Abstract
The purpose of this systematic review was to examine the application of event-related potentials (ERPs) to investigate neural processes of swallowing functions in adults with and without dysphagia. Computerized literature searches were performed from three search engines. Studies were screened using Covidence (Cochrane tool) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards (PRISMA-2009). A total of 759 studies were initially retrieved, of which 12 studies met inclusion criteria. Electrophysiological measures assessing swallowing functions were identified in two major ERP categories: (1) sensory potentials and (2) pre-motor potentials. Approximately 80% of eligible studies demonstrated strong methodological quality, although most employed a case series or case–control study design. Pharyngeal sensory-evoked potentials (PSEPs) were used to assess pharyngeal afferent cortical processing. The temporal sequence of the PSEP waveforms varied based on the sensory stimuli. PSEPs were delayed with localized scalp maps in patients with dysphagia as compared to healthy controls. The pre-motor ERPs assessed the cortical substrates involved in motor planning for swallowing, with the following major neural substrates identified: pre-motor cortex, supplementary motor area, and primary sensorimotor cortex. The pre-motor ERPs differed in amplitude for the swallow task (saliva versus liquid swallow), and the neural networks differed for cued versus non-cued task of swallowing suggesting differences in cognitive processes. This systematic review describes the application of electrophysiological measures to assess swallowing function and the promising application for furthering understanding of the neural substrates of swallowing. Standardization of protocols for use of electrophysiological measures to examine swallowing would allow for aggregation of study data to inform clinical practice for dysphagia rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing is a complex and dynamic sensorimotor process, which involves the timely integration of an estimated 30 pairs of head and neck muscles innervated by five cranial nerves receiving information from various cortical and subcortical structures. Dysphagia (disordered swallowing) is a highly prevalent symptom in various patient populations, including neurogenic conditions (e.g., stroke, Parkinson’s disease), cancers of the head and neck, and pulmonary conditions (e.g., chronic obstructive pulmonary disease) [1,2,3]. Dysphagia can substantially impact the quality of life, often leading to health care burden due to hospitalizations resulting from aspiration pneumonia, malnutrition, and dehydration [4,5,6].
Over the past 20 years, research in swallowing assessment and rehabilitation has increasingly employed functional neuroimaging techniques to better understand the neural substrates of swallowing and neural reorganization [7,8,9,10,11,12,13,14,15]. Neuroimaging techniques include functional magnetic resonance imaging (fMRI), transcranial magnetic stimulation (TMS), positron emission tomography (PET), electroencephalography (EEG), and magnetoencephalography (MEG). Limitations in using neuroimaging techniques to evaluate swallowing functions have been described in previous literature [7,8,9, 16]. For example, fMRI can be elicited only in a supine position, which is not reflective of what occurs during normal eating behaviors and is not considered an optimal position because of alterations in swallowing biomechanics and related pressures that impact swallow safety and efficiency [8]. Further, the limited temporal resolution eliminates monitoring of swallowing neural responses before and after swallow [14]. Although MEG provides high temporal resolution, the myoelectric discharges affect swallowing-related brain activity of interest [7].
EEG is a non-invasive and relatively inexpensive electrophysiological technique that uses scalp electrodes to measure the underlying brain electrical activity that reflects post-synaptic potentials generated from neurons that have a similar radial orientation with the scalp [17]. Event-related potentials (ERPs), which are digitally extracted from the EEG, measure time-locked neural activity in response to sensory, motor, or cognitive events [18]. The evoked potential recordings measure the current from cortical dipoles generated with the activation of specific regions of cortical neurons [19, 20]. ERPs provide excellent temporal synchronization in response to the neural conduction and integration of the cortical changes related to both motor (efferent) and/or sensory (afferent) behavioral changes [21,22,23,24,25,26,27,28,29,30,31,32]. Accordingly, ERPs provide the ability to measure the dynamic swallowing mechanism with millisecond precision. Therefore, ERPs afford the enhanced ability to study the cortical neural processing for motor planning, motor, and sensory pathways of swallowing compared to other aforementioned techniques [21,22,23,24,25,26,27,28,29,30,31,32].
