Abstract
Tongue strength and its role in the pathophysiology of dysphagia in adults are well accepted and studied. An objective and reliable measurement of tongue strength in children necessitates equally good methodology, knowledge of influencing factors, and normative data. Only limited data on testing tongue strength in children are available thereby limiting its potential use. The present study examined tongue strength and several parameters known to be important in adults in the largest sample of healthy children from 3 to 11 years old to date using the Iowa Oral Performance Instrument with standard bulbs. Tongue strength increases markedly for children between 6 and 7 years, with slower increases before and after this age. Unlike adults, no influence of sex or location was found on the maximum tongue strength in children, and visual feedback was found to be counterproductive in obtaining the highest tongue pressures. The normative data obtained can be used for objective assessment of tongue weakness and subsequent therapy planning in dysphagic children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of normally developing children will never experience serious or prolonged swallowing problems. Whenever dysphagia is present in children, an interdisciplinary approach based on objective data is warranted to diagnose and treat medical conditions, problems with oral motor control and function, and behavioral and/or sensory issues that may interfere with normal feeding and swallowing [1]. The tongue not only plays a role in bolus formation but is also the primary propulsive agent for the food bolus and therefore crucial for bolus clearance in the oral phase; both an effective oral and a pharyngeal phase are needed to accomplish an efficient and safe oropharyngeal swallow [2, 3]. In adults, it is clear that diminished lingual strength and coordination contribute to dysphagia [4,5,6]; this relation in children, however, remains less proven. Conflicting evidence exists on the relation between tongue strength and speech disorders in children, while data linking tongue strength to pediatric dysphagia are anecdotal [7, 8]. Objective evaluation of lingual functioning provides a valid and necessary addition to the clinical swallowing evaluation and should ideally replace unreliable subjective assessments [5]. Progress in this field has been slow thereby forcing clinicians to act often on data and insights extrapolated from adults and/or anecdotal evidence [9,10,11]. Appropriate instruments to measure physiologic events in children need to be available, not only in research settings but also in clinical practice. The Iowa Oral Performance Instrument (IOPI) was conceptualized originally to measure tongue strength of children by Dr. Erich Luschei in 1988 (personal communication). Nowadays the use of both the IOPI-device and its standard tongue bulbs is deemed suitable in both children and adults [12, 13]. To make the most efficient use out of objective tongue strength values, researchers and clinicians alike should be able to compare their results with normative data, preferably obtained in a large group of healthy subjects with additional information on several methodological issues and influences that are known to impact tongue strength [13]. Cross-national research in children is warranted based on previous research in adults [13], and new information needs to be compared with prior investigations [12] to determine agreement and replication. Therefore, the current study aimed to provide the first European data on tongue strength in children and to supplement the exploration of age- and sex-related differences in these measures. The influence of additional parameters was also evaluated to address potential methodological questions; the rationale for these parameters is discussed next.
30–60 s
The optimal interval duration between consecutive maximum lingual strength efforts is a valid research question. A previous study remarked that due to a decrease in the best trial within a testing block, 30 s may not be adequate time for recovery between trials in healthy children [12]. The answer to this question can provide important methodological information; if lingual maximum isometric pressure (MIP) values are similar for 30 and 60 s intervals, the total testing time in children can be greatly reduced with probable increases in acceptability, feasibility, and planning of the test.
Repeated Trials
The standard procedure in adults used by almost all researchers involves three consecutive trials to determine the MIP [13, 14]. Little information can be found on the need to adhere to this procedure in children. Potter reported that although the first trial of each measurement session did not consistently produce the greatest score, the first trial was the highest on average, followed by the second and third trials [12]. Robin et al., however, reported consistent performance across trials, albeit for a small sample of six school-aged children [8]. Additional data are needed to improve the methodology of tongue strength testing in children.
Age and Sex
The available dataset of MIP in healthy children is still limited [12], making the exact influence of both age and sex unclear. Since age was found to influence MIP in a sample where several age groups were missing [12], additional data are needed. Further investigation of potential sex differences in MIP is also needed since such differences were previously documented in adults [13], but were absent in children within the age range studied here [12].
Visual Feedback
The influence of visual feedback (VFB) on MIP in adults has previously been demonstrated [13], but no clear data on the impact of feedback in children are available. A previous study on lingual MIP in children did not directly compare the effect of VFB on MIP and took an ad-hoc approach, whereby older children and adolescents watched the IOPI display and younger children were motivated primarily by the examiner’s encouragement [12]. No systematic comparison or operational definition of younger versus older is provided in the manuscript.
Bulb Position (Location)
All prior data on tongue strength in children using the standard tongue bulb focused solely on anterior tongue strength [12], notwithstanding the important propulsive function of the posterior tongue [15]. This study will for the first-time address both anterior and posterior tongue body function in children (abbreviated as MIPA and MIPP, respectively).
Order of Testing
From a methodological point of view, it would be interesting to investigate the order of testing on MIPA and MIPP. No such an effect was found in adults [13] but has never been investigated in children.
Methods
Participants
To be included in the study, the participants had to be of Caucasian descent, within the specified age range (3 to 11 years), speak Dutch as the mother tongue, and report being in good general physical and mental health (by the account of their parents or legal counsel). General exclusion criteria were a history of speech–language therapy for any language and/or articulation disorders [8, 13, 16, 17], and being born with a cleft lip and/or palate. Any child with a possible history of dysphagia was excluded because this could be indicative of an as-yet undocumented underlying abnormal tongue function or strength. An active orthodontic treatment was also considered an exclusion criterion since it could point to malocclusion [18] and/or abnormal palatal dimensions [19]; avoidance of any possible damage to the orthodontic equipment by the bulb and connecting tube was another safety consideration to exclude these children. Naturally occurring variations in dentition were accepted when there was no interference with the oral mechanism examination. Further exclusion criteria were any history of major medical illnesses, such as respiratory disease; neurologic trauma, disease, or insult; major head or neck surgery; or any form of cancer. The presence of exclusion criteria was determined by questionnaire for the parents/legal counsel and an oral mechanism examination. The use of medication was allowed provided the underlying condition was not an exclusion criterion.
There were two parts to the study. In part one, the effect of the interval duration between consecutive trials was evaluated in 18 children (2 for each age from 3 to 11 years). They were selected using a convenience sample, with some effort to obtain an equal sex distribution to prevent any possible sex effects (resulting in the inclusion of 10 girls and 8 boys).
The total number of children included in the second and main part of this study was 198, with an equal sex and age distribution among the nine age categories (N = 22 for every year from 3 to 11 years) to allow maximum power for detecting possible age-related influences, unlike available data where the number was limited to 10 for a selection of ages (namely 4, 6, 8, and 10 years) [12]. Children were recruited as a convenience sample in kindergartens and primary schools, sporting and dancing clubs, etc. Informed consent was obtained for every child included in the study through the parents or legal counsel. This procedure was in compliance of the institution’s procedures and policies for human-subjects research and in accordance to the norms of The Declaration of Helsinki (2013 Version) and following the Directives of the European Union on Good Clinical Practice (111/3976/88 of July 1990).
Instrumentation
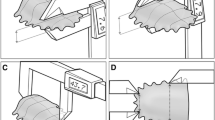
The IOPI (model 2.1; IOPI Medical LLC, Carnation, WA; Fig. 1a) was used to measure the tongue strength. The IOPI is a portable device measuring the amount of pressure exerted on a small air-filled PVC bulb, measuring 35 mm from the base to the tip. Pressures obtained are digitally displayed (expressed in kPa) via an LCD panel located on the instrument. As an instrument measuring tongue function, the IOPI has been utilized in a large number of published experiments [4, 5, 8, 12, 13, 20,21,22,23,24] and has an established high inter- and intrajudge reliability [5, 25]. A new bulb was used for every participant because of hygienic concerns and to minimize measurement error due to possible compliance variations of the bulb after extended use. The use of the IOPI in children has previously been demonstrated as excellent; children as young as 3 years of age were able to tolerate the IOPI standard tongue bulb [12]. IOPI calibration was checked weekly, as recommended by the manufacturer, to ensure accurate measurement.
