Swallow Impairment Profile: breaking ground on a 52,726-patient, clinical data set. J Speech Lang Hear Res. 2022;65(5):1659–70. https://doi.org/10.1044/2022_JSLHR-21-00554.
Martin-Harris B, Humphries K, Garand KLF. The Modified Barium Swallow Impairment Profile (MBSImP™©)—innovation, dissemination and implementation. Perspect ASHA Spec Interest Groups. 2017;2(13):129–38. https://doi.org/10.1044/persp2.SIG13.129.
Motawar B, Stinear JW, Lauer AW, Ramakrishnan V, Seo NJ. Delayed grip relaxation and altered modulation of intracortical inhibition with aging. Exp Brain Res. 2016;234(4):985–95. https://doi.org/10.1007/s00221-015-4527-y.
Humbert IA, Robbins J. Dysphagia in the elderly. Phys Med Rehabil Clin N Am. 2008;19(4):853–x. https://doi.org/10.1016/j.pmr.2008.06.002.
Theou O, Edwards D, Jones GR, Jakobi JM. Age-related increase in electromyography burst activity in males and females. J Aging Res. 2013. https://doi.org/10.1155/2013/720246.
Ding R, Logemann JA, Larson CR, Rademaker AW. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. J Speech Lang Hear Res. 2003;46(4):977–89. https://doi.org/10.1044/1092-4388(2003/076).
Madhavan A. Preclinical dysphagia in community dwelling older adults: what should we look for? Am J Speech Lang Pathol. 2021;30(2):833–43. https://doi.org/10.1044/2020_AJSLP-20-00014.
Jardine M, Miles A, Allen J. A systematic review of physiological changes in swallowing in the oldest old. Dysphagia. 2019;35(3):509–32. https://doi.org/10.1007/s00455-019-10056-3.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. 2014;57(3):768–78. https://doi.org/10.1044/2014_JSLHR-S-13-0152.
Brates D, Steele CM, Molfenter SM. Measuring Hyoid excursion across the life span: anatomical scaling to control for variation. J Speech Lang Hear Res. 2020;63(1):125–34. https://doi.org/10.1044/2019_JSLHR-19-00007.
Mancopes R, Gandhi P, Smaoui S, Steele CM. Which physiological swallowing parameters change with healthy aging? OBM Geriatr. 2021. https://doi.org/10.21926/obm.geriatr.2101153.
Garand KLF, Hill EG, Amella E, Armeson K, Brown A, Martin-Harris B. Bolus airway invasion observed during videofluoroscopy in healthy, non-dysphagic community-dwelling adults. Ann Otol Rhinol Laryngol. 2019;128(5):426–32. https://doi.org/10.1177/0003489419826141.
Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume on swallow-induced hyoid movement in normal subjects. AJR Am J Roentgenol. 1988;150(6):1307–9. https://doi.org/10.2214/ajr.150.6.1307.
Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(3):146–52. https://doi.org/10.1007/s004550010017.
Nagy A, Molfenter SM, Péladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. Biomed Res Int. 2014. https://doi.org/10.1155/2014/738971.
Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol. 1981;241(2):G129–36. https://doi.org/10.1152/ajpgi.1981.241.2.G129.
Sifrim D, Jafari J. Deglutitive inhibition, latency between swallow and esophageal contractions and primary esophageal motor disorders. J Neurogastroenterol Motil. 2012;18(1):6–12. https://doi.org/10.5056/jnm.2012.18.1.6.
Aydogdu I, Tanriverdi Z, Ertekin C. Dysfunction of bulbar central pattern generator in ALS patients with dysphagia during sequential deglutition. Clin Neurophysiol. 2011;122(6):1219–28. https://doi.org/10.1016/j.clinph.2010.11.002.
Veiga HP, Fonseca HV, Bianchini EM. Sequential swallowing of liquid in elderly adults: cup or straw? Dysphagia. 2014;29(2):249–55. https://doi.org/10.1007/s00455-013-9503-8.
Acknowledgements
This work was partially supported by the Veterans Affairs CDA-1 (RR&D1IK1RX001628-01A1 to Kendrea Garand), the National Institute on Deafness and Other Communication Disorders (K24DC12801 to Bonnie Martin-Harris), the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, National Center for Advancing Translational Sciences (TL1 TR000061 to Kathleen Brady, Project PI: Kendrea Garand), the American Speech-Language-Hearing Foundation to Kendrea Garand, and the Evelyn Trammell Trust (to Bonnie Martin-Harris).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Ethical Approval
IRB approval was initially obtained from the Medical University of South Carolina (Protocol #: 00011566).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ambrocio, K.R., Miles, A., Bhutada, A.M. et al. Defining Normal Sequential Swallowing Biomechanics. Dysphagia 38, 1497–1510 (2023). https://doi.org/10.1007/s00455-023-10576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-023-10576-z