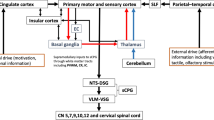
Over the past two decades (Fig. 1), the swallowing literature has employed ERPs to assess cortical neural substrates involved in swallowing behaviors and assess dysphagia treatment-induced cerebral reorganization [19,20,21,22,23,24,25,26,27,28,29,30]. Although the conceptualization of ERPs commenced with employing sensory ERPs in the early 1990s, the studies between 2003 and 2009 assessed pre-motor ERPs, more specifically, the neural substrates for motor-readiness potentials [20,21,22,23]. Pre-motor ERPs assess the cortical neural substrates involved in the motor planning and initiation of swallowing prior to the regulation of the motor plan by the central pattern generators [16]. The pre-motor ERPs used included the movement-related cortical potential (MRCP)/Bereitschafts potential (BP) and contingent negative variation (CNV) (Table 1) [22,23,24,25]. MRCP and BP are motor-readiness potentials that were used to evaluate the neural substrates in the planning/preparatory phase of swallow, while CNV assessed differences in the cognitive processes (attention) during cued versus non-cued tasks.
Since 2011, however, there has been a transition towards exploring the cortical processing of the afferent pathways of swallowing by measuring sensory ERPs. These studies have employed sensory ERPs to measure cortical processing of oropharyngeal afferent information during stimulation of oropharyngeal structures via different stimulations (mechanical, electrical, chemo-sensory) (Table 1). One type of sensory ERP, the pharyngeal sensory-evoked potentials (PSEPs), has been specifically used in swallowing research.
A 2015 review by Jestrovic et al. [16] highlighted EEG analysis techniques and summarized the utility of various EEG components to investigate various swallowing functions limited to neurotypical adults. Unfortunately, this review did not appraise eligible studies for study quality. Further, since that review was published, several studies have employed ERPs to evaluate swallowing functions, particularly in neurogenic dysphagia populations, to understand treatment-induced neuroplastic changes by employing several swallowing-related kinematic and timing measures as observed on videofluoroscopy (i.e., videofluoroscopic swallow study or VFSS). The current study aimed to examine the application of ERPs in assessing neural processes of swallowing function in adults with and without dysphagia, and to summarize and critically appraise the research employing ERPs in the swallowing literature.
Method
For reporting, the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses were followed [39].
Search Strategy
A search strategy was developed and implemented in three electronic bibliographic databases (PubMed, Scopus, and CINAHL). Next, the search strategy results were imported to an online platform (Covidence: www.covidence.org, Melbourne, VIC, Australia) for independent review. All parameters of interest were extracted using a spreadsheet. We reviewed all studies retrieved from the selected database from inception until September 2020 based on eligibility. The key search terms used in this review were “Deglutition” OR “Swallowing” OR “Deglutition disorders” OR “Oropharyngeal dysphagia” AND “EEG” OR “Electroencephalography” OR “Evoked Potentials” OR “Event-Related Potentials.”
Eligibility Criteria
A single author with previous experience in performing systematic reviews (AB) independently screened articles by title, abstract, and full text. Questionable inclusion was discussed with another author (TD), and a consensus judgment was made to determine eligibility. Inclusion criteria were as follows: (1) original article; (2) adult population; and (3) investigation employed ERPs to study swallowing behaviors. Exclusion criteria were as follows: (1) use of another neuroimaging technique to EEG; (2) ERPs measures were not collected for purposes of studying swallowing behavior; (3) study examined additional physiologic behaviors (e.g., respiration); (4) study lacked sufficient details of ERP methodology employed; (5) duplicated article in search engine results; or (6) non-English article.
Data Extraction
We obtained the following data from each included study based on our aim of the study: (a) study identification: first author, year; (b) design characteristics: type of study design and type of ERPs; (c) study sample characteristics: sample size, demographic data (age, gender), healthy versus patients with oropharyngeal dysphagia; (d) ERP elicitation characteristics: stimulus used for ERP elicitation, EEG setup, sampling rate, electrodes details, epochs, reference electrodes, and filters; (e) swallowing evaluation characteristics: use of instrumentation, swallowing outcome measures; and (f) Scalp topography: ERPs identified and neural substrates. Due to the wide variety of study types, patient populations, and types of ERPs and swallowing evaluation methods, currently it was not possible to analyze the data across studies quantitatively. Instead, a descriptive (narrative) synthesis of the findings across studies was completed while critically evaluating the risk of bias of their methods and results. The results are grouped based on type of ERPs (motor vs sensory), and a more global comparison of the results is also presented to explain the salient findings and conclusions for ERPs and swallowing outcomes of the test.