Hand strength for both dominant (defined as the hand used for writing) and nondominant hand were obtained using a Lafayette hydraulic hand dynamometer Jamar and expressed as kilogram.
For every child, several additional parameters were obtained, namely date of birth permitting the calculation of age in months, and body weight using a digital scale.
Procedures
Tongue strength was measured by obtaining maximal tongue-to-palate elevation pressures. Instructions to the participants were to “place this bulb in your mouth on the midline of your tongue and push it against the roof of your mouth as hard as you can.” In order to maximize standard placement, the examiner demonstrated placing the bulb along the central groove of the tongue blade; for additional details on placement, please refer to the “Bulb Position (Location)” section further on in the manuscript. Since previous research indicated that maximal measures of tongue strength and endurance are best assessed with an unconstrained jaw, participants were encouraged to gently rest their incisors on the tubing of the IOPI bulb [4, 20, 26]. All trials were motivated by verbal encouragement from the examiner [12, 13, 20] and lasted about 3 to 10 s. The strength measurement was completed three times, with a brief resting period of about 30 s between each trial, while the examiner recorded the peak pressure obtained. This procedure is similar to previous research, both in children and adults [12, 13].The highest pressure across the three trials was used as the participants’ maximal isometric pressure (MIP) instead of using the mean pressure, as other researchers do [21, 27]. Since the correlation between averaged and maximal pressure is high and both are similarly related to oral-phase swallowing function [5], the use of the maximal pressure is more efficient in a clinical setting because it requires no calculation.
30–60 s Interval
The first part of this study was designed to solve an initial methodological question: what is the effect of the interval between three repetitive MIP measurements (30 vs. 60 s)? Every child was tested twice on the same day starting with three trials with either a 30 or 60 s interval in the morning (both MIPA and MIPP) and this protocol was repeated using the alternate interval in the afternoon, in an effort to account for a potential day-to-day variability in tongue strength due to lack of sleep, previous activities, or other.
The Remainder of the Study
No adaptations to the study methods were made other than the choice for the 30 s interval between repeated trials.
Factors
To investigate if similar factors, known to influence MIPA and MIPP in adults [13] also played a role in children, several conditions were evaluated, including the need for and stability of the three trials, age, sex, bulb position, VFB, and the order of testing.
Repeated Trials
Children performed repetitive maximum tongue strength measures with both VFB and location randomized; an interval of 30 s was used and 2–5 min of rest between conditions. A possible interaction effect with sex was examined.
Age and Sex
The age range of the participants covered every year of the whole childhood period. The year of age used when comparing between-groups differences; the exact age in months was used when evaluating correlations between MIPs and hand strength measures.
Visual Feedback
Based on the existing literature on the development of the sense of numbers and the need to comprehend double-digit figures on the LCD screen of the IOPI, only children of 7 years or older were included in the VFB analyses as to maximally accommodate any differences in mathematical learning skills [28,29,30,31]. No formal assessment of mathematical skills was performed as its scope was deemed outside this protocol.
To evaluate the influence of VFB, tongue strength was measured with and without feedback in a randomized starting order. When allowed VFB, the participants got a clear view on the LCD display of the IOPI during each of the three trials. Verbal encouragement was provided in both conditions.
Bulb Position (Location)
To measure MIPA, the bulb was placed longitudinally along the hard palate just posterior to the upper alveolar ridge, where compression was exerted by the anterior tongue (~ 10 mm posterior to the tongue tip). After placing the bulb, cautious traction was applied to the connecting tube in order to ensure consistent placement of the base of the bulb against the oral aspect of the upper incisors. MIPP was measured with the tip of the bulb placed at the posterior edge of the hard palate, where contact is made by the posterior tongue (~ 10 mm anterior to the most posterior circumvallate papilla; Fig. 1b). The posterior measurement was only performed in children of age 6 years or older, since the dimensions of the oral cavity of younger children were too small to have a meaningful difference in distance between anterior and posterior [32]. Once the bulb was appropriately positioned on the posterior tongue, the researcher marked the point where the tubing running from the intraoral bulb to the connective tube met the labial aspect of the upper incisors using a permanent marker and a piece of tape, providing both a visual reference for the researcher and a tactile reference for the child (Fig. 1c). The distance between the base of the bulb (corresponding to the anterior position) and the marking for the posterior position was measured in millimeters (AP-distance). There were no participants who could absolutely not tolerate the posterior position because of a gag response. While the anatomy of the participants clearly varied due to differences in the shape of the upper alveolar ridge and palate, the consistent instruction to the participants and the placement demonstration and visual inspection of the individual markings by the researchers allowed for reliable bulb placement between trials and across participants. A similar approach was used by other authors [13, 33,34,35].
Order of Testing
To determine whether there was an effect caused by the order of testing, all participants were randomized to a particular sequence, i.e., anterior or posterior tongue location or lips (results of the lip dataset will not be discussed in this manuscript) as the first test position.
Data Analysis
All data analyses were made using SPSS v24 (IBM, Armonk, NY, USA) with the additional WRS2 package allowing for robust tests [36] wherever possible, since modern robust methods provide improved techniques for dealing with outliers, skewed distribution, and heteroskedasticity. Robust tests provide substantial gains in power as well as a more accurate and nuanced analysis of data [37]. Data on anterior and posterior tongue strengths were analyzed for normal distribution using the Shapiro–Wilk test. Descriptive statistics [means and 95% confidence intervals (CIs) of the mean, medians, and minimum and maximum values] were calculated for all variables. To investigate a possible influence of the interval between consecutive MIP measurements (either 30 or 60 s), a robust test of two dependent means was used [37, 38]. The reliability of the three trials during the different conditions was evaluated using the robust ANOVA tests of 20% trimmed means [36, 37]. The effect of age and order was studied using ANOVA with appropriate post-hoc procedures [36, 37]. The comparison between girls and boys was done using a robust independent t test since the assumption of normality was violated in both conditions without VFB [36,37,38].
All within-group comparisons (VFB and bulb location) employed a robust test of two dependent means since the normal distribution of the differences between the two measurements was violated [37, 38]. To investigate a possible influence of the order of testing on the different MIP measures, a one-way ANOVA with order as a between-groups variable was performed.
Pearson correlation coefficients with bootstrapping (to account for nonnormal distributions) were calculated between MIPA and MIPP in both feedback conditions, between MIPs, and hand strength (both dominant and nondominant), between MIPs and weight, and between age and the AP-distance difference. Multiple regression was performed for MIPA without VFB with the predictors nondominant hand strength, age, and body weight using forced entry, based on prior research [12]. An α of 0.05 was used to determine significance for all comparisons. The effect size was calculated using r, \(\eta_{\text{p}}^{2}\), and ω2, depending on the statistical test used.
Results
Feasibility
Feasibility in this study was excellent since > 99% of the protocol could be completed in the study population. There were three children (all 6-year olds) in whom one trial in the posterior location could not be completed due to minor gagging.
30–60 s Interval
When comparing the difference of MIPA with VFB between 30 s intervals (Mdn = 58 kPa) versus 60 s intervals (Mdn = 54 kPa), no statistical significant difference was found (rt(4) = 1.12, 95% CI [− 4.75, 11.15], p = 0.326, r = 0.18 (small; 95% CI for r = − 0.57 to 0.77). Similar comparison of MIPA without VFB between 30 s intervals (Mdn = 50.5 kPa) versus 60 s intervals (Mdn = 50.5 kPa) also showed statistical equivalence, rt(5) = − 0.71, 95% CI [− 19.99, 11.33], p = 0.51, r = − 0.508 (large; 95% CI = − 0.85 to 0.14).
The difference between MIPP with VFB using 30 (Mdn = 54 kPa) versus 60 s intervals (Mdn = 49 kPa) was not statistically significant, rt(4) = 0.07, 95% CI [− 15.82, 16.62], p = 0.949, r = − 0.21 (small; 95% CI = − 0.78 to 0.55), nor was there a statistically significant difference when comparing MIPP without VFB between 30 s intervals (Mdn = 53 kPa) and 60 s intervals (Mdn = 51 kPa), rt(4) = − 0.18, 95% CI [− 12.83, 11.23], p = 0.862, r = − 0.21 (small, 95% CI 0.78 to 0.55).
Since no significant difference was found in any of the conditions, an interval of 30 s between repeated MIP efforts was chosen for the remainder of this study. No attempt was made to investigate the possible influence of sex, body weight, or the use of VFB since these were part of the second study.
Main Study
Descriptive statistics for the different tongue strength variables by age group, sex, and feedback condition in the main study are provided in Table 1.
Repeated Trials
The overview of the descriptive statistics for both MIPA and MIPP in each VFB condition is presented in Table 2.
MIP Anterior with Visual Feedback
Normality testing of the pressures obtained during each of the tree trials indicated that the first trial demonstrated a nonnormal distribution (p ≤ 0.005). Robust tests of 20% trimmed means showed that MIPA using VFB did significantly differ across the three trials, Ft(1.67, 76.79) = 9.44, p = 0.0005. Post-hoc testing revealed that the second trial had significantly higher tongue pressure than the first trial (p = 0.021; smaller than pcrit of 0.05), and similarly the third trial reached higher pressures than the second trial (p = 0.019; smaller than pcrit of 0.025). There was no interaction effect with sex, F(1.8, 188.16) = 0.233, p = 0.766, \(\eta_{\text{p}}^{2}\) = 0.002.
MIP Anterior Without Visual Feedback
Analysis of the normal distribution of the tongue pressure results in each of the three trials showed significant deviations in every trial on the Shapiro–Wilk test (p < 0.001). Robust tests of 20% trimmed means showed that MIPA without using VFB did not significantly differ across the three trials, Ft(1.97, 159.51) = 0.60, p = 0.547. No follow-up multiple comparisons were performed. There was no interaction effect with sex, F(1.9, 378) = 1.289, p = 0.276, \(\eta_{\text{p}}^{2}\) = 0.007.
MIP Posterior with Visual Feedback
Normality testing of the pressures obtained during each of the tree trials showed significant deviations in two out of three trials on the Shapiro–Wilk test (p ≤ 0.003). Robust testing showed that MIPP using VFB did significantly differ for the three trials conducted, Ft(1.95, 91.8) = 4.833, p = 0.0106. Follow-up post-hoc testing revealed that the difference in pressure between trials 1 and 2 was not significant (p = 0.09; greater than pcrit of 0.05), but the difference between trials 1 and 3 was (p = 0.0007; smaller than pcrit of 0.0169), as was the difference between trials 2 and 3 (p = 0.0074; smaller than pcrit of 0.025), with the third trial reaching the highest mean pressure. There was no interaction effect with sex, F(1.8, 199.599) = 2.822, p = 0.066, \(\eta_{\text{p}}^{2}\) = 0.025.
MIP Posterior Without Visual Feedback
Analysis of the normal distribution of the tongue pressure results in each of the three trials showed significant deviations in every trial on the Shapiro–Wilk test (p ≤ 0.016). The results of the robust testing show that MIPP without VFB did not significantly change over the three trials conducted [Ft(1.91, 108.99) = 0.012, p = 0.986], therefore no follow-up multiple comparisons were performed. There was, however, an interaction effect for trial by sex, F(1.9, 243.211) = 3.878, p = 0.024, \(\eta_{\text{p}}^{2}\) = 0.029. Subsequent analysis of the contrasts revealed that this effect was manifested between trials 1 and 2 where boys reached mean pressures of 49.42 and 47.35 kPa versus mean pressures of 45.35 and 46.71 kPa in girls, respectively, F(1, 130) = 9.408, p = 0.003, η 2p = 0.067. No interaction effect was present when comparing trials 2 and 3, F(1130) = 0.311, p = 0.578, \(\eta_{\text{p}}^{2}\)= 0.002.
Age
MIP Anterior with Visual Feedback
Data for this specific testing condition were available in 110 children, due to the age requirements (age ≥ 7 years) for the VFB-condition. Robust comparison of the mean tongue strength across age indicated no significant effect of age on the MIPA when using VFB, Ft = 1.45, p = 0.278, ω2 = 0.034 (small effect size). No post-hoc tests were performed. The data are represented in Fig. 2.
MIP Anterior Without Visual Feedback
Data for this specific testing condition were available in all of the 198 included children. The robust ANOVA results indicated a statistically significant difference in the effect of age categories on MIPA without VFB, Ft = 18.64, p < 0.001, ω2 = 0.372 (large effect size). Pairwise post-hoc comparisons demonstrated several significant differences between ages (Table 3). The data are represented in Fig. 3.
MIP Posterior with Visual Feedback
Data for this specific testing condition were available in 110 children. Robust ANOVA results indicate a significant effect of age on MIPP with VFB, Ft = 3.75, p = 0.019, ω2 = 0.099 (medium effect size); pairwise post-hoc comparisons identified significant pairwise comparisons between ages 7, 8, and 9 versus 10 and 11 years (Table 3). The data are represented in Fig. 2.
MIP Posterior Without Visual Feedback
Data for this specific testing condition were available for 132 children. The robust ANOVA results indicate a significant effect of age on MIPP without VFB, Ft = 18.23, p < 0.001, ω2 = 0.419 (large effect size). Pairwise comparisons demonstrated several significant differences between ages (Table 3). A graphic representation of the data can be found in Fig. 3.
Correlation Between MIP and Age
The Pearson’s r correlation coefficients with bootstrapped CIs between age (expressed in months) and MIPA and MIPP in both feedback conditions are summarized in Table 4. All correlations are significant but with small effect sizes when considering the ‘with VFB’ condition, and with large effect sizes in the ‘no VFB’ condition. Anterior and posterior MIPs were highly correlated.
Sex
MIPA with VFB (N = 110) in girls (Mdn = 49 kPa) did not differ significantly from boys (Mdn = 54 kPa), rt = − 1.19, 95% CI [− 7.50, 2.04], p = 0.25, r = 0.10 (small). Similarly, there was no statistically significant difference in MIPA without VFB (N = 198) between girls (Mdn = 40 kPa) and boys (Mdn = 44 kPa), rt = − 0.97, 95% CI [− 8.82, 3.15], p = 0.34, r = 0.07 (small).
Looking at possible differences in MIPP with VFB (N = 110), girls (Mdn = 55 kPa) had similar pressures to boys (Mdn = 55 kPa), rt = − 0.57, 95% CI [− 5.81, 3.32], p = 0.57, r = 0.05 (small). MIPP in the no VFB condition (N = 132) demonstrated once again that girls (Mdn = 52 kPa) attained similar pressures than boys (Mdn = 53.5 kPa), rt = − 0.07, 95% CI [− 5.94, 5.54], p = 0.93, r = 0.03 (small). Even when broken down by age, no significant differences between girls and boys were found in any condition.