Quality Assessment
Two authors (AB and TD) independently judged the strength of evidence and level for each eligible article. If there was disagreement, a third author (KLG) resolved the conflict(s) to establish consensus. To determine the level of evidence based on study design, “The Oxford Centre for Evidence-Based Medicine Levels of Evidence” [40] was employed. In addition, study quality was assigned using the 14-item QualSyst critical appraisal tool [41]. The following scoring criteria were used for each applicable parameter: a score of “2” was awarded if criteria were completely met; “1” was awarded for partial criteria; and “0” was awarded if criteria were not met. Items that did not qualify for judgment were labeled “not applicable” [41]. Cumulative scores were calculated for each study, and a percentage score was then determined. Each study was subsequently judged based on quality: a score > 80% was considered strong quality; a score between 60 and 79% was considered good quality; a score between 50 and 59% was considered average quality, and a score < 50% was designated as poor quality [41].
Results
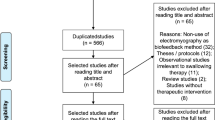
The search strategy initially identified 759 papers. Of these, 12 studies met eligibility criteria. Details of extraction are represented in Fig. 2. We extracted and grouped the data by the type of ERPs to better understand the relationship between the influence of swallow tasks and its impact on scalp topography/neural substrates. The results are provided to address the aim of the study to assess the application of ERPs assessing neural processes of swallowing function in adults with and without dysphagia.
Study Design and Methodological Quality
As outlined in Table 2, the majority (n = 7; 58%) of studies were case series (level 4). Three studies (25%) were case–control design (3b), and the remaining two studies (17%) employed a low-quality randomized control design (2b). The majority of the studies (n = 10; 83%) demonstrated “strong” quality evidence. The majority (n = 8; 67%) of studies lacked sufficient description of participant selection and related methodological aspects, such as sampling strategy, adequate demographic details, and details about informed consent.
Participant Characteristics
Seven studies (58%) investigated healthy adults, including three studies that used healthy individuals as a control group and a single study that also included persons with epilepsy as a subgroup. Five studies (40%) investigated various patient populations with oropharyngeal dysphagia, including stroke, Parkinson’s disease, and other neurogenic causes.
For studies involving healthy adults, the sample sizes ranged from 7 to 30, with an overall sample size of 112. The sample size for patient population-based studies ranged from 6 to 21, with an overall sample size of 147. The age range was 18–72 years old in healthy participants, and 65–82 years old for patient populations. The majority of studies (60%; n = 7) had an equal distribution of sex, while two studies did not provide sex distribution of participants.
Types of ERPs
Tables 3 and 4 and Fig. 1 describe the types of ERPs and the electrophysiological protocol used in the investigation. Eight studies (66%) measured sensory potentials that evaluated the oropharyngeal sensory mechanism. A single study that measured sensory potentials from faucial pillar stimulation was termed glossopharyngeal evoked potential (GP) [21]. Wheeler-Hegland and colleagues [26] first identified the sensory-evoked potentials from pharyngeal stimulation as pharyngo-sensory-evoked potentials (PSEP). Four studies (30%) elicited PSEPs with intrapharyngeal electrical stimulation, whereas the other three (25%) studies used intrapharyngeal mechanical stimuli (air puffs) (Table 3). Collectively, the PSEPs and GP were used to understand the role of the cortical neural substrates involved in the afferent pharyngeal swallowing mechanism, determine sensory thresholds for the urge to swallow, and assess the efficacy of novel pharmacological treatments using transient receptor potentials (TRP) agonist (capsaicin, TRPA1) on swallow safety and neural reorganization in persons with dysphagia [31, 32].
Studies that evaluated cortical processing for motor readiness/motor preparation and/or effects of cognition were categorized into pre-motor potentials. Pre-motor potentials were measured in approximately four (30%) of the studies and included MRCP, BP, and CNV [22,23,24,25]. These potentials measured the pre-motor activity at approximately 500 ms prior to swallow onset. The cortical networks for motor planning and initiation differed based on swallow tasks and cued versus volitional swallowing tasks.
ERP Waveform Morphology
The PSEP latencies varied based on the stimuli used to evoke PSEPs. The PSEPs elicited by mechanical stimulation were characterized as early-mid latencies within the range of 60–170 ms for the healthy population. The temporal sequence was P1 = 50–70 ms, N1 = 80–110 ms, P2 = 100–122 ms, and N2 = 145–170 ms [26]. On the other hand, the PSEPs evoked via intrapharyngeal electrical stimulation were characterized as mid-late latencies within the range of 70–315 ms, with the negative polarity of the first peak and temporal sequence of N1 = 56–80 ms, P1 = 120–150 ms, N2 = 220–270 ms, and P2 = 300–350 ms [29, 30]. Interestingly, a single-study eliciting sensory-evoked potentials via mechanical stimulation to the faucial pillars and lips (termed as “Glossopharyngeal evoked potential” or GP) generated P1, N1, P2, N2, and P3 components within the range of 10–35 ms [21].