Visual Feedback
There was no statistically significant difference in anterior tongue strength with VFB (Mdn = 51 kPa) than without (Mdn = 43 kPa), N = 110, rt(65) = − 1.05, 95% CI [− 2.68, 0.83], p = 0.298, r = 0.07 (small). When analyzed by years of age, similar nonsignificant results were obtained except for children of 10 years old; they demonstrated a significant difference (Mdn VFB = 52 kPa, Mdn no VFB = 60 kPa) with a large effect size (rt(13) = − 4.38, 95% CI [− 8.85, − 3.01], p = 0.001, r = 0.58 (large)). A graphical representation can be found in Fig. 4.
Similar results were found for the posterior location between the VFB (Mdn = 55 kPa) and the non-VFB condition (Mdn = 53 kPa), N = 110, rt(65) = 0.47, 95% CI [− 1.13, 1.83], p = 0.64, r = − 0.03 (small). A graphical representation can be found in Fig. 5.
Location
Overall, when VFB was provided, the posterior tongue (Mdn = 55 kPa) was able to reach a higher pressure than the anterior tongue (Mdn = 51 kPa), N = 110, rt(65) = − 3.14, 95% CI [− 6.17, − 1.37], p = 0.003, r = 0.26 (small). Without VFB, there also was a significant difference between MIPA (Mdn = 43 kPa) and MIPP (Mdn = 50.53 kPa), N = 132, rt(79) = − 2.03, 95% CI [− 5.12, − 0.06], p = 0.045, r = 0.14 (small). When analyzed by years of age, no significant differences between MIPA and MIPP using VFB were found except for children of 7 and 10 years. Children of 7 years old demonstrated a significant difference (Mdn MIPA = 50 kPa, Mdn MIPP = 55.5 kPa) with a medium effect size, rt(13) = − 2.35, 95% CI [− 11.79, − 0.49], p = 0.035, r = 0.42 (medium, Fig. 6). Children of 10 year showed a significant difference (Mdn MIPA = 52 kPa, Mdn MIPP = 62.5 kPa) with a large effect size, rt(13) = − 4.17, 95% CI [− 12.80, − 4.06], p = 0.001, r = 0.61 (large, Fig. 6).
Similar analysis in the no VFB condition revealed no significant differences across the age spectrum. These data are illustrated in Fig. 7.
When looking at the influence of sex when using VFB, overall MIPA in girls (Mdn = 49 kPa) was significantly lower than MIPP (Mdn = 55 kPa), N = 55, rt(32) = − 2.45, 95% CI [− 8.38, − 0.77], p = 0.02, r = 0.31 (medium). No significant differences were found in boys, MIPA (Mdn = 54 kPa), MIPP (Mdn = 55 kPa), N = 55, rt(32) = − 1.87, 95% CI [− 6.45, 0.27], p = 0.007, r = 0.20 (small). When no VFB was provided, there were a significant difference between MIPA and MIPP in girls (Mdn MIPA = 49 kPa; Mdn MIPP = 52 kPa, N = 66, rt(39) = − 2.03, 95% CI [− 6.50, − 0.01], p = 0.049, r = 0.16 (small)) but not in boys (Mdn MIPA = 51 kPa; Mdn MIPP = 53.5 kPa, N = 66, rt(39) = − 0.96, 95% CI [− 5.83, 2.07], p = 0.343, r = 0.12 (small)).
The difference between the markers for MIPA and MIPP ranged from 0 to 20 mm in the 131 children where this metric was available and showed a significant correlation with age, but not with sex (see Table 4). The mean difference in position increased from 5 mm in 6-year olds to 13 mm in 11-year olds, corresponding to the growing dimensions of the hard palate.
Order of Testing
There was no significant effect of order on MIPA with VFB, F(2, 109) = 2.76, p = 0.068, ω2 = 0.031 (small effect size). Similarly, no effect was found for any of the other conditions: MIPA without VFB, F(4, 193) = 1.83, p = 0.077, ω2 = 0.022 (small effect size); MIPP with VFB, F(2, 107) = 1.947, p = 0.148, ω2 = 0.017 (small effect size), and MIPP without VFB, F(2, 129) = 2.98, p = 0.054, ω2 = 0.029 (small effect size).
Multiple Regression
A multiple linear regression was calculated to predict MIPA without VFB based on age (expressed in months), nondominant hand strength, and body weight, based on the results from Table 4. Age and weight were found be not significant predictors and were subsequently excluded from the model. A significant regression equation was found [F(2, 79) = 30.34, p < 0.001], with a coefficient of determination (R2) of 0.434. Participants’ predicted MIPA without VFB is equal to 15.7 + 0.81 * nondominant hand strength (kg), with 95% CI for Bconstant = 7.1–24.4, and 95% CI for Bnondominant hand strength = 0.407–1.211.
Discussion
30–60 s Interval
The results revealed no statistically significant differences between the 30 and 60-s interval in 18 healthy children, independent of location or VFB. This provides additional support for the interval of 30 s as chosen in similar studies, both in children [12] and adults [13], and allows for an expedited procedure during clinical assessment. Nonetheless, confirmation of this finding in children with dysphagia or neurological development disorders (NDDs) is needed since their bulbar musculature and central nervous system could react differently; to the best knowledge of the authors, such a study has not yet been reported. Data looking at skeletal muscles’ characteristics indicate these are not affected by NDD, with several studies even demonstrating a higher resistance against fatigue when performing repetitive voluntary contractions than typically developing children, albeit set off by a lower maximal muscle strength [39, 40].
Repeated Trials
When analyzing the multiple trials in an effort to obtain a reliable maximum tongue pressure of both the anterior and posterior tongue in different feedback conditions, a similar effect of VFB is present at both locations. Performing MIP measurements in children with the use of VFB results in highly variable results, with the final trial reaching the highest pressure, thereby contradicting the findings of Potter and Short [12], although the use of VFB was not a specific hypothesis in their study. Such variability was completely absent in the no VFB condition.
Our results suggest that children systematically performed with better stability when not using additional external feedback (apart from the standard motivational verbal encouragement); the execution of a maximal muscle effort while simultaneously trying to decode the VFB resulted in suboptimal muscle performance. These findings are the opposite of the findings in adults where permitting VFB resulted in more stable maximal pressures [13]. The effect of VFB will be discussed further on in the manuscript. Our results can support the use of a single, well-executed MIP measurement without VFB when clinicians are faced with time constraints or poor child cooperation.
Age
When looking at the effect of age on MIPA when using VFB, no significant differences were found in that subset of children between 7 and 11 years old. The maximum number of children is included in the MIPA without VFB condition where the results clearly divide the children into two age groups with lower MIPs in 3–6-year olds versus higher pressures in 7–11-year olds; children of 10 years of age reached the highest MIPs.
At the posterior location, a small difference is found between children of 7, 8, and 9 years and their stronger counterparts of 10 years in the MIPP with VFB. Analyzing the maximum number of children in the posterior location in the MIPP without VFB subgroup, a similar effect of age was found as in the MIPA without VFB condition, namely, 6-year-old children have lower MIPs than 7–11-year-old children, with 10-year-old children again achieving the highest MIPs.
Overall, the effect of age on MIP in different conditions can be summarized as a rather slow increase in tongue strength between the ages of 3 and 6, a marked increase between ages of 6 and 7 years and again a subtle increase as children age further, with peak values at the age of 10 years. Comparison with available data (limited to MIPA without VFB) shows a similar trend, whereby tongue strength increases suddenly around the age of 6–7 years [12].
Data on skeletal muscle strength confirm this increase of muscle strength with age in healthy Dutch children from 8 to 20 years old [41]. In a study looking at lower extremity muscle strength in American 6- to 8-year-old children, the authors found that the strength produced by the 8-year-old children was significantly greater than the strength produced by the 7-year-old children, and similarly the strength of 7-year-old was greater than 6-year-old children [42].
Sex
This dataset provides no arguments for differences in MIP, regardless of feedback or location, between girls and boys. This corresponds to the findings of Potter and Short [12]. No further data on sex differences in bulbar muscle strength in children are available on this topic to the best of knowledge of the authors. This finding indicates a remarkable difference between bulbar muscles and skeletal muscles where pronounced sex differences are found. When assessing lower limb strength through a standing jump protocol in preschool children (age 3–6 years) [43] and in prepubertal children (5- to 11-year old) [44, 45], boys had greater muscle strength than girls. A similar sex difference was noted in upper-extremity muscular power (as assessed by a ball throw test), although the rate of strength development showed a significant sex by age interaction effect, where girls demonstrated a slightly larger performance progression compared to boys [46].
Visual Feedback
The impact of VFB on MIP in children has not been studied before, so comparable data are unavailable. Our results suggest that—unlike adults—children have no clear benefit of using VFB. The lack of performance improvements for repetitive MIP measurements with VFB could signal this combination to be an overwhelming experience resulting in suboptimal performance. To understand the impact of VFB in this setting, its influence can be broken down into several components.
Sense of Numbers and Visual Feedback
While it is known that the human brain is equipped from birth with a rudimentary number sense, it is limited mostly to small numbers. Higher mathematical levels are reached by virtue of language and symbolic representation for large numbers, and the acquirement of awareness of ordinal relationships between numbers. Children of the first grade show individual differences in working memory capacity and counting knowledge and research has indicated that counting proficiency, and mature number sense do not emerge until the age of 8 years [47, 48]. The exact mechanisms of number learning are still under study [49]; symbolic numerical representation in young and older children recruits the same network of brain regions as adults but also recruits higher-order brain mechanisms early in development [50]. It is therefore not a complete surprise result that the VFB condition did not lead to higher MIP values since the cortical mechanisms to observe and interpret the VFB to guide motor output may not be fully matured in younger children.
The effects of VFB on enhancing isometric maximal voluntary contractions (MVCs) in different movements are complex, and their impacts can be muscle dependent [51]. In theory, assuming a positive effect of VFB on performance, its use will standardize the execution, evaluation, and comparison of results between studies when MVC is the outcome measure of maximal performance or is applied for normalization. However, the application in children when combined with numerical displays seems counterproductive. With the implementation of game-based learning modules, mastery of number concepts and number operations comprehension can be expected to be achieved faster and better [52]. The finding that discontinuing VFB seemed to have no effect on force production or the variability of the force output (and in some cases may actually diminish the variability) has been observed by other authors [53].
Verbal Encouragement and Psychological Factors
The effects of verbal feedback on muscle performance have already been studied, albeit not in children. When upper body performance in a resistance training session was studied [54], results indicate that providing verbal feedback produced acute improvements in power output of even well-trained athletes, a population in which even small improvements in power are often difficult to achieve. Additionally, Jung and Hallbeck [55] reported an increase in peak handgrip strength of approximately 5% when verbal or VFB encouragement was given reinforcing the concept that psychological strategies can improve the performance of limb-based movements [55,56,57,58].
Tongue MIP measurements require similar performances and could therefore be susceptible to the same strategies. These psychological techniques can be classified as either intrinsic (e.g., self-talk, ‘psyching-up’) or extrinsic (e.g., visual and verbal feedback and knowledge of results or performance, and encouragement), and although the exact mechanisms are unclear, improvements may be because of a combination of enhanced neuromuscular activation, intent, focus of attention, levels of arousal, and improved skill performance and learning [55,56,57,58,59]. ‘Psyching-up’ has been shown to increase isokinetic bench press strength by 11.8% when compared to a mental distraction control [58].
Outcomes in adult tongue motor performance, however, do not appear to be subject to several psychological conditions such as motivation, fun, pain, or fatigue [60], in contrast to previous findings [61]; again, this finding could indicate that children need an adapted but still fun and challenging feedback modality in order to maximize MIP measurements.
Location
There are no comparable data available in the literature on the effect of bulb location in children, since all previous studies measured only the anterior portion of the tongue (“just posterior to the alveolar ridge”). Since the posterior tongue provides critical forces for transferring food and liquid from the oral cavity into the pharynx, establishing its strength is warranted. Our data show that in children there is only a statistically significant difference between the anterior and posterior part of the tongue in the VFB condition, with the posterior tongue reaching the highest pressures. When comparing this finding to the available adult data, opposite results are found since in general the anterior tongue is the strongest in adults [13]; however, some older and smaller studies in adults demonstrated higher values in the tongue blade [23] or posterior part [62]. In this dataset, 10-year-old children demonstrated higher posterior pressures, regardless of the feedback condition, as did girls in the VFB condition. Overall, it seems fair to conclude there is no significant difference although a tendency to higher posterior pressures is present. Future studies are needed to address this question.
The correlations between anterior and posterior MIPs are strong, especially in the no VFB condition. This can be partly explained by the relative size of the 35 mm IOPI bulb to both the length of the hard palate and the length of the tongue. An AP-distance difference of 5 mm in a 6-year old child would correspond to a small (14%) change in bulb–palate contact area, while the maximum AP-distance difference of 20 mm would correspond to a change of 36% in contact area when taking the data by Vorperian as age-controlled reference values for hard palate length [32].
The strong correlation between the age and the distance between the markers for MIPA and MIPP is suggestive of a small increase in hard palate length in growing children from the ages of 6–11 years. This corresponds with available anthropometric data indicating that the upper aerodigestive tract not only increases more than twofold in length from infancy to adulthood but also changes in geometric proportions, the so-called ‘anatomic restructuring’ [32]. Most structures have an ongoing growth from age of 2 weeks to age of 7 years with a somewhat more rapid growth during approximately the first 18 months of life. At about age of 18 months, the hard palate length is at 80% of the adult size, while the tongue length only reaches 70% of its final dimension at the age of 6 years, and undergoes considerable growth until it reaches adult size. Our finding that the AP-distance difference was not sex-dependent also corresponds to these data [32, 63].
Another possible explanation is a lack of independent control of subregions of the tongue (tip, blade, dorsum, and lateral margins) in young children [64]. Relatively independent control of these regions has been documented in adult speakers [65, 66] and was reported as lacking in young children with articulation/phonological disorders [67]. These undifferentiated lingual gestures can result in the simultaneous contact of the whole of the tongue with the bulb and palate during sophisticated lingual movements where tongue tip and body are supposed to operate independently.
A further confounding factor is potential racial differences in the development of the vocal tract [68, 69] and future research can provide additional information.
Order of Testing
Similar to adults, there was no significant effect of order on the different MIPs, allowing the clinician to tailor the approach to any individual patient.
Comparison Between Children and Adults
In summary, when comparing the impact of similar factors on lingual MIP between children and adults, both similarities and differences were found. The effect of repeated trials in adults shows equivalent pressures across the three trials when using VFB but a significant higher MIP during the first trial without VFB (when compared to the second and third trial who are similar) [13]. This clearly differs from children where VFB resulted in the third trial being the final MIP while no difference was found in the no feedback condition. Age-effects are present in both categories and are mirror-like; children between 3 and 6 years old have lower MIPs and adults at the other end of the age-spectrum similarly have lower MIPs (70+ years old) [13, 20]. Sex differences were absent in children but sparingly present in adults, where males had higher MIPA values than females [13]. Comparing MIP between the anterior and posterior location in adults demonstrated significantly higher values anteriorly [13, 22]; such marked difference is absent in children where even a tendency to higher MIP values at the posterior location was noted. In both children and adults, no effect of order of testing was noted [13].