Similar to measuring motor preparation activity for limb movements, the motor-readiness potentials MRCPs/BP and CNV were measured 1.5 and 2.0 s before the onset of the suprahyoid muscle in both command and volitional swallowing tasks [25]. There were differences in the early slope for the BP based on the swallowing task (liquid vs saliva swallow). For example, the early slope for BP was not present for the liquid swallowing task, and the amplitude of positive potential was larger for liquid swallow when compared to saliva swallow. Further, the swallow-related MRCPs differed from the limb-related motor tasks in terms of polarity and amplitudes (Table 4). The CNV differed based on the swallow command and swallow task and had a larger amplitude for the command swallow task when compared to the volitional swallow task (Table 4).
Swallowing Neural Substrates and Scalp Topography
The number of electrodes employed to obtain the scalp signals ranged from 3 to 68 electrodes. All studies adhered to the International 10–20 classification system for electrode placement. In PSEP studies, the most frequently used electrode sites were in the fronto-central-parietal regions (F3, Fz, F4, C3, Cz, C4, CP3, CPz, CP4, P3, P4, Pz), (Table 3). In contrast, pre-motor ERP studies typically used electrode sites located in fronto-central regions (C3, Cz, C4, F3, Fz, F4) (Table 4).
The Standardized Low-Resolution Brain Electromagnetic Tomography (sLORETA) was utilized in four of the total studies (30%) to identify the neural substrates for the sensory ERPs [29,30,31,32]. One study also applied cortical stimulation mapping along with ERPs for functional cortical mapping of the motor ERPs [23]. Table 5 describes the neural substrates across both healthy adults and adults with dysphagia. In studies assessing PSEPs in healthy adults employing the intrapharyngeal electrical stimulation, the P1 was more localized to the prefrontal cortex and inferior frontal gyrus; N1 was localized to the primary motor (BA 4), and sensory cortex (BA 2 and 3), and supplementary motor area [(SMA) BA 6]; P2 was localized to the cingulate cortex; and N2 was localized in primary somatosensory cortex (BA 2 and 3), the primary motor cortex (BA 4), and SMA (BA 6) [29, 30]. The scalp topography in a healthy population for P1 was located more posterior-central/lateral, while the N1 peak was generated in midline/pre-central regions. The N2 generators were in posterior-central regions and N2 was more diffusely spread (Fig. 3). On the other hand, in patients with oropharyngeal dysphagia, the peaks were asymmetrical and more localized distributed to a hemisphere [30].
The pre-motor ERP studies assessed the motor preparedness/planning prior to the swallow. The most frequent neural substrates identified for pre-motor evoked potentials were the primary somatosensory [Brodmann areas (BA) 2 and 3] and primary motor cortices (BA 4) supplementary motor area (BA 6), and anterior cingulate cortex (BA 32 and 24) [22, 23, 25].
Swallow Tasks and Identification of Swallow Signals
The swallow tasks employed during investigations were dependent upon the study purpose (Table 6). One third of studies assessing PSEP used mechanical stimulation to the pharynx via an air puff or used pharyngeal electrical stimulation while recording scalp signals [26,27,28,29,30,31,32] (Table 3). Four studies (30%) all assessing pre-motor ERPs identified a swallow signal using a surface electromyogram (sEMG) [22,23,24,25]. The pre-motor ERP studies (30%) employed either saliva/dry swallows or thin liquids swallow for determining neural substrates in terms of the motor preparatory phase of swallowing. Nanako et al. [25] were the only studies that evaluated neural substrates based on swallow commands (cued versus non-cued swallowing). The frequency of swallow trials employed ranged from a single trial to over 50 trials.
Association of Swallowing Safety, Biomechanics, and ERP Components
Four studies (30%) employed videofluoroscopy to assess swallow safety and efficiency in addition to measuring ERPs, although all studies collected VFSS and ERP measures on different visits. Out of three studies evaluating PSEPs, N1 and N2 latencies were delayed in patients with oropharyngeal dysphagia. An association was observed between delayed LVC and delayed latencies/reduced amplitude of PSEPs, demonstrating impaired afferent processing/feedback of sensory stimuli for safe and efficient swallowing (Table 3) [30,31,32].