Cross-Cultural Comparison
Since previous research in adults has indicated that the MIPs of healthy adult Belgians appear to be lower than their USA peers [13], a comparison was made with the limited available USA data in children [12]. When comparing MIPA without using VFB, only a single statistical significant difference was found using an independent t-test based upon N, mean and SD; American 8-year olds have a higher MIPA than Belgian children (Table 5). No American data were available for the ages of 7, 9, and 11. The question remains when and how the apparent MIP-differences in adults originate since they don’t exist in childhood. Regarding the possible predictors for MIPA without VFB obtained in our sample population, similar results are obtained in both Belgian and USA children [12], namely nondominant hand strength as sole predictor while both age and weight came out as nonsignificant; no R2 is provided in the Potter study for comparison.
Limitations
There is a potential shortcoming in this study that needs to be discussed. The oropalatal dimensions of these children were not studied although they are known to influence anterior and posterior tongue strengths in adults [19]. The technique of using dental casts was considered too invasive and unreliable in this population since overall dimensions are considerably smaller than adults leading to a possible increase in measurement error. It is, however, clear that in the growing child the assessment of palatal dimensions is only one aspect since also tongue dimensions should be considered [32], an aspect unstudied in adults. Future studies could look at using modern digital imaging technology (intraoral scanning) as used in orthodontic clinics since these are both noninvasive and highly reliable, but not yet extensively studied in children [70, 71].
Future Directions
Deglutition and its disorders in children have longtime been evaluated using mostly subjective measures [72]; this very challenging patient population deserves more objective diagnostics, ultimately permitting better therapy [73]. Incrementally, instrumental techniques are being used to study normal and disordered deglutition in children [74]. A complete evaluation of pediatric swallowing should therefore include an objective assessment of tongue strength as there is no valid reason to assume it is less relevant to swallowing than in adults [75]. Measurement data early in life can facilitate long-term individual follow-up aiming to expedite diagnosis and possible early intervention, maybe prolonging functional swallowing and quality of life. Future studies could potentially advance our understanding of the interaction between tongue strength, tongue length and palatal dimensions. These data would probably benefit children with a history of cleft palate (since the tongue in cleft palate shows a different behavior than normal during the first phase of deglutition [76]), craniofacial malformations or neurological conditions involving bulbar musculature, both for the development of speech and swallowing. Initial data on a small number of children with repaired unilateral cleft lip and palate showed no marked differences in MIPA when compared to a small group of controls [77]; data on MIPP or in children with bilateral cleft lip and palate are not yet available.
Obstructive sleep apnea is the most severe clinical type of sleep-disordered breathing and can be caused by oral breathing, leading to a secondary malposition of the tongue, finally influencing the tongue strength [78, 79]. Knowledge of normative data may allow tongue MIP measurements and training to be stratified and randomized for intervention studies, both in children [78, 80] and adults [81] with obstructive sleep apnea.
Malocclusions are growth and developmental abnormalities in the musculoskeletal components of the skull that may result in changes in the positioning of the mandible, the hyoid bone, and the tongue [82]. However, numerous researchers have found that the tongue strength was similar among different types of malocclusion; while genetics is accepted to be the most important etiologic factor, lips and cheeks are probably more important than the tongue [83]. Consensus about the relationship between the types of malocclusion and perioral pressures has not been achieved yet, and recent research in adults indicates that MIP is affected by oropalatal dimensions [19], although it is not yet clear what is cause and effect since other authors did not find such a relationship [84].
Conclusions
Evaluating tongue function is important in the assessment and rehabilitation of swallowing disorders in adults and children. Standardized, reliable, sensitive yet practical methods to obtain quantitative measurements of lingual muscle strength for children are needed when assessing dysphagia. The authors feel that the IOPI is uniquely suited for this challenge. The clinical and scientific utility of this measure ultimately depends on a correct methodology and the availability of normative data. This article may be the next stepping-stone in this evolution and can allow clinicians to perform well-designed studies.
References
Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118–27. https://doi.org/10.1002/ddrr.17.
McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98(1):71–8. https://doi.org/10.1288/00005537-198801000-00015.
Dodds WJ. Physiology of swallowing. Dysphagia. 1989;3(4):171–8.
Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–56. https://doi.org/10.1044/1058-0360(2007/019).
Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol. 2003;12(1):40–50. https://doi.org/10.1044/1058-0360(2003/051).
Yoshida M, Kikutani T, Tsuga K, Utanohara Y, Hayashi R, Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21(1):61–5. https://doi.org/10.1007/s00455-005-9011-6.
Cooper-Brown L, Copeland S, Dailey S, Downey D, Petersen MC, Stimson C, Van Dyke DC. Feeding and swallowing dysfunction in genetic syndromes. Dev Disabil Res Rev. 2008;14(2):147–57. https://doi.org/10.1002/ddrr.19.
Robin DG, Somodi LB, Luschei ES. Measurement of tongue strength, endurance in normal and articulation disordered subjects. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: perspectives on management. Baltimore: P.H. Brookes Publ. Co.; 1991. p. 173–84.
Lefton-Greif MA, Arvedson JC. Pediatric feeding/swallowing: yesterday, today, and tomorrow. Semin Speech Lang. 2016;37(4):298–309. https://doi.org/10.1055/s-0036-1587702.
Frazier JB. Effect of tactile stimulation on lingual motor function in pediatric lingual dysphagia. Dysphagia 2007;22(4):340–2; author reply 343–52. https://doi.org/10.1007/s00455-007-9106-3.
Rogers B, Arvedson J. Assessment of infant oral sensorimotor and swallowing function. Ment Retard Dev Disabil Res Rev. 2005;11(1):74–82. https://doi.org/10.1002/mrdd.20055.
Potter NL, Short R. Maximal tongue strength in typically developing children and adolescents. Dysphagia. 2009;24(4):391–7. https://doi.org/10.1007/s00455-009-9215-2.
Vanderwegen J, Guns C, Van Nuffelen G, Elen R, De Bodt M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia. 2013;28(2):159–66. https://doi.org/10.1007/s00455-012-9425-x.
Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009;24(1):57–65. https://doi.org/10.1007/s00455-008-9171-2.
McConnel FM, Cerenko D, Mendelsohn MS. Manofluorographic analysis of swallowing. Otolaryngol Clin N Am. 1988;21(4):625–35.
Bradford A, Murdoch B, Thompson E, Stokes P. Lip and tongue function in children with developmental speech disorders: a preliminary investigation. Clin Linguist Phon. 1997;11(5):363–87. https://doi.org/10.1080/02699209708985201.
Murdoch BE, Attard MD, Ozanne AE, Stokes PD. Impaired tongue strength and endurance in developmental verbal dyspraxia: a physiological analysis. Eur J Disord Commun. 1995;30(1):51–64.
Sakaue K, Fukui T, Sasakura C, Hori K, Ono T, Saito I. Tongue pressure production during swallowing in patients with mandibular prognathism. J Oral Rehabil. 2016;43(5):348–55. https://doi.org/10.1111/joor.12379.
Pitts LL, Stierwalt JAG, Hageman CF, LaPointe LL. The influence of oropalatal dimensions on the measurement of tongue strength. Dysphagia. 2017;32(6):759–66. https://doi.org/10.1007/s00455-017-9820-4.
Clark HM, Solomon NP. Age and sex differences in orofacial strength. Dysphagia. 2012;27(1):2–9. https://doi.org/10.1007/s00455-011-9328-2.
Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A. 1996;51(5):M247–50.
Gingrich LL, Stierwalt JA, Hageman CF, LaPointe LL. Lingual propulsive pressures across consistencies generated by the anteromedian and posteromedian tongue by healthy young adults. J Speech Lang Hear Res. 2012;55(3):960–72. https://doi.org/10.1044/1092-4388(2011/10-0357).
Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A. 1995;50(5):M257–62.
Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. J Speech Hear Res. 1992;35(6):1239–45.
Adams V, Mathisen B, Baines S, Lazarus C, Callister R. Reliability of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument with healthy adults. Dysphagia. 2014;29(1):83–95. https://doi.org/10.1007/s00455-013-9486-5.
Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. J Speech Lang Hear Res. 2004;47(3):584–94. https://doi.org/10.1044/1092-4388(2004/045).
Utanohara Y, Hayashi R, Yoshikawa M, Yoshida M, Tsuga K, Akagawa Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia. 2008;23(3):286–90. https://doi.org/10.1007/s00455-007-9142-z.
Geary DC, vanMarle K, Chu FW, Rouder J, Hoard MK, Nugent L. Early conceptual understanding of cardinality predicts superior school-entry number-system knowledge. Psychological Science. 2018;29(2):191–205. https://doi.org/10.1177/0956797617729817.
Desoete A, Vanderswalmen R, De Bondt A, Van Vreckem C, Van Vooren V, Vander Beken I, Van Dycke S, Baert J. Dyscalculie. Dyscalculie. Gent: Academia; 2015. p. 96–106.
Haesaert V, Desoete A. Welke tests gebruikt men bij de diagnose van dyscalculie en waarom?. Ghent: Ghent University; 2011.
Desoete A, Ceulemans A, De Weerdt F, Pieters S. Can we predict mathematical learning disabilities from symbolic and non-symbolic comparison tasks in kindergarten? Findings from a longitudinal study. Br J Educ Psychol. 2012;82(Pt 1):64–81. https://doi.org/10.1348/2044-8279.002002.
Vorperian HK, Kent RD, Lindstrom MJ, Kalina CM, Gentry LR, Yandell BS. Development of vocal tract length during early childhood: a magnetic resonance imaging study. J Acoust Soc Am. 2005;117(1):338–50.
Kays SA, Hind JA, Gangnon RE, Robbins J. Effects of dining on tongue endurance and swallowing-related outcomes. J Speech Lang Hear Res. 2010;53(4):898–907. https://doi.org/10.1044/1092-4388(2009/09-0048).
Palmer PM, Neel AT, Sprouls G, Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. J Speech Lang Hear Res. 2010;53(6):1567–78. https://doi.org/10.1044/1092-4388(2010/09-0068).
Van den Steen L, Schellen C, Verstraelen K, Beeckman AS, Vanderwegen J, De Bodt M, Van Nuffelen G. Tongue-strengthening exercises in healthy older adults: specificity of bulb position and detraining effects. Dysphagia. 2018;33(3):337–44. https://doi.org/10.1007/s00455-017-9858-3.
Mair P, Schoenbrodt F, Wilcox RR. WRS2: Wilcox robust estimation and testing. R package version (Version 0.4-0), 2015.
Wilcox RR. Introduction to robust estimation and hypothesis testing. 4th ed. Burlington: Elsevier; 2016.
Yuen KK. The two-sample trimmed t for unequal population variances. Biometrika. 1974;61(1):165–70. https://doi.org/10.1093/biomet/61.1.165.
Eken MM, Dallmeijer AJ, Houdijk H, Doorenbosch CA. Muscle fatigue during repetitive voluntary contractions: a comparison between children with cerebral palsy, typically developing children and young healthy adults. Gait Posture. 2013;38(4):962–7. https://doi.org/10.1016/j.gaitpost.2013.05.004.
Eken MM, Harlaar J, Dallmeijer AJ, de Waard E, van Bennekom CA, Houdijk H. Squat test performance and execution in children with and without cerebral palsy. Clin Biomech (Bristol Avon). 2017;41:98–105. https://doi.org/10.1016/j.clinbiomech.2016.12.006.
Wind AE, Takken T, Helders PJ, Engelbert RH. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. 2010;169(3):281–7. https://doi.org/10.1007/s00431-009-1010-4.
Macfarlane TS, Larson CA, Stiller C. Lower extremity muscle strength in 6- to 8-year-old children using hand-held dynamometry. Pediatr Phys Ther. 2008;20(2):128–36. https://doi.org/10.1097/pep.0b013e318172432d.
Latorre Roman PA, Moreno Del Castillo R, Lucena Zurita M, Salas Sanchez J, Garcia-Pinillos F, Mora Lopez D. Physical fitness in preschool children: association with sex, age and weight status. Child Care Health Dev. 2017;43(2):267–73. https://doi.org/10.1111/cch.12404.
Grund A, Dilba B, Forberger K, Krause H, Siewers M, Rieckert H, Muller MJ. Relationships between physical activity, physical fitness, muscle strength and nutritional state in 5- to 11-year-old children. Eur J Appl Physiol. 2000;82(5–6):425–38. https://doi.org/10.1007/s004210000197.
Tambalis KD, Panagiotakos DB, Arnaoutis G, Sidossis LS. Endurance, explosive power, and muscle strength in relation to body mass index and physical fitness in Greek children aged 7–10 years. Pediatr Exerc Sci. 2013;25(3):394–406.
Golle K, Muehlbauer T, Wick D, Granacher U. Physical fitness percentiles of German children aged 9–12 years: findings from a longitudinal study. PLoS ONE. 2015;10(11):e0142393. https://doi.org/10.1371/journal.pone.0142393.
Geary DC, Hoard MK, Byrd-Craven J, DeSoto MC. Strategy choices in simple and complex addition: contributions of working memory and counting knowledge for children with mathematical disability. J Exp Child Psychol. 2004;88(2):121–51. https://doi.org/10.1016/j.jecp.2004.03.002.
Geary DC, Bailey DH, Littlefield A, Wood P, Hoard MK, Nugent L. First-grade predictors of mathematical learning disability: a latent class trajectory analysis. Cogn Dev. 2009. https://doi.org/10.1016/j.cogdev.2009.10.001.
Rips LJ, Bloomfield A, Asmuth J. From numerical concepts to concepts of number. Behav Brain Sci. 2008;31(6):623–42; discussion 642–87. https://doi.org/10.1017/s0140525x08005566.
Cantlon JF, Libertus ME, Pinel P, Dehaene S, Brannon EM, Pelphrey KA. The neural development of an abstract concept of number. J Cogn Neurosci. 2009;21(11):2217–29. https://doi.org/10.1162/jocn.2008.21159.
Toumi A, Jakobi JM, Simoneau-Buessinger E. Differential impact of visual feedback on plantar- and dorsi-flexion maximal torque output. Appl Physiol Nutr Metab. 2016; 41(5):557–9. https://doi.org/10.1139/apnm-2015-0639.
Chin LC, Zakaria E. Understanding of number concepts and number operations through games in early mathematics education. Creat Educ. 2015;06(12):1306–15. https://doi.org/10.4236/ce.2015.612130.
Athreya DN, Van Orden G, Riley MA. Feedback about isometric force production yields more random variations. Neurosci Lett. 2012;513(1):37–41. https://doi.org/10.1016/j.neulet.2012.02.002.
Argus CK, Gill ND, Keogh JW, Hopkins WG. Acute effects of verbal feedback on upper-body performance in elite athletes. J Strength Cond Res. 2011;25(12):3282–7. https://doi.org/10.1519/jsc.0b013e3182133b8c.
Jung M-C, Hallbeck MS. Quantification of the effects of instruction type, verbal encouragement, and visual feedback on static and peak handgrip strength. Int J Ind Ergon. 2004;34(5):367–74. https://doi.org/10.1016/j.ergon.2004.03.008.