Discussion
This systematic review highlights the different types of electrophysiological measures, ERP recording methodology, and swallowing stimuli used in assessing swallowing neural substrates. We have identified studies assessing swallowing functions employing ERPs in terms of two established themes: (1) sensory potentials assessing cortical neural correlates for pharyngeal afferent pathways; and (2) pre-motor potentials assessing the role of the cerebral cortex. Studies differed in methodology for eliciting ERPs; hence, it is difficult to compare studies or aggregate data to employ additional statistical analyses to perform a meta-analysis.
Sensory ERPs
The sensory ERPs assessed the neural networks for the pharyngeal afferent mechanism to determine the pharyngeal thresholds for swallow initiation and investigate cerebral reorganization following sensory enhancement pharmacological treatments in patients with oropharyngeal dysphagia [26,27,28,29,30,31,32]. The P1 and N1 peaks characterize the arrival of the afferent information from the oropharyngeal regions to the sensory cortex; the later peaks, P2 and N2, may reflect integration with the motor cortex [26]. We observed differences in PSEP waveform morphology and latencies based on type of stimuli and varying ERP acquisition methods. On mechanical stimulation of the pharynx in healthy adults, the PSEPs were identified as earlier latencies within the range of 60–160 ms, with an initial positive peak, sequenced as P1, N1, P2, and N2. However, intrapharyngeal electrical stimulation elicited late latencies PSEPs ranging from 70 to 315 ms in healthy adults, with an initial negative peak, sequenced as N1, P1, N2, and P2 [30]. We attribute these morphology and latency differences to the task that may have activated different sensory receptor channels. The transient receptor potential channel ankyrin 1 (TRPA1) is responsible for recognition of the mechanosensory functions [42], whereas the voltage-gated ion channels of the pharyngeal branch of the glossopharyngeal nerve are responsive for intrapharyngeal electrical stimulation [50]. Because of different sensory receptors and voltage-gated ions, there might be differences in the generation of action potentials for the afferent nerves that then elicit differences in the morphology and latencies of the PSEPs. In addition, we attribute the differences in scalp topography and morphology across the PSEP studies to their differences in their reference electrodes. As outlined in Table 3, the reference electrodes differed ranging from C7 vertebrae, linked earlobe, and a single earlobe across studies. Further, there is wide heterogeneity across the number of electrodes, signal-processing methods, swallowing stimuli used, and application of signal-processing measures. We recommend, therefore that future investigations take caution and not cross compare PSEPs that differ in stimulus techniques.
Pre-motor ERPs
Four studies (25%) evaluated the role of the cerebral cortex in motor planning and initiation of a volitional swallow by evaluating the motor-readiness potential [30,31,32,33]. Interestingly, studies evaluating pre-motor potentials are limited to the early 2000s and only involved healthy participants. Surprisingly, the lack of subsequent contributions may be due to the application of other neuroimaging such as transcranial magnetic stimulation [43, 44]. The motor-readiness potential expanded on the role of the supplementary motor area in swallow preparation and initiation. In addition, task-specific differences in the neural substrates of cortical processing were noted such as differences in cued/command swallow versus volitional swallowing. The influence of cueing on cortical swallowing processing was also established via investigating CNV potentials suggesting additional cognitive processes are involved during cued swallow tasks because of anticipation for the stimuli. These findings expand our understanding of differences noted during normal swallowing based on swallow commands and may also have potential clinical implications for dysphagia rehabilitation specifically for the population with cognitive decline. The neural substrates highlighted via pre-motor ERPs are in agreement with the information identified via other neuroimaging techniques [11, 45,46,47]. We believe the limitations of pre-motor event-related potentials research could be due to the confabulation of myogenic potentials from the tongue and jaw movements involved during swallowing. These additional movements restrict the abilities to procure quality event-related potential recordings. Further, there is growing evidence of the use of other neuroimaging techniques such as TMS, to study the pre-motor potentials and that have advantageous over the aforementioned shortcomings [43,44,45,46].