Kim HJ, Kramer JF. Effectiveness of visual feedback during isokinetic exercise. J Orthop Sports Phys Ther. 1997;26(6):318–23. https://doi.org/10.2519/jospt.1997.26.6.318.
McNair PJ, Depledge J, Brettkelly M, Stanley SN. Verbal encouragement: effects on maximum effort voluntary muscle action. Br J Sports Med. 1996;30(3):243–5.
Tod DA, Iredale KF, McGuigan MR, Strange DE, Gill N. “Psyching-up” enhances force production during the bench press exercise. J Strength Cond Res. 2005;19(3):599–603. https://doi.org/10.1519/14263.1.
Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Med Educ. 2010;44(1):75–84. https://doi.org/10.1111/j.1365-2923.2009.03421.x.
Kothari M, Svensson P, Huo X, Ghovanloo M, Baad-Hansen L. Motivational conditions influence tongue motor performance. Eur J Oral Sci. 2013;121(2):111–6. https://doi.org/10.1111/eos.12022.
Kothari M, Svensson P, Huo X, Ghovanloo M, Baad-Hansen L. Force and complexity of tongue task training influences behavioral measures of motor learning. Eur J Oral Sci. 2012;120(1):46–53. https://doi.org/10.1111/j.1600-0722.2011.00894.x.
Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A. 2000;55(11):M634–40.
Vorperian HK, Wang S, Chung MK, Schimek EM, Durtschi RB, Kent RD, Ziegert AJ, Gentry LR. Anatomic development of the oral and pharyngeal portions of the vocal tract: an imaging study. J Acoust Soc Am. 2009;125(3):1666–78. https://doi.org/10.1121/1.3075589.
Cheng HY, Murdoch BE, Goozee JV, Scott D. Electropalatographic assessment of tongue-to-palate contact patterns and variability in children, adolescents, and adults. J Speech Lang Hear Res. 2007;50(2):375–92. https://doi.org/10.1044/1092-4388(2007/027).
Green JR, Wang YT. Tongue-surface movement patterns during speech and swallowing. J Acoust Soc Am. 2003;113(5):2820–33.
Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14(6):413–29. https://doi.org/10.1177/154411130301400604.
Goozee J, Murdoch B, Ozanne A, Cheng Y, Hill A, Gibbon F. Lingual kinematics and coordination in speech-disordered children exhibiting differentiated versus undifferentiated lingual gestures. Int J Lang Commun Disord. 2007;42(6):703–24. https://doi.org/10.1080/13682820601104960.
Durtschi RB, Chung D, Gentry LR, Chung MK, Vorperian HK. Developmental craniofacial anthropometry: assessment of race effects. Clin Anat. 2009;22(7):800–8. https://doi.org/10.1002/ca.20852.
Xue SA, Hao JG. Normative standards for vocal tract dimensions by race as measured by acoustic pharyngometry. J Voice. 2006;20(3):391–400. https://doi.org/10.1016/j.jvoice.2005.05.001.
Goracci C, Franchi L, Vichi A, Ferrari M. Accuracy, reliability, and efficiency of intraoral scanners for full-arch impressions: a systematic review of the clinical evidence. Eur J Orthod. 2016;38(4):422–8. https://doi.org/10.1093/ejo/cjv077.
Chalmers EV, McIntyre GT, Wang W, Gillgrass T, Martin CB, Mossey PA. Intraoral 3D scanning or dental impressions for the assessment of dental arch relationships in cleft care: which is superior? Cleft Palate Craniofac J Off Publ Am Cleft Palate Craniofac Assoc. 2016;53(5):568–77. https://doi.org/10.1597/15-036.
Hudspeth MP, Holden KR, Crawford TO. The “slurp” test: bedside evaluation of bulbar muscle fatigue. Pediatrics. 2006;118(2):e530–3. https://doi.org/10.1542/peds.2006-0043.
Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. The effects of oral-motor exercises on swallowing in children: an evidence-based systematic review. Dev Med Child Neurol. 2010;52(11):1000–13. https://doi.org/10.1111/j.1469-8749.2010.03707.x.
Arvedson JC, Lefton-Greif MA. Instrumental assessment of pediatric dysphagia. Semin Speech Lang. 2017;38(2):135–46. https://doi.org/10.1055/s-0037-1599111.
van den Engel-Hoek L, Erasmus CE, Hendriks JC, Geurts AC, Klein WM, Pillen S, Sie LT, de Swart BJ, de Groot IJ. Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment. J Neurol. 2013;260(5):1295–303. https://doi.org/10.1007/s00415-012-6793-y.
Malek R. Cleft lip and palate, lesions, pathophysiology and primary treatment. London: Martin Dunitz; 2001.
Van Lierde KM, Bettens K, Luyten A, Plettinck J, Bonte K, Vermeersch H, Roche N. Oral strength in subjects with a unilateral cleft lip and palate. Int J Pediatr Otorhinolaryngol. 2014;78(8):1306–10. https://doi.org/10.1016/j.ijporl.2014.05.017.
Villa MP, Evangelisti M, Martella S, Barreto M, Del Pozzo M (2017) Can myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing? Sleep Breath. 2017;21(4):1025–32. https://doi.org/10.1007/s11325-017-1489-2.
de Felicio CM, da Silva Dias FV, Folha GA, de Almeida LA, de Souza JF, Anselmo-Lima WT, Trawitzki LV, Valera FC. Orofacial motor functions in pediatric obstructive sleep apnea and implications for myofunctional therapy. Int J Pediatr Otorhinolaryngol. 2016;90:5–11. https://doi.org/10.1016/j.ijporl.2016.08.019.
Van Dyck C, Dekeyser A, Vantricht E, Manders E, Goeleven A, Fieuws S, Willems G. The effect of orofacial myofunctional treatment in children with anterior open bite and tongue dysfunction: a pilot study. Eur J Orthod. 2016;38(3):227–34. https://doi.org/10.1093/ejo/cjv044.
Verma RK, Johnson JJ, Goyal M, Banumathy N, Goswami U, Panda NK. Oropharyngeal exercises in the treatment of obstructive sleep apnoea: our experience. Sleep Breath. 2016;20(4):1193–201. https://doi.org/10.1007/s11325-016-1332-1.
Regalo SCH, de Lima Lucas B, Diaz-Serrano KV, Frota NPR, Regalo IH, Nassar MSP, Righetti MA, Oliveira LF, Goncalves LMN, Siessere S, Palinkas M. Analysis of the stomatognathic system of children according orthodontic treatment needs. J Orofac Orthop Organ/Off J Dtsch Ges Kieferorthop. 2018;79(1):39–47. https://doi.org/10.1007/s00056-017-0117-x.
Partal I, Aksu M. Changes in lips, cheeks and tongue pressures after upper incisor protrusion in Class II division 2 malocclusion: a prospective study. Prog Orthod. 2017;18(1):29. https://doi.org/10.1186/s40510-017-0182-0.
da Silva JB, Giglio LD, Regalo SH, de Mello-Filho FV, Trawitzki LV. Effect of dentofacial deformity on maximum isometric tongue strength. J Oral Rehabil. 2013;40(4):247–51. https://doi.org/10.1111/joor.12020.
Acknowledgements
The authors wish to thank Ellen De Keersmaecker and Janne Fret for their invaluable help in collecting the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vanderwegen, J., Van Nuffelen, G., Elen, R. et al. The Influence of Age, Sex, Visual Feedback, Bulb Position, and the Order of Testing on Maximum Anterior and Posterior Tongue Strength in Healthy Belgian Children. Dysphagia 34, 834–851 (2019). https://doi.org/10.1007/s00455-019-09976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-019-09976-x