Experiment Recording and Design Considerations
The studies included in this review varied with the use of electrode sites for data collection of ERPs from 3 to 68 electrodes. Studies that employed fewer electrodes provide less informative topographical maps, and therefore, essential to acquire signals from a larger cerebral surface area [12,13,14]. The current best practice guidelines suggest 32 electrode sites are appropriate for effective signal acquisition [48]. Another factor essential for the fidelity of data collection is to control for the eye blinks (alpha control). The EEG signals are often confabulated by eye blinks, myogenic artifacts, and environmental noise [17]. These can affect the signal-to-noise ratio and, thus, the overall reliability of data analysis. Unfortunately, verbal instructions for alpha control were reported in half of the studies [21,22,23,24, 31,32,33,34,35,36]. Based on current best practices, verbal instructions to participants should be provided so that they remain alert during the ERP recordings, and this leads to a reduction in alpha waves and better identification of the desired ERPs.
Highly relevant to patient populations, an individual with dysphagia may present with drooling and an open-mouth posture. These extraneous myogenic movements are other important movement considerations that potentially cause artifacts during ERP acquisitions. There are cross-system interactions between the respiratory and swallowing systems; therefore, while designing experiments, it is important to control for the confabulation of respiratory-related event potentials (RREPs) [49,50,51]. Despite heterogeneity for data acquisition across studies, the findings of his review formulate the need for the development of reliable and standardized ERP experimental protocols for future research investigations. Further, we postulate that graduate programs in speech-language pathology generally do not provide education related to ERP experiments and signal processing for swallowing behaviors. Thus, it would be beneficial to develop tutorials/guidelines for the training and application of these paradigms considering the utility of ERPs in terms of ease of availability and cost effectiveness. There is also a need for studies simultaneously assessing both motor-readiness potentials and sensory ERPs. Further studies may benefit from pre-motor ERPs to understand different neural networks based on dual-task paradigm, compensatory techniques such as chin tuck, effects of cueing and bolus volume, and viscosity differences. At last, PSEPs can be used to enhance our understanding of the sensory pathways from the pharynx to the cortex and serve as an outcome tool for novel treatment approaches such as that focus on the sensory enhancement of oropharyngeal regions especially transient receptor potentials [transient receptor potential vanilloid 1 (TRPV1), transient receptor potential ankyrin 1 (TRPA1), and transient receptor potential melastatin 8 (TRPM8)] utilizing pharmacological agents such as capsaicin and piperine [52].
Limitation
We acknowledge that there are limitations to the review. First, due to the heterogeneous nature of the studies, we were unable to perform a meta-analysis. Second, the studies included were restricted to papers published in the English language. Further, we did not include studies that used another imaging technique to assess neural substrates of swallowing. We acknowledge that studies in this review were limited to ERPs and did not include EEG articles of swallowing that employed advanced EEG analyses related to network theories and motor imagery to investigate swallowing rehabilitation outcomes [53,54,55,56,57]. Future, studies may benefit including qualitative EEG and network-based EEG methods to assess utility of swallow functions along with EEG. In addition, the data extraction procedures were performed by a single author; however, quality assignment was performed by two authors, and in case of conflict, it was resolved by a third author. Finally, the current study was not registered under systematic review registry.
Conclusion
ERPs can enhance understanding of the cortical neural substrates in the swallowing mechanism. This systematic review provides an overview of the application of ERPs to assess central neural substrates involved in swallowing functions. The ERPs elicited differed based on the stimuli used for acquisition, and there was considerable heterogeneity across studies in terms of ERPs of interest, stimuli, and methods. Yet, the scope of ERPs is promising to elucidate the cognitive processes depending on the swallowing task, novel dysphagia treatment-induced neuroplasticity, and related biomechanical changes on the swallowing physiology specifically because of cost effectiveness and accessibility of EEG setup in most research and hospital settings.
References
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18:311–5.
Hutcheson KA, Nurgalieva Z, Zhao H, Gunn GB, Giordano SH, Bhayani MK, et al. Two-year prevalence of dysphagia and related outcomes in head and neck cancer survivors: an updated SEER-medicare analysis. Head Neck. 2019;41:479–87.
Attrill S, White S, Murray J, Hammond S, Doeltgen S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv Res. 2018;18:594.
Allen J, Greene M, Sabido I, Stretton M, Miles A. Economic costs of dysphagia among hospitalized patients. Laryngoscope. 2020;130:974–9.
Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Ciucci MR, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus Off J Int Soc Dis Esophagus. 2018;31:1–7.
Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–39.
Malandraki GA, Johnson S, Robbins J. Functional magnetic resonance imaging of swallowing function: from neurophysiology to neuroplasticity. Head & Neck. 2011;33:S14-20.
Loose R, Hamdy S, Enck P. Magnetoencephalographic response characteristics associated with tongue movement. Dysphagia. 2001;16:183–5.
Michou E, Raginis-Zborowska A, Watanabe M, Lodhi T, Hamdy S. Repetitive transcranial magnetic stimulation: a novel approach for treating oropharyngeal dysphagia. Curr Gastroenterol Rep. 2016;18:10.
Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–71.
Maezawa H. Cortical mechanisms of tongue sensorimotor functions in humans: a review of the magnetoencephalography approach. Front Hum Neurosci. 2017;11:134.
Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, et al. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–55.
Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–95.
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–50.
Jestrović I, Coyle JL, Sejdić E. Decoding human swallowing via electroencephalography a state-of-the-art review. J Neural Eng. 2015;12:051001.
Luck SJ. An introduction to the event-related potential technique. 2nd ed. Cambridge, MA, USA: A Bradford Book; 2014.
Woodman GF. A brief introduction to the use of event-related potentials (ERPs) in studies of perception and attention. Atten Percept Psychophys. 2010;72(8):2031–46.
Handy TC. Event-related potentials: A methods handbook. Cambridge: MIT Press; 2005.
Chan P-YS, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105:1106–13.
Fujiu M, Toleikis JR, Logemann JA, Larson CR. Glossopharyngeal evoked potentials in normal subjects following mechanical stimulation of the anterior faucial pillar. Electroencephalogr Clin Neurophysiol. 1994;92:183–95.
Huckabee M-L, Deecke L, Cannito MP, Gould HJ, Mayr W. Cortical control mechanisms in volitional swallowing: the bereitschaftspotential. Brain Topogr. 2003;16(1):3–17.
Satow T, Ikeda A, Yamamoto J, Begum T, Thuy DHD, Matsuhashi M, et al. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: a movement-related cortical potential study. Am J Physiol-Gastrointest Liver Physiol. 2004;287:G459–70.
Hiraoka K. Movement-related cortical potentials associated with saliva and water bolus swallowing. Dysphagia. 2004;19(3):155–9.
Nonaka T, Yoshida M, Yamaguchi T, Uchida A, Ohba H, Oka S, et al. Contingent negative variations associated with command swallowing in humans. Clin Neurophysiol. 2009;120:1845–51.
Wheeler-Hegland K, Pitts T, Davenport PW. Peak morphology and scalp topography of the pharyngeal sensory-evoked potential. Dysphagia. 2011;26:287–94.
Wheeler-Hegland K, Pitts T, Davenport PW. Cortical gating of oropharyngeal sensory stimuli. Front Physiol. 2011;1:167.
Pitts T, Hegland KW, Sapienza CM, Bolser DC, Davenport PW. Alterations in oropharyngeal sensory evoked potentials (PSEP) with Parkinson’s disease. Respir Physiol Neurobiol. 2016;229:11–6.
Rofes L, Ortega O, Vilardell N, Mundet L, Clavé P. Spatiotemporal characteristics of the pharyngeal event-related potential in healthy subjects and older patients with oropharyngeal dysfunction. Neurogastroenterol Motil. 2017;29:e12916.
Cabib C, Ortega O, Vilardell N, Mundet L, Clavé P, Rofes L. Chronic post-stroke oropharyngeal dysphagia is associated with impaired cortical activation to pharyngeal sensory inputs. Eur J Neurol. 2017;24:1355–62.
Tomsen N, Ortega O, Rofes L, Arreola V, Martin A, Mundet L, et al. Acute and subacute effects of oropharyngeal sensory stimulation with TRPV1 agonists in older patients with oropharyngeal dysphagia: a biomechanical and neurophysiological randomized pilot study. Ther Adv Gastroenterol. 2019;12:1756284819842043.
Tomsen N, Alvarez-Berdugo D, Rofes L, Ortega O, Arreola V, Nascimento W, et al. A randomized clinical trial on the acute therapeutic effect of TRPA1 and TRPM8 agonists in patients with oropharyngeal dysphagia. Neurogastroenterol Motil. 2020;32:e13821.
Shibasaki H, Hallett M. What is the bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56.
Deecke L. Planning, preparation, execution, and imagery of volitional action. Cogn Brain Res. 1996;3:59–64.
Walter WG, Cooper R, Aldridge VJ, Mccallum WC, Winter AL. Contingent negative variation: an electric sign of sensori-motor association and expectancy in the human brain. Nature. 1964;203:380–4.
Rektor I, Bareš M, Brázdil M, Kaňovský P, Rektorová I, Sochǔrková D, et al. Cognitive- and movement-related potentials recorded in the human basal ganglia. Mov Disord. 2005;20:562–8.
Brunner JF, Olsen A, Aasen IE, Løhaugen GC, Håberg AK, Kropotov J. Neuropsychological parameters indexing executive processes are associated with independent components of ERPs. Neuropsychologia. 2015;66:144–56.
Taylor BK, Gavin WJ, Davies PL. The test-retest reliability of the visually-evoked contingent negative variation (CNV) in children and adults. Dev Neuropsychol. 2016;41:162–75.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ British Medical Journal Publishing Group. 2021;372:n71.
Howick J, Phillips B, Ball C, Sackett D, Badenoch D, Straus S, et al. Oxford centre for evidence-based medicine levels of evidence. 2009. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/.
Kmet LM, Lee RC, Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Her Found Med Res. 2004. https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?ID=32004000313&ID=32004000313
Rofes L, Arreola V, López I, Martin A, Sebastián M, Ciurana A, et al. Effect of surface sensory and motor electrical stimulation on chronic poststroke oropharyngeal dysfunction. Neurogastroenterol Motil. 2013;25:888-e701.
Cabib C, Nascimento W, Rofes L, Arreola V, Tomsen N, Mundet L, et al. Neurophysiological and biomechanical evaluation of the mechanisms which impair safety of swallow in chronic post-stroke patients. Transl Stroke Res. 2020;11:16–28.
Magara J, Watanabe M, Tsujimura T, Hamdy S, Inoue M. Lasting modulation of human cortical swallowing motor pathways following thermal tongue stimulation. Neurogastroenterol Motil. 2021;33:3938.
Hamdy S. Role of cerebral cortex in the control of swallowing. GI Motil Online [Internet]. 2006.
Wilmskoetter J, Daniels SK, Miller AJ. Cortical and subcortical control of swallowing—can we use information from lesion locations to improve diagnosis and treatment for patients with stroke? Am J Speech Lang Pathol. 2020;29:1030–43.
Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22:266–75.
Woodman GF. A brief introduction to the use of event-related potentials in studies of perception and attention. Atten Percept Psychophys. 2010;72:2031–46.
von Leupoldt A, Keil A, Chan P-YS, Bradley MM, Lang PJ, Davenport PW. Cortical sources of the respiratory-related evoked potential. Respir Physiol Neurobiol. 2010;170:198–201.
Chan P-YS, Davenport PW. Respiratory related evoked potential measures of cerebral cortical respiratory information processing. Biol Psychol. 2010;84:4–12.
von Leupoldt A, Keil A, Davenport PW. Respiratory-related evoked potential measurements using high-density electroencephalography. Clin Neurophysiol. 2011;122:815–8.
Cabib C, Ortega O, Kumru H, Palomeras E, Vilardell N, Alvarez-Berdugo D, et al. Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function. Ann N Y Acad Sci. 2016;1380:121–38.
Cuellar M, Harkrider AW, Jenson D, Thornton D, Bowers A, Saltuklaroglu T. Time–frequency analysis of the EEG mu rhythm as a measure of sensorimotor integration in the later stages of swallowing. Clin Neurophysiol. 2016;127:2625–35.
Jestrović I, Coyle JL, Sejdić E. Characterizing functional connectivity patterns during saliva swallows in different head positions. J NeuroEng Rehabil. 2015;12:61.
Yang H, Guan C, Chua KSG, Chok SS, Wang CC, Soon PK, et al. Detection of motor imagery of swallow EEG signals based on the dual-tree complex wavelet transform and adaptive model selection. J Neural Eng. 2014;11:035016.
Jestrović I, Coyle JL, Sejdić E. Differences in brain networks during consecutive swallows detected using an optimized vertex-frequency algorithm. Neuroscience. 2017;344:113–23.
Jestrović I, Coyle JL, Perera S, Sejdić E. Influence of attention and bolus volume on brain organization during swallowing. Brain Struct Funct. 2018;223:955–64.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhutada, A.M., Davis, T.M. & Garand, K.L. Electrophysiological Measures of Swallowing Functions: A Systematic Review. Dysphagia 37, 1633–1650 (2022). https://doi.org/10.1007/s00455-022-10426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10426-4